活性炭吸附氦气式回热器低温比热与流阻特性的研究
2018-03-15陈六彪孔春辉吴显林王俊杰
陈六彪, 孔春辉, 吴显林, 周 远, 王俊杰
(1. 中国科学院 理化技术研究所,低温工程学重点实验室,北京100190; 2. 中国科学院大学,北京100049)
1 Introduction
The regenerator is one of the important components in refrigerator. The regenerator alternately repeats two heat transfer processes. During the heat blowing period, the hot working gas helium, coming from the compressor, flow to the regenerator and cooled by the regenerative materials, then enter to the expansion unit, at this time, the temperature of the working gas helium will decrease and the temperature of the regenerative materials will increase. During the cold blowing period, the cold working gas helium, coming out from the expansion unit, reverse flow through the regenerator and cool off the regenerative materials, then enter to the compressor. Therefore, the ideal regenerator is characterized by its high specific heat of regenerative materials, and low specific heat of the working gas. However, as shown in Fig. 1, in the temperature range of 4-30 K, the specific heat of the working gas helium is higher than that of the commonly used regenerative materials, such as the stainless steel wire mesh (SS), lead and magnetic materials Er3Ni, HoCu2, which severely limits the enhancement of the refrigerator performance[1-4]. To solve this problem, Zhejiang University carried out a series of researches on using ErNi alloy to adsorb hydrogen as regenerative unit[ 5-7]. In this paper, a part of working gas helium is directly adsorbed by activated carbon with a large specific surface area as the storage unit, and a testing device is set up to investigate the adsorbed helium amount by activated carbon in low temperature, and the specific heat capacity of the adsorbed unit was compared with that of the conventional rare-earth materials. In addition, the flow resistance impedance of the adsorbed unit and the magnetic materials Er3Ni and HoCu2were also tested by a self-developed flow resistance testing device.
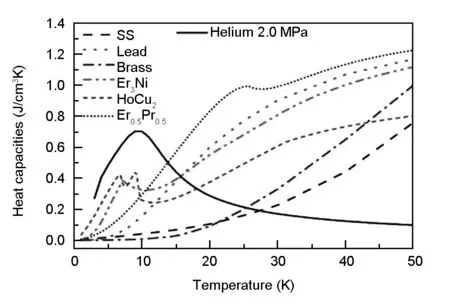
Fig.1 Volumetric heat capacities of the commonly used regenerative materials and working gas helium 4 (Data from National Institute of Standards and Technology (NIST)).
The presented work is a new exploration on the research of regenerative materials in a refrigerator. Before this, some scholars proposed using activated carbon to adsorb helium as an adsorbent compressor or hydrogen storage medium[8-10], the focus of which is the helium adsorption amounts, while more attention will be given to the specific heat capacities and the flow resistance characteristics of the activated carbon with adsorbed helium in the temperature below 30 K in this work.
2 Experimental
2.1 The developed adsorption capacity test device and its data processing methods
The developed adsorption capacity test device is shown in Fig. 2. It mainly includes the self-developed miniature pulse tube refrigerator (the lowest no-load temperature is 10 K)[2], vacuum systems (the highest vacuum up to 10-5Pa), adsorption chamber (the volume is 10 cm3), the calibrated volume (the volume is 611 cm3), the pressure sensors (JYB, 0-4 MPa) and the temperature sensors (pt100 is employed to measure room temperature, and calibrated rhodium-iron resistance thermometer is used to measure the cryogenic temperature).
