碳载金属单原子催化剂
2018-03-15章海霞闫晓丽许并社郭俊杰
李 海, 章海霞, 闫晓丽, 许并社, 郭俊杰
(新材料界面科学与工程教育部重点实验室,太原理工大学,山西 太原030024)
1 Introduction
In heterogeneous catalysis system, supported metal catalysts are widely used in many important industrial catalytic reactions. It has long been recognized that downsizing the metal particles is a key process to improve the performance of the supported metal catalysts (shown in Fig. 1)[1].
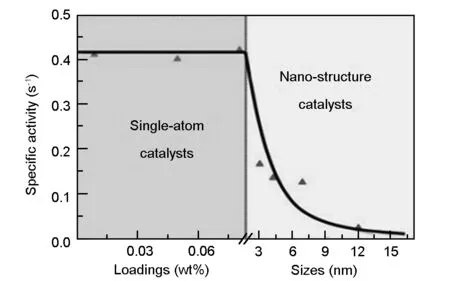
Fig. 1 Specific activity of catalysts as a function of metal loadings and sizes[1].
Extensive investigations have revealed that sub-nanometer clusters have a better catalytic activity or selectivity than larger particles[2-4]and, in particular, Qiao et al.[5]first prepared a well dispersed Pt single atom catalysts (SACs) supported on iron oxide with an improved catalytic activity and stability in the CO selective oxidation reaction. Isolating metal atoms greatly improved the utilization efficiency of the metal catalyst, and the adsorption/desorption selectivity of the active species on the different molecules can be changed, which affected the reaction kinetics[6]. Accordingly, metal SACs have recently attracted much attention owing to their incredible catalytic behaviors and the potential to explore new catalytic mechanism[7].
Nevertheless, reducing the size of metal particles to single atom level can result in extremely the high surface free energy[1]. Their high reactivity would lead to serious aggregation and catalyst deactivation in the preparation and catalysis process, which is an enormous challenge in the industrial applications of SACs. Adopting a high-surface-area support material that strongly interacts with the metal atoms could prevent their aggregation, creating finely dispersed stable metal SACs. Till now, in most single atom catalyst systems, the isolated metal atoms are uniformly anchored to supports such as metal surfaces, metal oxides and carbon materials. Recently graphene-based carbon materials have been adopted to disperse nanoparticles or single atoms for novel catalyst[8]owing to their large specific surface area (high catalyst loading), high electrical conductivity (facilitated electron transfer), and potential low manufacturing cost.
Herein, we introduce recent advances in the selection of carbon substrate, preparation methods, and the anchoring mechanism of metal SACs. Based on the understanding of single atom catalytic activity, we discuss the development trend and application prospect of this research field.
2 Selection of substrates
The improvement of catalyst substrate cannot be avoided in designing the catalyst system because the catalytic behavior of the catalyst can be greatly influenced by the properties of the support material. The effects of the substrate on catalysis include decorative effect, electronic effect, new alloy phase formation and generation of new interface sites[6]. When SACs are mentioned, their high mobility would result in serious aggregation and coarsening, interfering with the density of active sites and limiting the catalytic durability and efficiency[9, 10]. Accordingly, it is necessary to screen out suitable carriers to anchor metal single atoms to avoid the catalyst deactivation due to agglomeration.
Some metals, for example Cu, Au and Pd, have been used as substrates of SACs and exhibit improved catalytic performances[11-15], in which the single atoms interact with the host metal substrate to form monatomic alloys[16]. Various metal oxide, such as iron oxides, hydroxides[17], and oxide of anionic clusters[18], hollandite-type manganese oxide (HMO)[19], aluminum and cluster anions[20], cerium, titanium and zinc oxides, have also been proven to be good substrate candidates for SACs. It is found that surface defects of metal oxide could serve as anchoring sites for metal clusters or even single atoms[21-23]. In addition, molecular sieves[24, 25]have the advantage superior to metal oxides, providing highly homogeneous sites for the attachment of metal active components. Covalent triazine frameworks (CTFs)[26]and CTFs hybridized with carbon nanoparticles[27], silicon oxide[28]and silicate[29]have been used to load SACs. Recently, metal-organic frameworks (MOFs)[30]have also been widely considered as the substrates for SACs, which have great application prospects. However, the above mentioned SAC supports are of disadvantages including low loading density, instability, or poor tolerance, which could be conquered by using carbon materials instead.

Table 1 Loadings of different metal single atoms on different carriers
Graphene, a unique structure of two-dimensional (2D) carbon sheet with one-atomic layer thick[47], is considered to be the building block of many carbon materials such as carbon nanotubes, carbon nanoonions[48]and nanoporous carbon[49]. It is expected that graphene-based materials with unique electric and microstructural characteristics will offer a new type of carbon-metal nanocomposite for the next generation of catalysts[50-53]. Sun et al.[54]observed the Pt single atoms and sub-nanometer clusters on graphene nanosheet (GNS) by high-angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) (Fig. 2). The Pt/GNS sample prepared by atomic layer deposition (ALD) for 50 cycles exhibited a peak current density of 22.9 mA·cm-2, which was 9.5 times higher than Pt/C catalyst (2.41 mA·cm-2). And the CO oxidation peak for 50 ALD Pt/GNS could not be observed until an exposure time of 2 min, indicating the better CO tolerance.

Fig. 2 (a, b, c) HAADF-STEM images of Pt/GNS samples with 50, 100, and 150 ALD cycles, respectively; (d) CVs of methanol oxidation on Pt/GNS samples; (e) CO stripping voltammogram as a function of CO poisoning time for the sample with 50 ALD cycles[55].
Yan et al.[56]atomically dispersed Pd on graphene, which showed excellent catalytic performance in selective hydrogenation of 1,3-butadiene (Fig. 3). More importantly, metal atom aggregation was not observed by HAADF-STEM after either 100 h reaction, or annealing at 400 ℃ in Ar for 1 h.

Fig. 3 HAADF-STEM images of Pd/graphene at (a) low and (b) high magnifications; Catalytic performances of prepared samples in selective hydrogenation of 1,3-butadiene; (c) Butenes selectivity as a function of conversion by changing the reaction temperatures; (d) the distribution of butenes at 95% conversion; (e) Propene conversion and (f) the distribution of butenes at 98% 1,3-butadiene conversion in hydrogenation of 1,3-butadiene in the presence of propene[56].
