Disinfection of dairy wastewater effluent through solar photocatalysis processes
2018-11-15MojtAfshrniMojtKinmehrHmedBiglriAdollhDrghiAdolrezKrimi
Mojt Afshrni,Mojt Kinmehr,Hmed Biglri,Adollh Drghi,Adolrez Krimi*
aFaculty of Health,Gonabad University of Medical Sciences,Gonabad 9691793718,Iran
bFaculty of Medicine,Gonabad University of Medical Sciences,Gonabad 9691793718,Iran
cFaculty of Health,Kermanshah University of Medical Sciences,Kermanshah 6716139663,Iran
dFaculty of Engineering,Qom University of Technology,Qom 9183673531,Iran
Abstract Due to the strict regulations and reuse policies that govern wastewater's use as an irrigation water resource for agricultural purposes,especially in dry climates,optimization of the disinfection process is of the utmost importance.The effects of solar radiation along with Titanium dioxide(TiO2)nanoparticles applied to optimization of the photolysis and photocatalysis processes for inactivating heterotrophic bacteria were investigated.Temperature,pH,and dissolved oxygen fluctuations in the dairy wastewater effluent treated by activated sludge were examined.In addition,different dosages of TiO2were tested in the solar photocatalysis(ph-C S)and concentrated solar photocatalysis(ph-C CS)processes.The results show that the disinfection efficiencies of the solar photolysis(ph-L S)and concentrated solar photolysis(ph-L CS)processes after 30 min were about 10.5%and 68.9%,respectively,and that the ph-C S and ph-C CS processes inactivated 41%and 97%of the heterotrophic bacteria after 30 min,respectively.The pH variation in these processes was negligible.Using the ph-L CS and ph-C CS processes,the synergistic effect between the optical and thermal inactivation caused complete disinfection after three hours.However,disinfection was faster in the ph-C CS process than in the ph-L CS process.Significant correlations were found between the disinfection efficiency and the variation of the dissolved oxygen concentration in the ph-C S and ph-C CS processes,while the correlations between the disinfection efficiency and temperature variation were not significant in the ph-L S and ph-C S processes.Moreover,the oxygen consumption rate was greatest(3.2 mg·L-1)in the ph-C CS process.Hence,it could be concluded that the ph-C CS process is an efficient photocatalysis process for disinfection of dairy wastewater effluent.
©2018 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:Dairy wastewater;Disinfection;Photocatalyst;Solar;Photolysis and photocatalysis;TiO2
1.Introduction
Food industries use a large volume of water and produce a large volume of wastewater(Sivrioglu and Yonar,2015).Nowadays,recycling wastewater is a priority in many parts of the world,especially in dry climates(Biglari et al.,2016;Khosravi et al.,2017).Disinfection is critical in wastewater reuse for any purposes(Agunwamba et al.,2013).Several methods have been introduced for inactivating microorganisms in water(Alipour et al.,2017).Most of them use the same fundamental mechanisms,such as heat,chemicals,radiation,or a combination of several of the above(Pinho et al.,2015).Recently,advanced oxidation processes(AOPs)have been used for the tertiary treatment,specifically for the disinfection of wastewater(Biglari et al.,2017;Sajjadi et al.,2016).The development of AOPs is based on producing highly reactive·OH with a high electrochemical oxidation potential and non-selective attacking properties(Fotiou et al.,2015;Zhang et al.,2014;Demirel et al.,2005).Consequently,they are highly effective for a wide variety of contaminants in water and wastewater(Ortega-Gomez et al.,2014).Of the AOPs,solar photocatalysis and photolysis processes as well as solar disinfection(SODIS)have