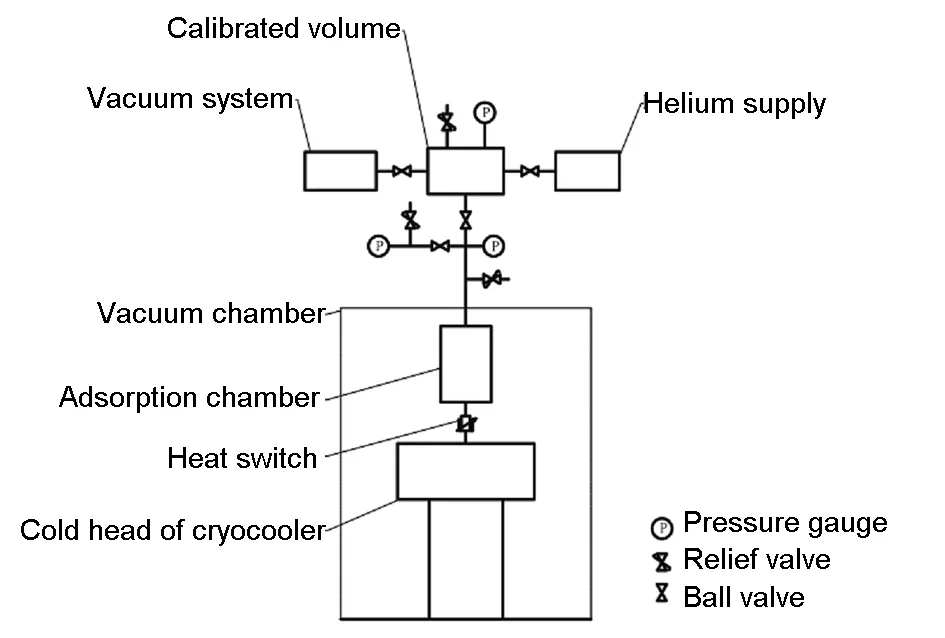
Fig. 2 A schematic illustration of the developed adsorption capacity test device.
The specific testing procedure is as follow. Firstly, a vacuum operation was performed to reduce the system vacuum to 10-4Pa. Secondly, the calibrated volume and the adsorption volume was filled with a certain pressure of helium, and the pressure P1 and room temperature T1 were recorded. Thirdly, the refrigerator was started to cool off the adsorption chamber to the set temperature, and the temperature of the adsorption Tc, the room temperature T2 and the pressure of the calibrated volume P2 were recorded.
The data processing procedure is as follows. The density and heat capacities of the helium at a certain temperature and pressure can be achieved by the REFPROP database of NIST. And the mass of helium is the product of density and volume. Then the amount of helium adsorbed to the adsorption chamber is equal to the mass difference of the calibrated volume before and after cooling. The helium in the adsorption chamber contains two parts. The helium adsorbed by the activated carbon and the helium filled in the macroscopic volume between the activated carbon particles. Noticed that the macroscopic volume is equal to the product of the porosity and the total volume of the adsorption chamber, so the helium mass filled in the macroscopic volume can be achieved according to the test temperature and pressure of the adsorption chamber. Then the mass of helium adsorbed in the activated carbon is equal to the total helium mass in the adsorption chamber minus the helium mass filled in the macroscopic volume.
In order to determine the heat capacities of the adsorbed helium in activated carbon, the temperature and the density should be given. For the temperature of the adsorbed helium, it is equal to that of the adsorption chamber. For the density of the adsorbed helium, the tested temperature and pressure in the presented work is much higher than the helium critical parameters (Tc=5.2 K,Pc=0.23 MPa), i.e., it is an above-critical adsorption process. However, the mechanism of above-critical adsorption is not clear, there is no mature method to determine the density of the adsorbed phase at present[11]. The density of the adsorbed phase is roughly determined by a simplified method here. The total volume of the activated carbon and its microscopic volume is equal to the volume of the adsorption chamber minus the volume of the macroscopic volume between the activated carbon particles, while the mass of activated carbon can be tested and its absolute density is equal to that of graphite (2.2 g/cm3)[12], then the volume of the adsorbed helium and its density can be determined further.
Table 1 gives the specific information of the samples in the tests.