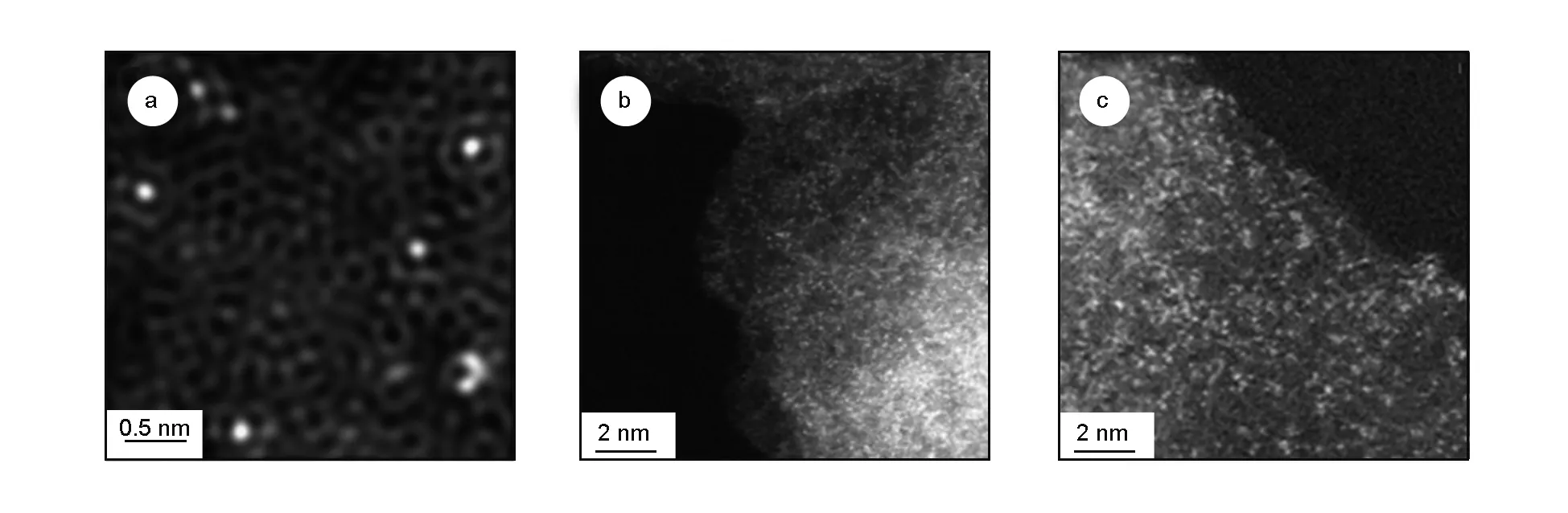
Fig. 4 HAADF-STEM images of the Co-N-C: (a)[57], (b)[58], (c)[59]
On the other hand, a lot of efforts have been focused on searching for the substitutes for noble metal-based catalysts. Cobalt single atoms on nitrogen-doped graphene (Co-NG)[57]was found to work as extraordinary catalysts towards hydrogen evolution reaction (HER) in both acidic and basic water. Yin et al.[58]achieved stable Co single atoms on nitrogen-doped porous carbon, which exhibited a superior oxygen reduction reaction (ORR) performance with a half-wave potential (0.881 V) to commercial Pt/C. Liu et al.[59]proposed the Co-N4structure in graphene, in which the single Co atom was strongly coordinated with four pyridinic nitrogen atoms within graphitic layers. Such a unique structure exhibited an excellent activity, chemoselectivity and stability for the synthesis of aromatic azo compounds through hydrogenative coupling of nitroarenes.
Single Fe sites confined in a graphene matrix also showed an excellent catalytic performance for the four-electron reduction of dioxygen to water[60]and oxidation of benzene[61]. The similar structure of FeN4with a Fe atom center and four surrounding N atoms was embedded into the graphene matrix. Qiu et al.[62]synthesized single-atom nickel dopants anchored to three-dimensional nanoporous graphene, which could be used as catalysts of HER in acidic solution. They observed by STEM that the Ni atoms were physically adsorbed onto the hollow centers of the graphene lattice.
Recently Guo et al. reported the one-step synthesis of Nb SACs[63]and W SACs[64]trapped in onion-like carbon shells as catalysts for the ORR. The atomic scale observation by STEM indicated that metal single atoms incorporated in graphite layers were the active sites responsible for high catalytic ORR performance. This structure effectively ensured the electrochemical stability of catalytically active single atom sites. In addition, high density of defects in carbon shells allowed easy O2penetration and reaction at single metal atom sites. The chronoamperometric curves recorded at -0.40V and a rotation rate of 1 600 rpm in an O2-saturated 0.1 mol/L KOH solution have been used to evaluate the durability of the Nb-in-C complex. The residual current after 30 000 s still remained at 92% of the original value, which indicated that the Nb single atoms were stabilized in graphitic layers (Fig. 5b).
