哇巴因体外逆转肝癌耐药Bel-7402/ADM细胞的实验研究
2019-11-14杨明镇赵逵
杨明镇 赵逵
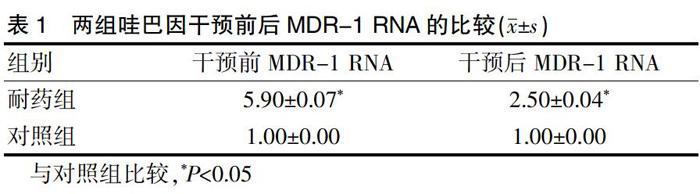
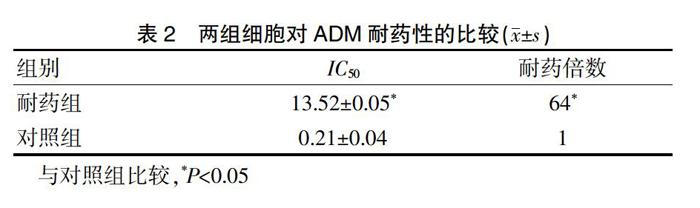

[摘要]目的 研究哇巴因體外逆转肝癌耐药Bel-7402/(阿霉素)ADM细胞机制。方法 选用人肝癌Bel-7402细胞株,正常Bel-7402作为对照组,ADM大剂量间断冲击法建立Bel-7402/ADM模型作为耐药组,采用Real-Time-PCR检测哇巴因干预前后肿瘤多药耐药性(MDR-1)RNA表达,噻唑蓝溴化四唑(MTT)检测哇巴因耐药性并计算其耐药倍数、逆转倍数。结果 耐药组对ADM耐药性显著高于对照组(P<0.05),耐药组耐药倍数显著高于对照组(P<0.05),哇巴因干预后对照组和耐药组IC50均显著低于干预前(P<0.05),耐药组逆转效果显著高于对照组(P<0.05),耐药组在哇巴因干预前后MDR-1RNA表达均显著高于对照组(P<0.05),干预后MDR-1RNA表达显著低于干预前(P<0.05)。结论 哇巴因可通过降低MDR-1RNA表达体外逆转肝癌耐药Bel-7402/ADM细胞,值得临床推广,但哇巴因是否还参与其他耐药机制还待进一步研究。
[关键词]哇巴因;肝癌;耐药;阿霉素
[中图分类号] R735.7 [文献标识码] A [文章编号] 1674-4721(2019)9(b)-0060-03
Experimental study on the reversal of hepatocarcinoma-resistant Bel-7402/ADM cells by Ouabain in vitro
YANG Ming-zhen1 ZHAO Kui2
1. Department of Intervention, Affiliated Hospital of Zunyi Medical College, Guizhou Province, Zunyi 563003, China; 2. Department of Gastroenterology, Affiliated Hospital of Zunyi Medical College, Guizhou Province, Zunyi 563003, China
[Abstract] Objective To study the mechanism of Ouabain in reversing the hepatocarcinoma-resistant Bel-7402/ Adriamycin (ADM) in vitro. Methods Human hepatoma Bel-7402 cell line was selected. A high-dose intermittent impact method was used to establish Bel-7402/ADM model as drug-resistant group and normal Bel-7402 was used as control group. Real-Time-PCR was used to detect tumor multidrug resistance (MDR-1) RNA expression before and after Ouabain intervention and Thiazolyl Blue tetrazolium bromide (MTT) was used to detect the effects of Ouabain resistance, calculating the drug resistance factor and reversal factor. Results The drug resistance of ADM was significantly higher in the drug-resistant group than that in the control group (P<0.05). The resistance factor in the drug-resistant group was significantly higher than that in the control group (P<0.05). IC50 in the control group and drug-resistant group after Ouabain intervention was decreased compared with pre-intervention (P<0.05), and the reversal effect of the drug-resistant group was significantly higher than that of the control group (P<0.05). The expression of MDR-1RNA in the drug-resistant group was significantly higher than that in the control group before Ouabain intervention (P<0.05); the expression of MDR-1RNA after intervention was significantly lower than that before intervention (P<0.05). Conclusion Ouabain reverses hepatocarcinoma-resistant Bel-7402/ADM cells in vitro by reducing MDR-1 RNA expression.