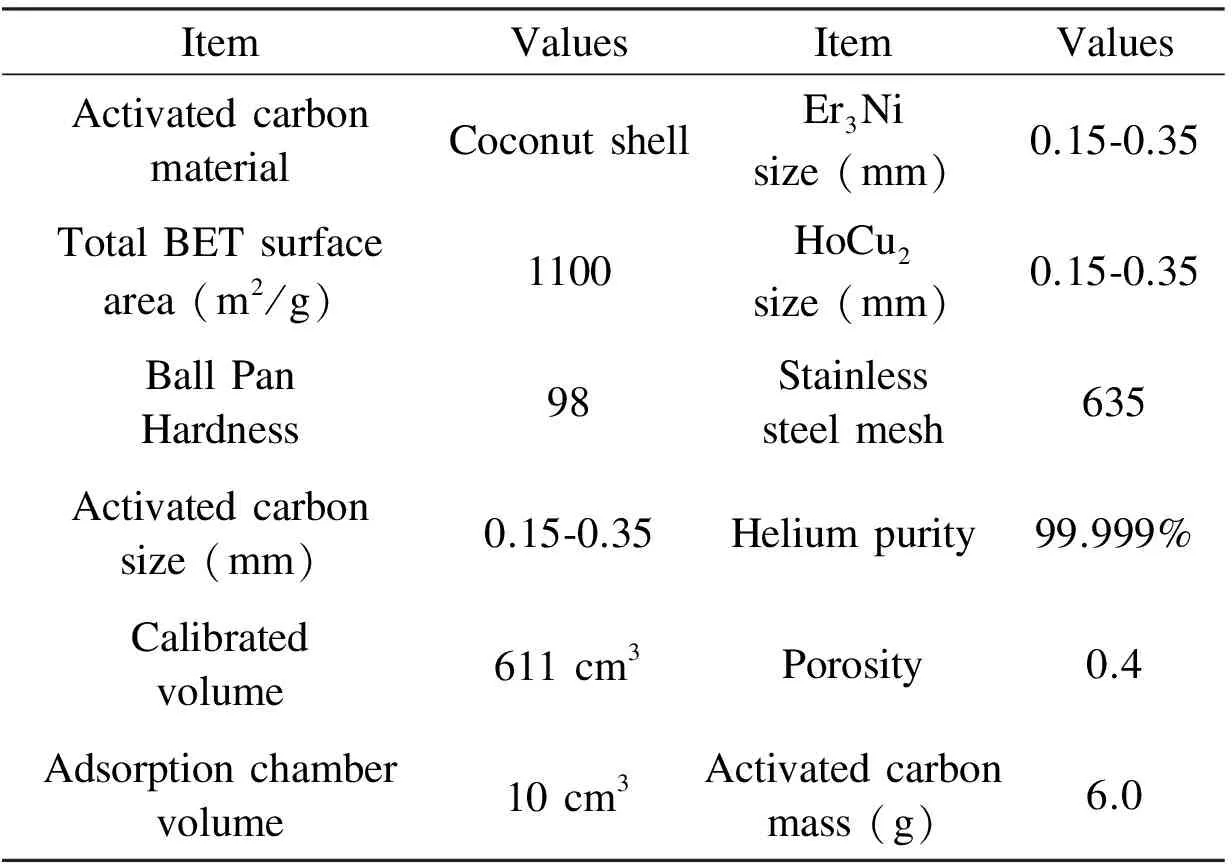
Table 1 The information of the samples in the tests.
2.2 The developed flow resistance test device
Fig. 3 shows a schematic illustration of the flow resistance test device. It mainly includes pressure source, pressure sensors, flowmeter and the sample unit. To make the test results more conducive to improve the design of a refrigerator, a coaxial pulse tube cold head was used to serve as the sample test system (outer diameter 18 mm, inner diameter 8.9 mm and length 60 mm). The flow resistance test procedure is as follows. The pressure of the gas entering into the sample test system was controlled by a pressure relief valve and a needle valve. Then the gas passed through the tube, straightener, sample unit and straightener in turn, then flowed out to the ambient. Meanwhile, the pressure and flow rate of the gas were recorded. Finally, the flow resistance impedance value was obtained by dividing the measured pressure difference between the two ends of the sample test system by the tested flow rate.