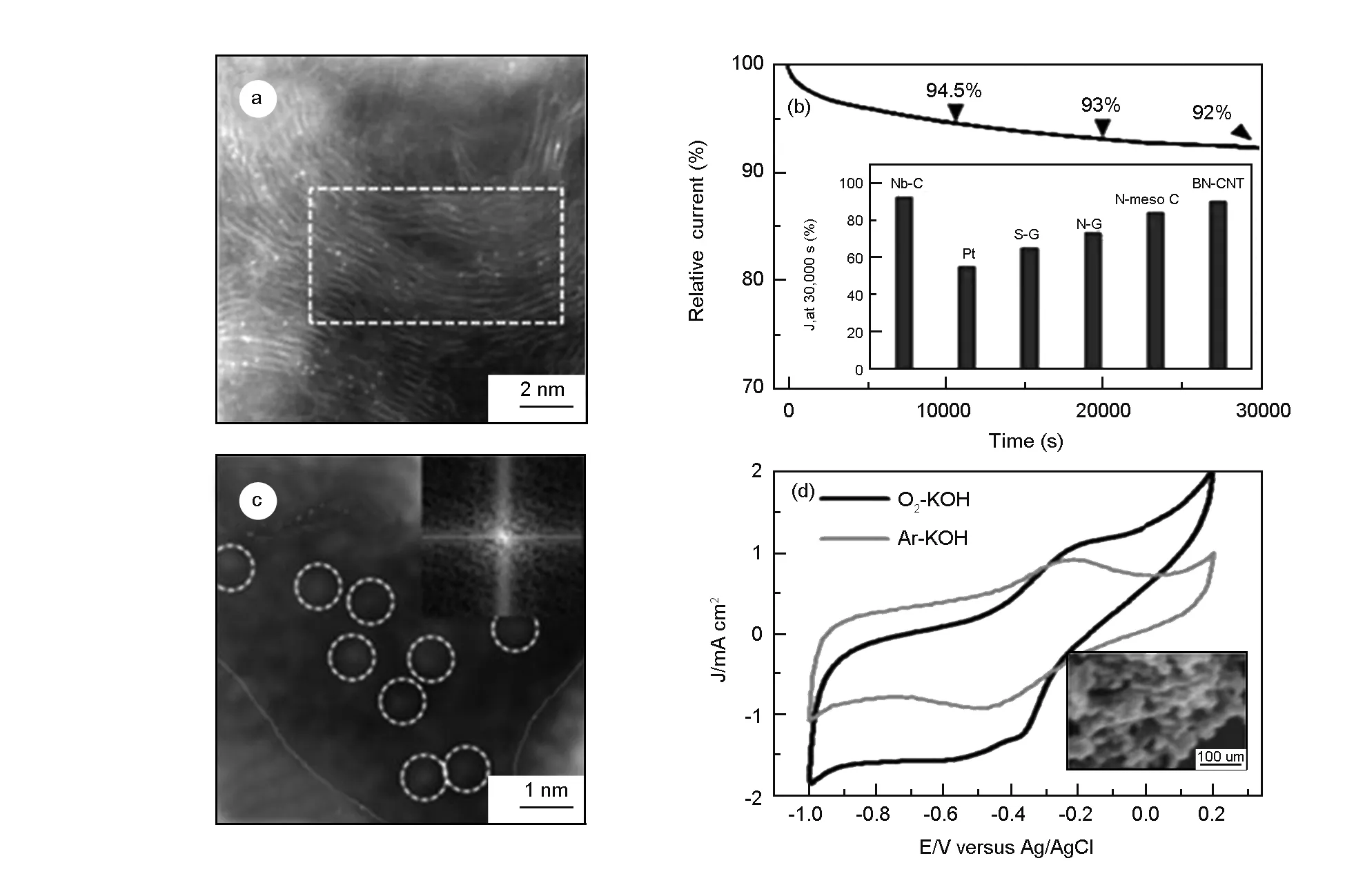
Fig. 5 ADF images of a single atom (a) Nb-in-C[63] and (c) W-in-C[64]; Chronoamperometric response curve of (b) Nb-in-C complex and (d) WC@C complex in 0.1 mol/L KOH solution with and without the addition of 1 mol/L methanol (CH3OH), at a scan rate of 100 mV·s-1.
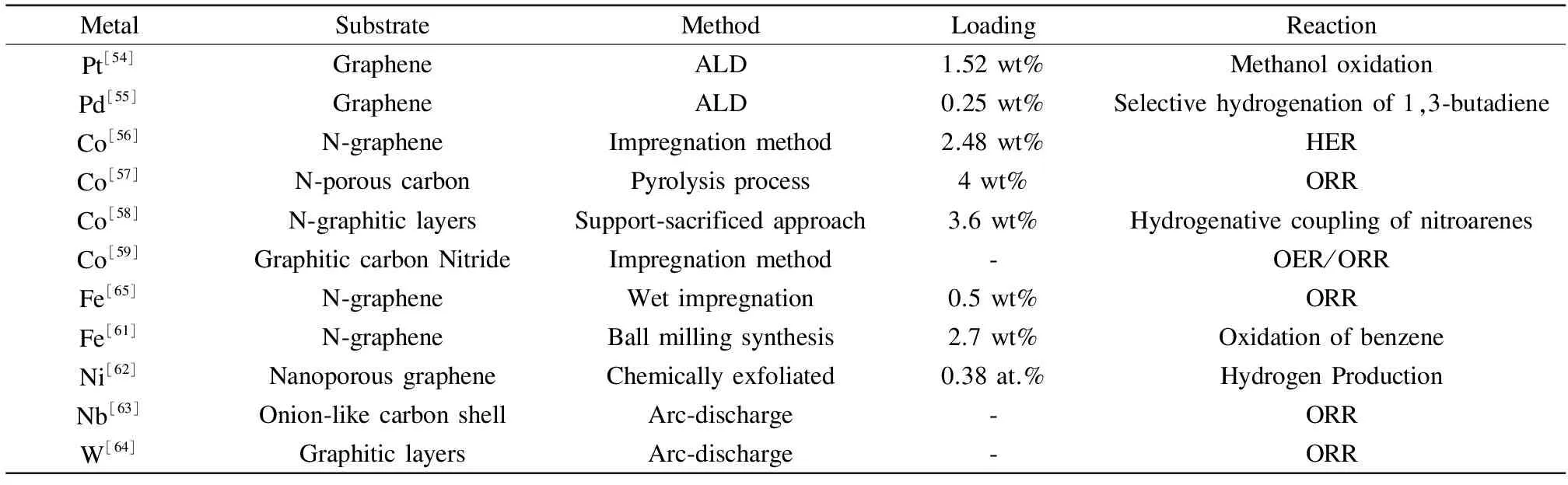
MetalSubstrateMethodLoadingReactionPt[54]GrapheneALD1.52wt%MethanoloxidationPd[55]GrapheneALD0.25wt%Selectivehydrogenationof1,3⁃butadieneCo[56]N⁃grapheneImpregnationmethod2.48wt%HERCo[57]N⁃porouscarbonPyrolysisprocess4wt%ORRCo[58]N⁃graphiticlayersSupport⁃sacrificedapproach3.6wt%HydrogenativecouplingofnitroarenesCo[59]GraphiticcarbonNitrideImpregnationmethod⁃OER/ORRFe[65]N⁃grapheneWetimpregnation0.5wt%ORRFe[61]N⁃grapheneBallmillingsynthesis2.7wt%OxidationofbenzeneNi[62]NanoporousgrapheneChemicallyexfoliated0.38at.%HydrogenProductionNb[63]Onion⁃likecarbonshellArc⁃discharge⁃ORRW[64]GraphiticlayersArc⁃discharge⁃ORR
3 Preparation methods
3.1 Mass-selected soft-landing technique
The mass-selected soft-landing technique is a powerful method to deposit metal single atoms and nanoparticles on supports. In this method, the metal is gasified by a high frequency laser. Abbet et al.[66]studied the cyclotrimerization of acetylene on size-selected Pdncluster (1≤n≤30) supported on thin MgO(100) films, in which the single Pd atom had a very high activity at low temperature (300 K). However, the ultrahigh vacuum and low production yield had limited its industrial application[1].