哇巴因[11-12]是细胞膜上钠钾泵(Na+/K+-ATP ase)抑制剂,钠钾泵作为细胞膜上ATP酶,通过消耗ATP可以将细胞外低浓度K+纳入细胞内,并将细胞内低浓度Na+外排出细胞,维持细胞膜两侧钠钾浓度,保持细胞处于静止电位,帮助传输和调整细胞体积。研究表明[13],P-糖蛋白(P-glycoprotein,P-gp)是MDR基因编码重要跨膜转运蛋白,广泛分布与全身各个器官及组织,参与药物体内转运。可利用ATP水解能量将疏水亲脂性化疗药物(如ADM)在尚未发挥作用时排出细胞,导致细胞耐药性。而P-gp外排需要依赖ATP水解能量,哇巴因[14]可通过与钠钾泵特异性结合抑制其ATP酶功能,导致细胞内ATP分解减少,Na+浓度上升,引起细胞膜超极化,Ca2+内流,其浓度升高,阻断MDR-1基因转录翻译P-gp,P-gp表达降低外排能力减弱,细胞外排化疗药物减少,从而减少其对化疗药物耐药性。
本研究采用RT-PCR测量MDR-1RNA表达及MTT测定耐药性,RT-PCR[15]检测法特异性强,敏感度高,可避免同位素污染,可从基因、蛋白水平反映LC细胞耐药本质,测得MDR-1RNA表达准确有效。其通过提取样本总RNA合成cDNA进行PCR扩增,扩增产物经琼脂糖电泳,紫外灯照像,激光扫描定量分析。因RNA提取、cDNA逆转率、PCR扩增等环节可能导致MDR-1 RNA定量精确度,因此选择与MDR-1基因扩增动力学相似的β1微球蛋白为内参标,以两者比值大小衡量MDR-1RNA水平高低,从而尽可能消除各种因素引起的定量误差。细胞线粒体脱氢酶通过将黄色MTT还原成紫色晶状物[16],溶解释放后检测吸光度,吸光度值的高低与活细胞数量成正比,通过与空白组对比可间接反映活细胞数量,计算耐药倍数、逆转倍数,结果准确可靠。结果显示耐药组对ADM耐药性显著高于对照组,哇巴因干预后对照组和耐药组IC50显著低于干预前,耐药组在哇巴因干预前MDR-1RNA表达显著高于对照组,干预后MDR-1RNA表达显著低于干预前(P<0.05),提示哇巴因有效降低MDR-1RNA表达,逆转细胞耐药性,其中对已耐药细胞更为显著。不足之处在于本实验未能直接检测LC细胞耐药前后及哇巴因干预前后ADM含量,其数值可准确反映P-gp作用水平,本研究因时间缘故采用MTT检测,只能大致反映P-gp功能,以ADM含量检测和MTT药敏性检测两相结合可以使数据更具说服力。
综上所述,哇巴因可通过降低MDR-1RNA表达体外逆转LC耐药Bel-7402/ADM细胞,值得临床推广,但哇巴因是否还参与其他耐药机制还待进一步研究。
[参考文献]
[1]文天夫.原发性肝癌诊疗规范(2017年版)解读[J].中国普外基础与临床杂志,2018,3(1):32-34.
[2]赵荣荣,邓永东,袁宏.236例原发性肝癌患者流行病学及临床特点分析[J].临床肝胆病杂志,2016,32(8):45-46.
[3]程红岩.肝癌介入治疗的现状与展望[J].临床肝胆病杂志,2016,20(1):3-8.
[4]程中华,熊文坚,冯珍,等.哇巴因对人食管癌细胞的增殖调控作用及其机制研究[J].胃肠病学,2015,20(9):30-31.
[5]Chen J,Ding Z,Peng Y,et al.HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-Glycoprotein[J].PLoS One,2014,9(6):35.
[6]张洪新.肝癌化疗栓塞术后综合征的中西医治疗探究[J].实用中西医结合临床,2017,17(1):45-46.
[7]Shindo Y,Fukuda S,Kataoka R,et al.A case of advanced gastric cancer with multiple liver metastases treated with curative conversion therapy after S-1 plus CDDP[J].Gan to Kagaku Ryoho,2018,45(3):480-482.
[8]Peng YC,Lu SD,Zhong JH,et al.Combination of 5-fluorouracil and 2-morphilino-8-phenyl-4H-chromen-4-one may inhibit liver cancer stem cell activity[J].Tumor Biol,2016,37(8):10 943-10 958.
[9]弓艷霞,张庆瑜,唐艳萍.化疗药物阿霉素靶向治疗肝癌的研究进展[J].中华全科医师杂志,2016,15(6):482.
[10]吴亚琼,方伟蓉,李运曼.肿瘤多药耐药机制及逆转药物的研究进展[J].药学与临床研究,2016,17(1):43-47.
[11]Song H,Karashima E,Hamlyn JM,et al.Ouabain-digoxin antagonism in rat arteries and neurones[J].J Physiol,2013, 592(5):941-969.
[12]李杰,徐忠伟,程世翔,等.哇巴因诱发人神经胶质瘤U251细胞凋亡的机制[J].武警后勤学院学报(医学版),2016,22(3):174-177.
[13]杨利娟,吕游,李柱虎.胃癌组织中p53,p16和P-gp蛋白的表达及其与病理学行为的相关性[J].延边大学医学学报,2016,14(4):249-252.
[14]Shen Y,Wang Q,Tian Y.Reversal effect of ouabain on multidrug resistance in esophageal carcinoma EC109/CDDP cells by inhibiting the translocation of Wnt/β-catenin into the nucleus[J].Tumor Biol,2016,37(12):15937-15947.
[15]Gautam R,Mijatovicrustempasic S,Esona MD,et al.One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains in stool samples[J].Peer J,2016,4(10):e1560.
[16]李永福,王莉,熊亮发.MTT法测定细胞因子信号负调控因子3稳转乳腺癌细胞株对他莫昔芬的耐药性[J].中国生物制品学杂志,2016,29(4):417-419.
(收稿日期:2019-01-14 本文编辑:崔建中)