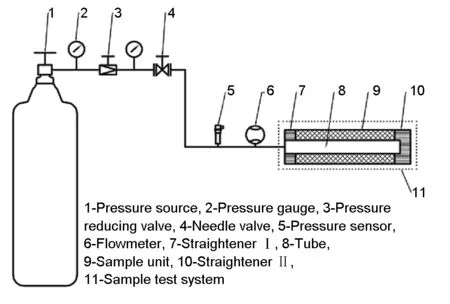
Fig. 3 A schematic illustration of the developed flow resistance test device.
3 Results and discussion
3.1 Comparison of the heat capacities of the activated carbons and rare-earth materials
Fig. 4 shows the test results of helium adsorption on activated carbon. It should be mentioned here that the helium pressure marked in the figure is the initial pressure in the calibrated volume before adsorption. It can be found that the lower the temperature, the greater the amount of helium adsorbed, this trend is consistent with the cooling needs of the refrigerator. It also can be seen from Fig. 4 that with the pressure increases, the amount of helium adsorbed by activated carbons also increases. But in fact, for a special refrigerator, there is an optimal pressure, so it cannot simply be used to increase the adsorption capacity by increasing the pressure to improve the cooling performance. But as the results indicate, one can design the refrigerator with a higher working pressure at the beginning.
Another thing should be pointed out that the amount of helium adsorbed depends on the kind of activated carbon used.A key parameter that can influence the amount of adsorption is the surface area[13-14]. The purpose of this paper is to explore whether it is feasible to use a helium-adsorbed activated carbon as the regenerative material. So an activated carbon with a medium surface area (1 100 m2/g) was selected to test. It is foreseeable that the adsorption amount will be larger by using activated carbon with a larger specific surface area.
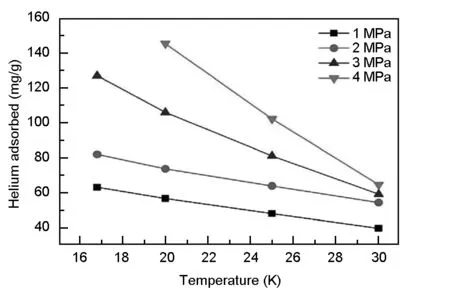
Fig. 4 The test results of helium adsorption on an activated carbon.
Fig. 5 shows the specific heat capacities of activated carbon after adsorption of helium at different temperatures, which were compared with that of stainless steel wire mesh, lead and rare-earth material Er3Ni, HoCu2. As shown in Fig. 5, the specific heat capacities of the helium stored in the macroscopic volume between the activated carbon particles has been subtracted from the specific heat capacities of the adsorbed unit. It can be seen that the higher the pressure and the lower the temperature corresponding to a greater the specific heat capacity. But different with the helium adsorption trend, the specific heat capacity increasing slope gradually slowed down with decreasing the temperature. There is an optimum value of pressure. The reason is that there is a maximum value for the specific heat capacity of helium with temperature as shown in Fig. 1, and the higher the pressure, the maximum value occurs at a higher temperature. It can be seen from the Fig. 5 that the specific heat capacity at the pressure of 2.0 MPa is higher than that of the stainless steel wire mesh, copper and the magnetic materials Er3Ni and HoCu2, but is lower than lead at 25 K. When the pressure increases to 3.0 MPa, the specific heat capacities of the activated carbon is higher than all the commonly used materials at 25 K. Therefore, based on the perspective of heat storage capacity, the specific heat capacities of the activated carbon after the adsorption of helium can improve the storage capacity of regenerator, thus further improving the cooling performance of the refrigerator.