drawn most of the attention,especially for degradation of the microorganism components(García-Ferńandez et al.,2015;Ferńandez-Ibãnez et al.,2015;Helali et al.,2014;Malato et al.,2016).Disinfection by solar radiation is a natural,simple,cheap,and ancient technology(Gelover et al.,2006;Helali et al.,2014).Sunlight destroys biomolecules directly and indirectly,as UV-A(wavelength of 320-400 nm)is absorbed by DNA and reactive oxygen species(ROSs)are produced by solar radiation in water or wastewater.UV-A and UV-B from sunlight and the reactive molecules kill or inactivate a wide range of microorganisms,such as Pseudomonas aeruginosa,Escherichia coli,Salmonella,and Shigella Flexneri(Polo-Lopez et al.,2011;Giannakis et al.,2015;Lawrie et al.,2015;Oates et al.,2003;Nalwanga et al.,2014;McGuigan et al.,2006).ROSs include free radicals,such as superoxide anion radicals,and non-radicals,such ashydrogen peroxide(Malato et al.,2009).These radicals cause the degradation of cellular contents,such as proteins,DNA,and cell membrane components,especially membrane lipids,which play an effective role in killing pathogens(Gelover et al.,2006;Reed,2004).In order to improve the SODIS process,prevent microorganism regrowth,and minimize exposure time,researchers have introduced the heterogeneous photocatalysis process(Barreca et al.,2015;Ndounla and Pulgarin,2014;Kalt et al.,2014;Malato et al.,2016).Titanium dioxide(TiO2)accelerates the SODIS process and kills cells under UVor solar radiation(Gelover et al.,2006;Helali et al.,2014;Li et al.,2012).Therefore,the use of TiO2offers an advantage over SODIS,accelerates bacteria death,and destroys undesirable organic matter at the same time(Gelover et al.,2006).TiO2nanoparticles(NPs)are excited by UV-A light in water,generating·OH in contact with dissolved oxygen(DO).DO is necessary for an efficient photocatalysis process.In addition,the generatedandare ROSs that can kill microorganisms(Krzemińska et al.,2015;García-Ferńandez et al.,2015).To illustrate the mechanism of this process more particularly,NPs are illuminated by the photo energy,which is greater in amount than the NP band gap,so NP electrons tend to be in the conduction band.This process generates a hole in the NP valence band,in which the generation of highly oxidizing properties of NPs originates.In fact,the presence of NP electrons in the conduction band and a hole in the valence band are responsible for highly oxidizing features of these NPs(Li et al.,2012).
The diary wastewater includes a variety of microorganisms due to its highly dense organic nature.The predominant species in heterotrophic bacterial strains are Acinetobacter spp.,Aeromonas spp.,Alcaligenes spp.,Comamonas spp.,Enterobacter spp.,Flavobacterium spp.,Klebsiella spp.,Moraxella spp.,Pseudomonas spp.,Sphingomonas spp.,Stenotrophomonas spp.,a typical Mycobacterium spp.,Bacillus spp.,and Nocardia spp.These bacteria are described as opportunistic pathogens that may be discovered in heterotrophic plate count(HPC)microbiota,including strains of Pseudomonas aeruginosa spp.,Acinetobacter spp.,Aeromonas spp.,and Klebsiella pneumoniae spp.(Bartram et al.,2003).Many similar organisms can cause serious diseases,such as microbial and viral gastroenteritis,hepatitis A,and giardiasis(Zazouli et al.,2013).The main aim of this study was to investigate the efficiency of using the concentrated and non-concentrated solar photolysis and photocatalysis processes to inactivate dairy wastewater pathogens,with a focus on inactivation of the heterotrophic bacteria index.The effects of those processes on the DO concentration,temperature,and pH in the effluent of dairy industrial wastewater were investigated as well.