3.2 Impregnation method
The traditional impregnation method is widely used to prepare heterogeneous catalysts, in which the carrier is impregnated in a precursor solution. The active substance is gradually adsorbed on the surface of the substrate. Fei et al[57]. reported the first achievement of Co SACs on graphene oxide (GO) using CoCl2·6H2O as precursor solution. Catalytically active Pt single atoms onθ-alumina[36, 67]or TiN nanoparticles[68]had also been achieved by this method.
3.3 Co-precipitation method
Co-precipitation method is widely used to synthesize the nano-metal catalyst[69-71]by mixing the metal precursor and the carrier, followed by filtering and drying processes. After the Pt single atoms were uniformly dispersed on a FeOxsupport by co-precipitation method[5], Zhang et al. achieved precipitation of different precious metals (Ir[34], Au[40]et al.) on iron oxide. Recently, they synthesized highly active, selective, and extremely stable CeO2-supported Au SACs (Au1/CeO2) for preferential oxidation of CO in H2-rich stream.
3.4 Atomic layer deposition
Atomic layer deposition (ALD)[72-74], a process that provides atomic level control of thin film growth using sequential, self-limiting surface reactions, has been widely used to prepare nanomaterials. Yan et al[54]. reported a practical synthesis for isolated single Pt atoms anchored to graphene nanosheet using the ALD technique. The prepared catalyst showed a much higher activity for methanol oxidation and better CO tolerance than the conventional Pt/C catalyst. Lu et al[56]. reported that atomically dispersed Pd on graphene could be fabricated by ALD technique. The single-atom Pd/graphene catalyst showed a selectivity of about 100% for butenes at a 95% conversion under a mild reaction condition.
3.5 Solid phase melting method
Guo et al[75]. reported the Fe©SiO2catalyst prepared by solid phase melting method, which showed a good reactivity after a 60 h test. Single iron sites embedded in the silica matrix could directly convert methane to ethylene and aromatics. They had found that the absence of adjacent iron sites activated the first C—H bond of methane and prevented catalytic C—C coupling.
3.6 Successive reduction method
The successive reduction method, also known as the seed mediated growth method, is effective in the size-controlled synthesis of transition metal nanoparticles[76-78]. Zhang et al.[14]synthesized colloidal Au/Pd SACs by a facile successive reduction method, which exhibited a significantly improved catalytic activity (up to 17 times) for glucose oxidation over that of Au nanoclusters (NCs).
3.7 Arc discharge method
The traditional carbon arc discharge method was originally used by Iijima[79]to produce multi-walled carbon nanotubes. The direct current arc operates between two graphite electrodes installed in a water-cooled chamber filled with helium gas at subatmospheric pressure. It is a very simple technique and is capable of massive production of carbon/metal nanocomposites[80, 81]. Guo et al.[63]prepared carbon nanoonion-supported Nb SACs by arc discharge between a Nb (99.9%) anode and a carbon cathode. The Nb rod was evaporated by arc-discharging and the product deposited on the chamber wall. They found that single Nb atoms were incorporated into onion-like carbon shells and played a key role in improving ORR catalytic performance.
4 Anchoring mechanism of carbon supported metal SACs
Although it is found that stabilizing metal SACs onto the surface of the substrate is effective to avoid their agglomeration and inactivation, the anchoring mechanism of metal single atoms remains unclear. The anchoring mechanism differs from the choice of substrate. For example, the metal single atoms interact with metal substrate by forming monatomic alloys[11-13]. The surface defects could serve as anchoring sites when the metal oxide is used as the substrate[5, 34, 82]. As carbon material is mentioned, some mechanisms have been proposed based on atomic resolution microscopy observations. Metal single-atom is confirmed to anchor to graphene lattice by direct bonding or with an intermediate bridge (shown in Fig. 6).
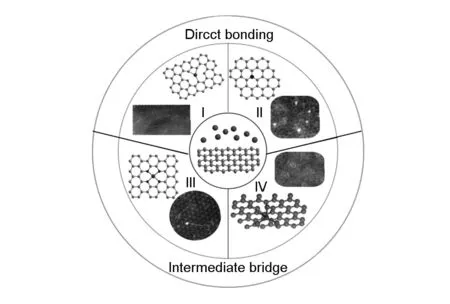
Fig. 6 Anchoring types of single metal atoms on graphene.
Atomic-resolution microscopy investigation by Guo et al.[83]on graphene-based nanoporous carbons demonstrated that they comprise isotropic, three-dimensional networks of wrinkled one-atom-thick graphene sheets(shown in Fig. 7). In each graphene plane, topological defects induced localized rippling of graphene sheets, which interfered with their graphitic stacking, forming nanopores to enhance adsorptions of molecules or metal atoms.
In the Nb SAC sample, single niobium atoms were observed by STEM, uniformly dispersed and stabilized in the highly defective graphitic shells (shown in Fig. 8). Based on the simulation, it was found that the single niobium atoms occupied substitutional sites of the carbon planes (Type I in Fig. 6). It was indicated that the most favorable substitution sites for single niobium atoms were the triple vacancy of graphene, which was consistent with the experimental observation. This Nb-in-carbon onion structure not only enhanced the overall conductivity for accelerating the exchange of ions and electrons in ORR, but also suppressed the agglomeration of metal single atom in the process of chemical/thermal reaction. The same anchored mechanism of metal single atoms was confirmed in the metal single atom tungsten catalysts stabilized in graphitic layers[64].