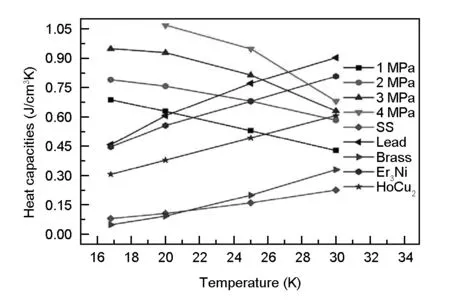
Fig.5 The specific heat capacities of different materials.
3.2 Test results of the flow resistance impedance of the activated carbons and rare-earth materials
The flow resistance is another important parameter of the regenerator besides the specific heat capacity. A higher specific heat capacity and a lower flow resistance are the ideal characteristics of the refrigerator regenerator. Fig. 6 shows the test flow resistance impedance of activated carbon, Er3Ni, HoCu2and stainless steel wire mesh at room temperature. It can be seen that the flow resistance impedance of activated carbon is almost the same with Er3Ni, and slightly higher than that of HoCu2, but much less than that of 635 mesh stainless steel wire mesh. As shown in Fig. 7, the flow resistance impedance of the activated carbon and Er3Ni is slightly higher than that of HoCu2because of their irregular shape, while the regular spherical shaped HoCu2has a smooth surface. However, in terms of flow resistance, this test result is sufficient to show that activated carbon can be used as a regenerator material.
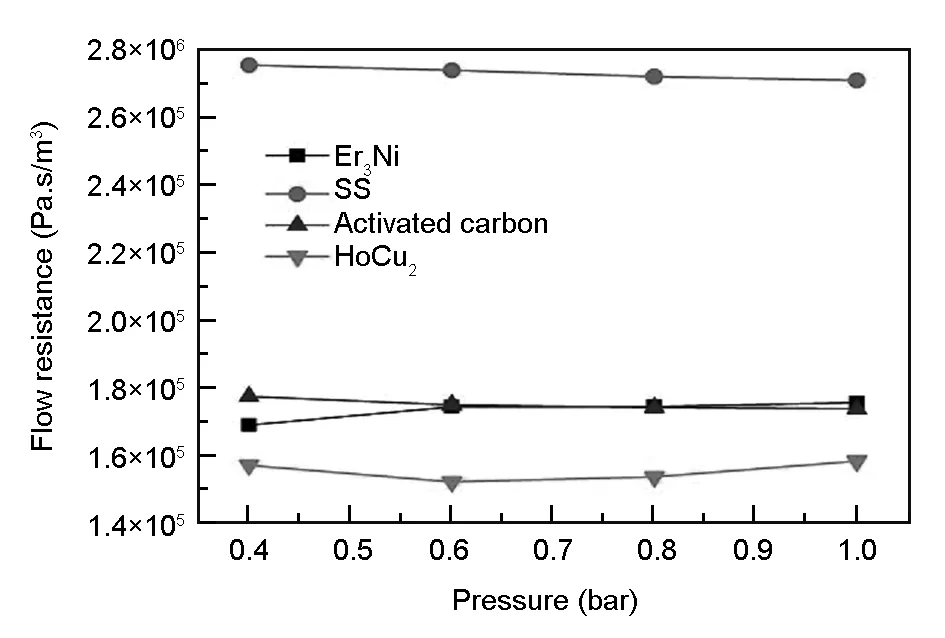
Fig. 6 The test results of the flow resistance impedance of the activated carbon, HoCu2, Er3Ni and stainless steel wire mesh.