2.Materials and methods
2.1.Chemicals and devices
Reasoner's 2A(R2A)agar culture medium from the Merck Company in Germany was used in this study.The TiO2NPs were provided by the US Research Nanomaterial(USNANO)Corporation and used in three different concentrations(0.25,0.5,and 1.0 mg·L-1)without pre-purification in the photocatalysis reactor.The TiO2NPs had a specific surface area of 200-240 m2·g-1,a particle size of 10-25 nm,and a purity of 99%.TiO2is widely used as a photocatalyst due to its optical and electrical properties.Before being solar radiated,TiO2NP suspensions were stirred for 30 min in a dark ambient until the adsorption equilibrium was reached.Afterwards,the samples were placed in photocatalytic reactors and exposed to the radiation source.The experimental period was from April 1 to September 30 in 2015.Throughout the experimental procedure,the weather was sunny in most cases,and the reaction time was from 10:30 to 14:30 for daytime with solar radiation.The intensities of sunlight,UV-A,and infrared(IR)radiation were measured by a solar meter(TES-1333,Korea),a UV meter(Hagner EC1 UV-A,Swiss),and an IR meter(Hagner EC1 IR,Swiss),respectively.The average and standard deviation of sunlight,IR,and UV-A over months are shown in Table 1.The pH and DO concentration in the effluent were determined by a portable device model(Multi 340iSET,Germany).Theturbidity of the samples was measured with a turbidity meter(HI 93703,Portugal).

Table 1 Average and standard deviation of sunlight,IR,and UV-A for different months.
2.2.Wastewater source
The samples of industrial wastewater were obtained from dairy industry effluent treated by the activated sludge process(extended aeration).The properties of the initial dairy wastewater were as follows:a pH of 6.5-7.5,a turbidity of 2-5 NTU,a DO concentration of 6.5-7.5 mg·L-1,a temperature of 20-25°C,and a colony number of microbes of 2300-2900 CFU·mL-1.
2.3.Concave dish concentrator
The concave dish concentrator was 90 cm in diameter and 10 cm in depth,and covered by highly reflective aluminum foil with a concentration factor of 1(95%total reflectivity).The focal point was obtained by Eq.(1)(García-Ferńandez et al.,2015):

where f is the focal length(cm),D is the dish diameter(cm),and d is the dish depth(cm).The volume of the photocatalytic reactor was 1000 mL,which was fixed at a focal point with a 5-cm focal area diameter.To avoid the sedimentation of the photocatalyst TiO2NPs in the reactor,the reactor was stirred continuously with a magnetic stirrer(Fig.1).
2.4.Experiments
To determine the concave dish efficiency in the photolysis and photocatalysis processes for disinfecting real effluent,four types of experiments were designed,in which samples containing TiO2NPs were exposed to sunlight in a solar photocatalysis(ph-C S)process and a solar photolysis(ph-L S)process,and exposed to concentrated sunlight produced by the concave dish in a concentrated solar photocatalysis(ph-C CS)process and a concentrated solar photolysis(ph-L CS)process,respectively.All experiments were conducted under natural sunlight in the city of Gonabad,at a latitude of 34°20N and longitude of 58°42E.

Fig.1.Sketch of photocatalytic reactor.
The R2A agar culture medium was applied to culturing heterotrophic bacteria.The culturing procedure was based on Rand et al.(1976).The heterotrophic bacteria were counted using the pour plate method.The samples were taken at regular intervals(every 30 min)over 240 min from 10:30 to 14:30 during the experimental period,and evaluated with the enumeration method.
3.Results and discussion
3.1.Effects of various processes on microbial photodegradation
Microbial disinfection of dairy wastewater was carried out in four processes:ph-C S,ph-C CS,ph-L S,and ph-L CS over the radiation time(240 min from 10:30 to 14:30 during the experimental period).Fig.2 was obtained from the average values of collected samples during the experimental period,with a TiO2concentration of 0.5 g·L-1.It shows the disinfection efficiency of those processes under solar radiation in the experiments.In Fig.2,N and N0are the initial and final bacteria colony numbers,respectively.