Qiu et al.[62]observed that Ni single atoms occupied carbon sites in the graphene lattices (Type II in Fig. 6) by STEM. The partial density of states projected to the Ni atom and the three surrounding C atoms, together with their overlapping, indicating strong C-Ni binding (shown in Fig. 9).

Fig. 7 Atomic-resolution ADF-STEM images of graphene-based nanoporous carbons[83].
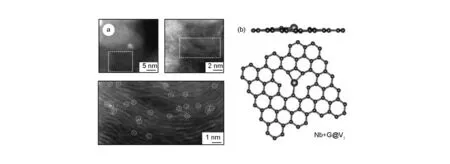
Fig. 8 Direct observation of single niobium atoms trapped in carbon onion structure. The schematic diagram of the most energetically advantageous configuration of single niobium atoms incorporated into defects of single-layer carbon plane[64].
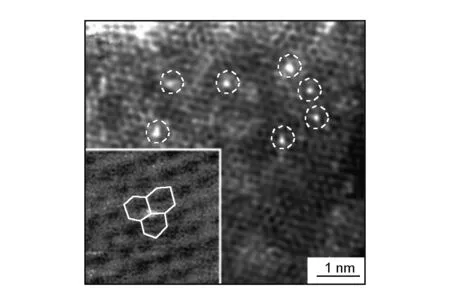
Fig. 9 HAADF-STEM image of Ni-doped graphene. Inset: Enlarged HAADF-STEM image (white circle), which shows a substitutional Ni atom (bright orange spot) occupying a carbon site in the graphene lattice (white lines)[62].
On the other hand, nitrogen-doped graphene has been used to stabilize Co[58]or Fe[61]atoms and a unique structure with a metal atom center and four surrounding N atoms embedded into graphene lattice (Type III in Fig. 6) has been suggested (shown in Fig. 10). Recently, this hypothesized structure was directly observed by Lin[84]using gentle STEM. They suggested that the structure tended to trap a series of transition metal atoms (Mg, Al, Ca, Ti, Cr, Mn, and Fe) as individual atoms.
In addition, Sun and Lu et al. prepared Pt[55], Pd[56]metal single atom by ALD and suggested that metal single atoms were connected to oxygen containing function groups on the surface of graphene (Type IV in Fig. 6). This hypothesis had been proved by the STEM observation of oxygen atoms in oxidized graphene. Guo et al.[85]found that the oxygen atoms constructed stable crown ether configurations within the graphene lattice. It was indicated that the crown ether in graphene tended to selectively bind various metal atoms depending on their ring size (Fig. 11). So their discovery could introduce a new wave of investigations and applications of chemically functionalized graphene.
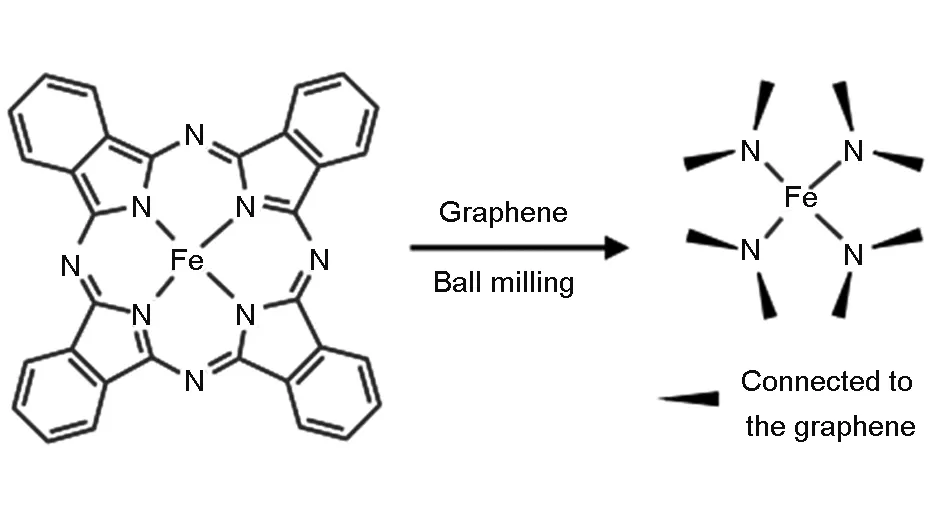
Fig. 10 Scheme of a proposed mechanism for synthesis of FeN4/GN via a facile ball milling method[61].
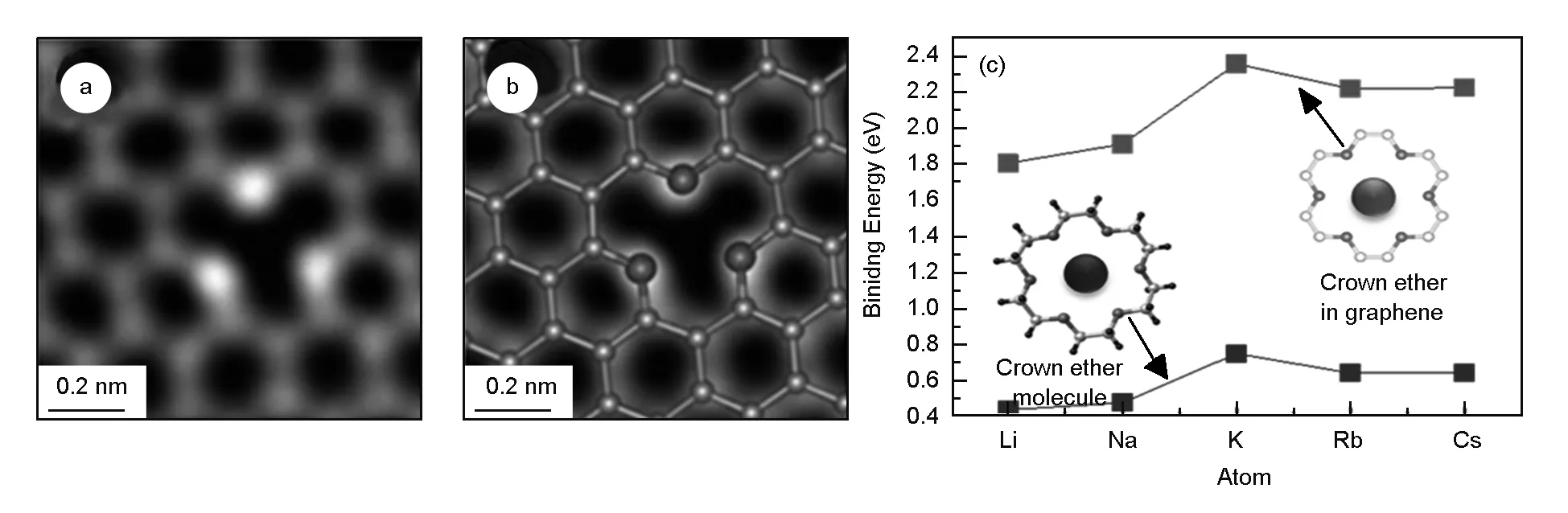
Fig. 11 Atomic structures of oxygen atoms incorporated in graphene multivacancies[85].