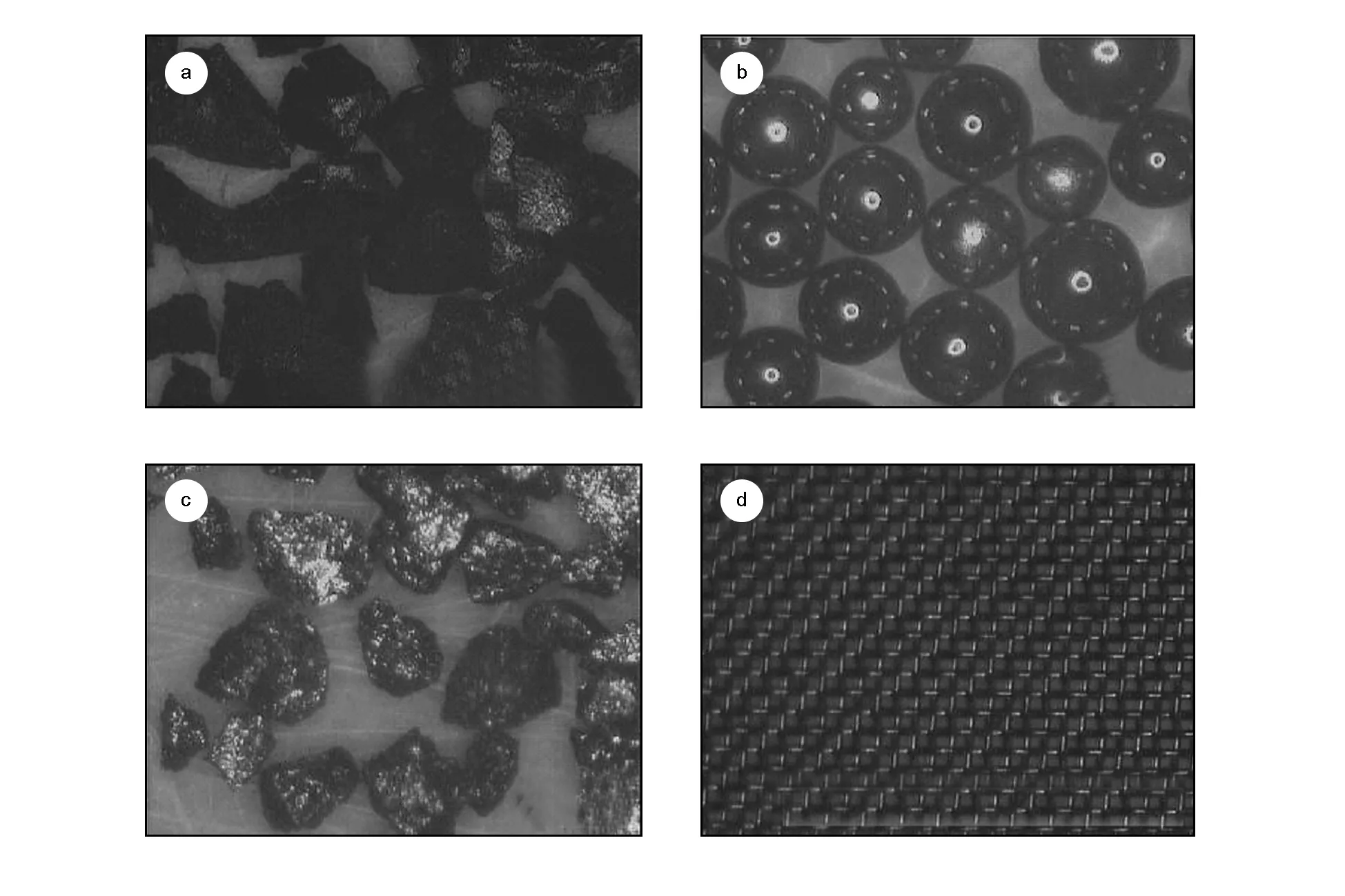
Fig. 7 The microscopic image of (a) activated carbon, (b)HoCu2, (c)Er3Ni and (d)stainless steel wire mesh.