Statistical analysis results show a considerable increase in the disinfection efficiency when using a concave dish.The disinfection efficiencies of the ph-C CS and ph-C S processes were significantly correlated with the solar intensity,with a Pearson correlation coefficient(PCC)of 0.987 and sig.(2-tailed)of 0.000.The correlation between the disinfection efficiency of the ph-L CS process and the UV-A intensity was significant,with a PCC value of 0.956 and sig.(2-tailed)of 0.044.The correlations between the disinfection efficiencies of the ph-C CS and ph-C S processes and the UV-A intensity were insignificant.The data show that the concave dish had an effective role in the photocatalys is processes,and the enhancement of the photocatalytic activity of the TiO2NPs completely depended on the radiation intensity(Gelover et al.,2006).In the ph-C CS process,almost 97%of the bacteria were killed after 30 min,while in the ph-C S process only 41%of the bacteria were killed during the same period.The disinfection efficiencies of the ph-L S and ph-L CS processes after 30 min were about 10.5%and 68.9%,respectively.
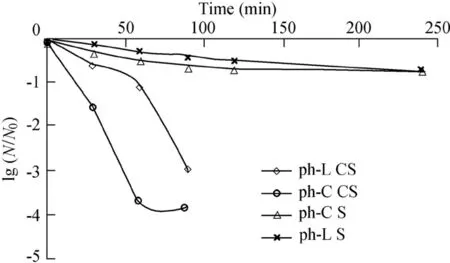
Fig.2.Disinfection efficiencies under different solar radiation conditions.
In the ph-L CS and ph-C CS processes,about three hours were needed for the complete removal of heterotrophic bacteria;the rate of photodegradation in the ph-C CS process was higher than that in the ph-L CS process,with the disinfection efficiencies of the ph-C CS and ph-L CS processes after one hour being 99.98%and 90.58%,respectively.However,the ph-C S process yielded a disinfection efficiency of 78%after four hours.According to Gelover et al.(2006)and Helali et al.(2014),the use of TiO2NPs as supplements of SODIS could accelerate bacteria death and destroy undesirable organic matter under UV or solar radiation,and the photocatalysis process produced more free radicals due to the use of TiO2NPs.Gelover et al.(2006)demonstrated that disinfection with TiO2under solar radiation was more effective than the SODIS process in removal of total coliforms as well as fecal coliforms.Méndez-Hermida et al.(2007)studied the disinfection of drinking water contaminated by Cryptosporidium parvum oocysts and confirmed that SODIS reactors with TiO2powder were more effective than those without TiO2powder.They reported that after eight hours oocyte viability was reduced to 37.7%and 81.3%in the SODIS reactor with and without using TiO2power under solar radiation,respectively.
3.2.Effect of TiO2dosage
The disinfection efficiencies of the ph-C S and ph-C CS processes at three different catalyst concentrations were evaluated and the results are listed in Table 2.The disinfection efficiency significantly increased along with the concentration ofTiO2NPs in the ph-C S process,with a PCC value of 0.997 and sig.(2-tailed)of 0.045.Ferńandez-Ibãnez et al.(2015)demonstrated that the highest disinfection efficiency was obtained at the catalyst concentration of 0.50 g·L-1.They considered that,under the radiation of visible light,more single oxygen molecules in TiO2NPs were produced in the reactor,which could affect the membrane of microorganism cells and accelerate their death.
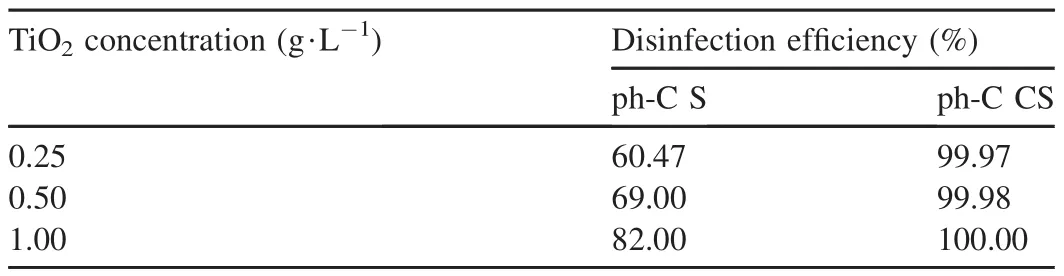
Table 2 Disinfection efficiencies of ph-C S and ph-C CS processes at different TiO2 concentrations after 120 min.