5 Conclusions and prospects
The maximum utilization of metal catalyst can be realized by downsizing the metal particles to isolated single atoms. Nevertheless, practical supported metal SACs are normally inhomogeneous and usually consist of a mixture of different sizes from nanoparticles to subnanometer clusters, which limits the accurate test of catalytic behaviors of SACs. Furthermore, most of the current metal SACs are limited by the extremely low metal loading and density of single active sites. The multi-layer of defective graphitic layers are rich in anchoring sites of single atoms, thus increasing the metal loading and catalytic efficiency[58, 59].
Now, the synthesis and characterization of well dispersed metal single atoms, as well as the test of reaction on single active site have been achieved. The catalytic mechanism may be significantly changed due to the low-coordination environment, quantum size effect, and the improved metal-support interactions. The better understanding of the metal-substrate reaction and single active site catalytic mechanism is necessary for designing new single-atom catalyst.
In the near future, we could make a significant progress in understanding the fundamental properties of supported metal SACs and realize the ultimate goal of manipulating individual atoms by innovative synthesis method, advanced characterization and theoretical calculation. It is believed that the superior catalytic performance and potential cost advantages will attract increasing attention in the related research fields.
[1] Yang X F, Wang A Q, Qiao B T, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research, 2012, 46(8): 1740-1748.
[2] Turner M, Golovko V B, Vaughan O P, et al. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters[J]. Nature, 2008, 454(7207): 981-983.
[3] Lei Y, Mehmood F, Lee S, et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects[J]. Science, 2010, 328(5975): 224-228.
[4] Qiao B T, Wang A Q, Li L, et al. Ferric oxide-supported Pt subnano clusters for preferential oxidation of CO in H2-rich gas at room temperature[J]. ACS Catalysis, 2014, 4(7): 2113-2117.
[5] Qiao B T, Wang A Q, Yang X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry, 2011, 3(8): 634-641.
[6] Poh C K, Lim S H, Lin J Y, et al. Tungsten carbide supports for single-atom platinum-based fuel-cell catalysts: First-principles study on the metal-support interactions and O2dissociation on WxC low-index surfaces[J]. The Journal of Physical Chemistry C, 2014, 118(25): 13525-13538.
[7] Liu P X, Zhao Y, Qin R X, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts[J]. Science, 2016, 352(6287): 797-801.
[8] Tang L, Wang Y, Li Y, et al. Preparation, structure, and electrochemical properties of reduced graphene sheet films[J]. Advanced Functional Materials, 2009, 19(17): 2782-2789.
[9] Uzun A, Ortalan V, Hao Y, et al. Nanoclusters of gold on a high-area support: Almost uniform nanoclusters imaged by scanning transmission electron microscopy[J]. ACS Nano, 2009, 3(11): 3691-3695.
[10] Uzun A, Ortalan V, Browning N D, et al. A site-isolated mononuclear iridium complex catalyst supported on MgO: Characterization by spectroscopy and aberration-corrected scanning transmission electron microscopy[J]. Journal of Catalysis, 2010, 269(2): 318-328.
[11] Kyriakou G, Boucher M B, Jewell A D, et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335(6073): 1209-1212.
[12] Lucci F R, Liu J, Marcinkowski M D, et al. Selective hydrogenation of 1,3-butadiene on platinum-copper alloys at the single-atom limit[J]. Nature Communications, 2015, 6: 8550.
[13] Zhang L L, Wang A Q, Miller J T, et al. Efficient and durable Au alloyed Pd single-atom catalyst for the ullmann reaction of aryl chlorides in water[J]. ACS Catalysis, 2014, 4(5): 1546-1553.
[14] Zhang H J, Kawashima K, Okumurac M, et al. Colloidal Au single-atom catalysts embedded on Pd nanoclusters[J]. Journal of Materials Chemistry A, 2014, 2(33): 13498-13508.
[15] Ge J, He D S, Chen W, et al. Atomically dispersed Ru on ultrathin Pd nanoribbons[J]. Journal of the American Chemical Society, 2016.
[16] Wang Z T, Matthew T D, Andrew J T, et al. Preparation, structure, and surface chemistry of Ni-Au single atom alloys[J]. The Journal of Physical Chemistry C, 2016, 120(25): 13574-13580.
[17] Lin J, Qiao B T, Liu J Y, et al. Design of a highly active Ir/Fe(OH)xcatalyst: Versatile application of Pt-group metals for the preferential oxidation of carbon monoxide[J]. Angewandte Chemie, 2012, 51(12): 2920-2924.
[18] Yuan Z, Li X N, and He S G. CO oxidation promoted by gold atoms loosely attached in AuFeO3- cluster anions[J]. The Journal of Physical Chemistry Letters, 2014, 5(9): 1585-1590.