4 Conclusions
The low temperature specific heat capacities and the flow resistance characteristics of the activated carbon adsorbed with helium and the commonly used regenerative materials such as stainless steel wire mesh, lead and magnetic materials Er3Ni, HoCu2were studied. It is concluded that the activated carbon can be employed as the regenerator material when temperature is below 25 K from the perspective of heat capacity and flow resistance. The specific conclusions are listed as follows:
(1) The lower the temperature, the higher the pressure, the greater the adsorption of activated carbon on the helium.
(2) After the adsorption of helium, the specific heat capacity of activated carbon exhibits a maximum with temperature. The specific heat capacities of the activated carbon with adsorbed helium is higher than that of rare-earth material Er3Ni and HoCu2at 3.0 MPa and 16 - 25 K. It shows that the specific heat capacities can satisfy the demand for further improving the heat storage capacity of the regenerator of the refrigerator.
(3) The flow resistance impedance of activated carbon is almost the same as Er3Ni, and slightly higher than that of HoCu2, but much less than that of 635 mesh stainless steel wire mesh. The flow resistance of activated carbon can satisfy the demand for a regenerator material.
[1] Chen L, Wu X, Liu X, et al. Numerical and experimental study on the characteristics of 4 K gas-coupled Stirling-type pulse tube cryocooler[J]. International Journal of Refrigeration, 2018, https://doi.org/10.1016/j.ijrefrig.2018.01.010.
[2] Liu S, Chen L B, Wu X, et al. 10 K high frequency pulse tube cryocooler with precooling[J]. Cryogenics, 2016, 77: 15-19.
[3] Zhou Q, Chen L, Zhu X, et al. Development of a high-frequency coaxial multi-bypass pulse tube refrigerator below 14 K[J]. Cryogenics, 2015, 67: 28-30.
[4] Chen L, Zhou Q, Jin H, et al. 386 mW/20 K single-stage stirling-type pulse tube cryocooler[J]. Cryogenics, 2013, 57: 195-199.
[5] Xu B, Jin T, Li C, et al. Study on reaction characteristics of Er3Ni hydrogenation[J]. Rare Metal Materials and Engineering, 2011, 40(1): 14-17.
[6] Jin T, Xu B, Li C, et al. Study on the hydrogen absorption capacity of magnetic regenerative material Er3Ni[J]. Journal of Engineering Thermophysics, 2011, 32(1): 6-8.
[7] Jin T, Li C, Tang K, et al. Hydro-genation-induced variation in crystal structure and heat capacity of magnetic regenerative material Er3Ni[J]. Cryogenics, 2011, 51: 214-217.
[8] D Lozano-Castello, M Jorda Beneyto, Cazorla-Amoros, et al. Characteristics of an activated carbon monolith for a helium adsorption compressor[J]. Carbon, 2010, 48: 123 -131.
[9] Gao F, Wang Y, Li C, et al. Surface modification of activated carbon for CO2adsorption[J]. New Carbon Materials, 2014, 29(2): 96-101.
[10] Song T, Liao J M, Xiao J, et al. Effect of micropore and mesopore structure on CO2adsorption by activated carbons from biomass[J]. New Carbon Materials, 2015, 30(2): 156-166.
[11] Dundar E, Zacharia R, Chahine R, et al. Modified potential theory for modeling supercritical gas adsorption[J]. International journal of hydrogen energy, 2012, 37: 9137-9147.
[12] Tang B, Hu G, Gao H, et al. Application of graphene as filler to improve thermal transport property of epoxy resin for thermal interface materials[J]. International Journal of Heat and Mass Transfer, 2015, 85: 420-429.
[13] Inagaki Michio. Pores in carbon materials-importance of their control[J]. New Carbon Materials, 2009, 24(3): 193-222.
[14] Chen Y, Zhou L, Hong Y, et al. Preparation of high surface area activated carbon from coconut shell fibers[J]. New Carbon Materials, 2010, 25(2): 151-155.