3.3.Effects of photocatalysis processes on effluent properties
The variations of the pH,temperature,and DO concentration of samples during the photolysis and photocatalysis processes of 240 min,with a TiO2concentration of 0.5 g·L-1,are shown in Fig.3.It should be noted that the data in Fig.3 are also the average values of collected samples during the experimental period.
A strong synergistic effect was observed between the optical and thermal inactivation in the photocatalysis processes under solar radiation(McGuigan et al.,2012).There was a significant correlation between the disinfection efficiency and temperature change in the ph-L CS process,with a PCC value of 0.997 and sig.(2-tailed)of 0.0005;about 0.2 mg·L-1of oxygen was consumed in 20 min.An electron acceptor(such as oxygen)is always used in the photocatalysis process to achieve a high oxidation efficiency.Borges et al.(2017)noted that in wastewater samples subject to solar photocatalysis,the DO concentration decreased and more than 90%of the contaminants were removed after five hours.According to Shukla et al.(2010)and Malato et al.(2009),using oxygen as the electron acceptor could help produce ROSs and accelerate contaminant oxidation.In this study,there were significant correlations between the oxygen consumption rate and the disinfection efficiency in the ph-C S and ph-C CS processes,with PCC values of 0.895 and 0.991,and sig.(2-tailed)values of 0.040 and 0.0015,respectively.The oxygen consumption rates after 120 min were observed to be 3.20,2.43,1.31,and 0.92 mg·L-1in the ph-C CS,ph-C S,ph-L CS,and ph-L S processes,respectively,with the highest rate occurring in the ph-C CS process,suggesting that oxygen played the most significant role in this process.It was reported that in the disinfection of F.solani spores with solar radiation,the DO concentration decreased from 8.5 mg·L-1to 5.9 mg·L-1as the catalyst dosage increased from 3 g·L-1to 5 g·L-1during the ph-C S process(Ferńandez-Ibãnez et al.,2015).In the ph-L S and ph-C S processes,the correlations between the disinfection efficiency and temperature were not significant,with sig.(2-tailed)being greater than 0.05.The pH variation during the experiments was negligible and consistent,and the pH variation in Ferńandez-Ibãnez et al.(2015)was not significant,either.

Fig.3.Variations of pH,temperature,and DO concentration of samples during solar photolysis and photocatalysis processes.
4.Conclusions
The photolysis and photocatalysis processes were used for disinfection of dairy wastewater effluent.The results indicate a definite disinfection effect in natural wastewater samples obtained from dairy effluent at almost neutral and alkaline pH(8.5±2.0)conditions with a low TiO2concentration(0.5 g·L-1).After a specific period of solar photocatalysis treatment,the reactivation or regrowth of bacteria was not observed.The disinfection efficiencies of the ph-L CS and ph-C CS processes were higher than those of the ph-L S and ph-C S processes.Furthermore,the highest disinfection efficiency was obtained in the ph-C CS process over a short period.Since the irreversible inactivation of bacteria after three hours was observed in both the ph-L CS and ph-C CS processes,these processes might be used as effluent disinfection methods in natural environments.
Acknowledgements
The authors are grateful to the Department of Environmental Health Engineering of the Gonabad University of Medical Sciences,in Iran,for technical support.
杂志排行
Water Science and Engineering的其它文章
- Application of a hybrid multiscalar indicator in drought identification in Beijing and Guangzhou,China
- On relationship between curve numbers and phi indices
- Analysis of influence of observation operator on sequential data assimilation through soil temperature simulation with common land model
- Common effluent treatment plant(CETP):Reliability analysis and performance evaluation
- Numerical study of hydrodynamic mechanism of dynamic tidal power
- Evaluation of numerical wave model for typhoon wave simulation in South China Sea