[19] Hu P P, Amghouz Z, Huang Z W, et al. Surface-confined atomic silver centers catalyzing formaldehyde oxidation[J]. Environmental Science & Technology, 2015, 49(4): 2384-2390.
[20] Zhao Y X, Li Z Y, Yuan Z, et al. Thermal methane conversion to formaldehyde promoted by single platinum atoms in PtAl2O4- cluster anions[J]. Angewandte Chemie, 2014, 53(36): 9482-9486.
[21] Chen M S, Goodman D W. The structure of catalytically active gold on titania[J]. Science, 2004, 306(5694): 252-255.
[22] Matthey D, Wang J G, Wendt S, et al. Enhanced bonding of gold nanoparticles on oxidized TiO2(110)[J]. Science, 2007, 315(5819): 1692-1696.
[23] Kwak J H, Hu J Z, Mei D H, et al. Coordinatively unsaturated Al3+centers as binding sites for active catalyst phases of platinum on g-Al2O3[J]. Science, 2009, 325(5948): 1670-1673.
[24] Lu J, Aydin C, Browning N D, et al. Imaging isolated gold atom catalytic sites in zeolite NaY[J]. Angewandte Chemie, 2012, 51(24): 5842-5846.
[25] Kistler J, Chotigkrai N, Xu P H, et al. A single-site platinum CO oxidation catalyst in zeolite KLTL: Microscopic and spectroscopic determination of the locations of the platinum atoms[J]. Angewandte Chemie, 2014, 53(34): 8904-8907.
[26] Kamai R, Kamiya K, Hashimoto K, et al. Oxygen-tolerant electrodes with platinum-loaded covalent triazine frameworks for the hydrogen oxidation reaction[J]. Angewandte Chemie, 2016, 55(42): 13184-13188.
[27] Kamiya K, Kamai R, Hashimoto K, et al. Platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles as methanol-tolerant oxygen reduction electrocatalysts[J]. Nature Communications, 2014, 5: 5040.
[28] Pei G X, Liu X Y, Wang A Q, et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene[J]. ACS Catalysis, 2015, 5(6): 3717-3725.
[29] Huang W X, Zhang S R, Tang Y, et al. Low-temperature transformation of methane to methanol on Pd1O4single sites anchored on the internal surface of microporous silicate[J]. Angewandte Chemie, 2016, 55: 1-6.
[30] Zhang H B, Jing W, Dong J C, et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework[J]. Angewandte Chemie, 2016, 55(46): 14310-14314.
[31] Kyriakou G, Boucher M B, Jewell A D, et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335(6073): 1209-1212.
[32] Wei H S, Liu X Y, Wang A Q, et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes[J]. Nature Communications, 2014, 5: 5634.
[33] Shi Y T, Zhao C Y, Wei H S, et al. Single-atom catalysis in mesoporous photovoltaics: the principle of utility maximization[J]. Advanced Materials, 2014, 26(48): 8147-8153.
[34] Lin J, Wang A Q, Qiao B T, et al. Remarkable performance of Ir1/FeOxsingle-atom catalyst in water gas shift reaction[J]. Journal of the American Chemical Society, 2013, 135(41): 15314-15317.
[35] He Q, Freakley S J, Edwards J K, et al. Population and hierarchy of active species in gold iron oxide catalysts for carbon monoxide oxidation[J]. Nature Communications, 2016, 7: 12905.
[36] Moses-DeBusk M, Yoon M, Allard L F, et al. CO oxidation on supported single Pt atoms: experimental and ab initio density functional studies of CO interaction with Pt atom on theta-Al2O3(010) surface[J]. Journal of the American Chemical Society, 2013, 135(34): 12634-12645.
[37] Ghosh T K, Nair N N. Rh1/γ-Al2O3single-atom catalysis of O2activation and CO oxidation: mechanism, effects of hydration, oxidation state, and cluster size[J]. ChemCatChem, 2013, 5(7): 1811-1821.
[38] Li Z Y, Yuan Z, Li X N, et al. CO oxidation catalyzed by single gold atoms supported on aluminum oxide clusters[J]. Journal of the American Chemical Society, 2014, 136(40): 14307-14313.
[39] Song W Y, Hensen E J M. Structure sensitivity in CO oxidation by a single Au atom supported on ceria[J]. The Journal of Physical Chemistry C, 2013, 117(15): 7721-7726.
[40] Qiao B T, Liu J X, Wang Y G, et al. Highly efficient catalysis of preferential oxidation of CO in H2-rich stream by gold single-atom catalysts[J]. ACS Catalysis, 2015, 5(11): 6249-6254.
[41] Guo L W, Du P P, Fu X P, et al. Contributions of distinct gold species to catalytic reactivity for carbon monoxide oxidation[J]. Nature Communications, 2016, 7: 13481.
[42] Gao D W, Zhang X, Yang Y, et al. Supported single Au(III) ion catalysts for high performance in the reactions of 1,3-dicarbonyls with alcohols[J]. Nano Research, 2016, 9(4): 985-995.
[43] Jones J, Xiong H F, DeLaRiva A T, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping[J]. Science, 2016, 353(6295): 150-154.
[44] Wang L, Zhang S R, Zhu Y, et al. Catalysis and in situ studies of Rh1/Co3O4nanorods in reduction of NO with H2[J]. ACS Catalysis, 2013, 3(5): 1011-1019.
[45] Li X N, Yuan Z, and He S G. CO oxidation promoted by gold atoms supported on titanium oxide cluster anions[J]. Journal of the American Chemical Society, 2014, 136(9): 3617-3623.
[46] Xie X W, Li Y, Liu Z Q, et al. Low-temperature oxidation of CO catalysed by Co3O4nanorods[J]. Nature, 2009, 458(7239): 746-749.
[47] Geim A K, Novoselov K S. The rise of graphene[J]. Nature Materials, 2007, 6(3): 183-191.
[48] Guo J J, Wang X M, Yao Y L, et al. Structure of nanocarbons prepared by arc discharge in water[J]. Materials Chemistry and Physics, 2007, 105(2-3): 175-178.
[49] Guo J J, Morris J R, Ihm Y, et al. Topological defects: Origin of nanopores and enhanced adsorption performance in nanoporous carbon[J]. Small, 2012, 8(21): 3283-3288.
[50] Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669.
[51] Scheuermann G M, Rumi L, Steurer P, et al. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the suzuki-miyaura coupling reaction[J]. Journal of the American Chemical Society, 2009, 131(23): 8262-8270
[52] Yin H J, Tang H J, Wang D, et al. Facile synthesis of surfactant-free Au cluster/graphene hybrids for high-performance oxygen reduction reaction[J]. ACS Nano, 2012, 6(9): 8288-8297.
[53] Machado B F, Serp P. Graphene-based materials for catalysis[J]. Catal Sci Technol, 2012, 2(1): 54-75.
[54] Sun S, Zhang G, Gauquelin N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Scientific Reports, 2013, 3.
[55] Sun S H, Zhang G X, Gauquelin N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Scientific Reports, 2013, 3.
[56] Yan H, Cheng H, Yi H, et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene[J]. Journal of the American Chemical Society, 2015, 137(33): 10484-10487.
[57] Fei H L, Dong J C, Arellano-Jime M J, et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation[J]. Nature Communications, 2015, 6: 8668.
[58] Yin P Q, Yao T, Wu Y, et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angewandte Chemie, 2016, 55(36): 10800-10805.
[59] Liu W G, Zhang L L, Yan W S, et al. Single-atom dispersed Co-N-C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes[J]. Chem. Sci., 2016, 7(9): 5758-5764.
[60] Zitolo A, Goellner V, Armel V, et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials[J]. Nature Materials, 2015, 14(9): 937-942.
[61] Deng D H, Chen X Q, Yu L, et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature[J]. Science Advances, 2015, 1(11): 1-9.
[62] Qiu H J, Ito Y, Cong W T, et al. Nanoporous graphene with single-atom nickel dopants: An efficient and stable catalyst for electrochemical hydrogen production[J]. Angewandte Chemie, 2015, 54(47): 14031-14035.
[63] Zhang X F, Guo J J, Guan P F, et al. Catalytically active single-atom niobium in graphitic layers[J]. Nature Communications, 2013, 4: 1924.
[64] Guo J J, Mao Z, Yan X L, et al. Ultrasmall tungsten carbide catalysts stabilized in graphitic layers for high-performance oxygen reduction reaction[J]. Nano Energy, 2016, 28: 261-268.
[65] Zitolo A, Goellner V, Armel V, et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials[J]. Nature Materials, 2015, 14(9): 937-942.
[66] Abbet S, Sanchez A, Heiz U, et al. Acetylene cyclotrimerization on supported size-selected Pdn clusters (1≤n≤ 30): one atom is enough![J]. Journal of the American Chemical Society, 2000, 122: 3453-3457.
[67] Narula C K, Allard L F, Stocks G M, et al. Remarkable NO oxidation on single supported platinum atoms[J]. Scientific Reports, 2014, 4: 7238.
[68] Yang S, Kim J, Tak Y J, et al. Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions[J]. Angewandte Chemie, 2016, 55(6): 2058-2062.
[69] Haruta M. Size- and support-dependency in the catalysis of gold[J]. Catalysis Today, 1997, 36(1): 153-166.
[70] Akolekar D B, Foranb G, and Bhargava S K. X-ray absorption spectroscopic studies on gold nanoparticles in mesoporous and microporous materials[J]. Journal of Synchrotron Radiation, 2004, 11(3): 284-290.
[71] Akolekar D B, Bhargava S K, Foran G, et al. Studies on gold nanoparticles supported on iron, cobalt, manganese, and cerium oxide catalytic materials[J]. J Mol Catal Chem, 2005, 238(1-2): 78-87.
[72] Leskela M, Ritala M. Atomic layer deposition chemistry: recent developments and future challenges[J]. Angewandte Chemie, 2003, 42(45): 5548-5554.
[73] King J S, Wittstock A, Biener J, et al. Ultralow loading Pt nanocatalysts prepared by atomic layer deposition on carbon aerogels[J]. Nano Letters, 2008, 8(8): 2405-2409.
[74] Liu C, Wang C C, Kei C C, et al. Atomic layer deposition of platinum nanoparticles on carbon nanotubes for application in proton-exchange membrane fuel cells[J]. Small, 2009, 5(13): 1535-1538.
[75] Guo X G, Fang G Z, Li G, et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen[J]. Science, 2014, 344(6184): 616-619.
[76] Jana N R, Gearheart L, Murphy C J. Seeding growth for size control of 5-40 nm diameter gold nanoparticles[J]. Langmuir, 2001, 17: 6782-6786.
[77] Gole A, Murphy C J. Seed-mediated synthesis of gold nanorods: role of the size and nature of the seed[J]. Chem Mater, 2004, 16: 3633-3640.
[78] Zhou W J, Yang L J. Highly active core-shell Au@Pd catalyst for formic acid electrooxidation[J]. Electrochemistry Communications, 2007, 9(7): 1725-1729.
[79] Iijima S. Helical microtubules of graphitic carbon[J]. Nature, 1991, 354(6348): 56.
[80] Hisn Y L, Hwang K C, Chen F R, et al. Production and insitu metal filling of carbon nanotubes in water[J]. Advanced Materials, 2001, 13: 830-835.
[81] Alekseyev N I, Dyuzhev G A. Fullerene formation in an arc discharge[J]. Carbon, 2003, 41(7): 1343-1348.
[82] Liang J X, Lin J, Yang X F, et al. Theoretical and experimental investigations on single-atom catalysis: Ir1/FeOxfor CO oxidation[J]. The Journal of Physical Chemistry C, 2014, 118(38): 21945-21951.
[83] Guo J, Morris J R, Contescu C I, et al. Atomic-scale imaging of graphene-based nanoporous carbon[J]. Microscopy and Microanalysis, 2012, 18(S2): 1528-1529.
[84] Lin Y C, Teng P Y, Yeh C H, et al. Structural and chemical dynamics of pyridinic-nitrogen defects in graphene[J]. Nano Lett, 2015, 15(11): 7408-7413.
[85] Guo J J, Lee J, Contescu C I, et al. Crown ethers in graphene[J]. Nature Communications, 2014, 5: 5389.
