含醇溶蛋白小麦回生抗性直支链淀粉性质分析
2018-03-09郭俊杰马乔治康海岐连喜军
郭俊杰,马乔治,康海岐,连喜军
含醇溶蛋白小麦回生抗性直支链淀粉性质分析
郭俊杰1,马乔治1,康海岐2,连喜军1※
(1. 天津商业大学,生物技术与食品科学学院,天津市食品生物技术重点实验室,天津 300134;2. 四川省农业科学院作物研究所,成都 610066)
为研究含醇溶蛋白小麦回生抗性直支链淀粉性质,该文采用醇溶法从小麦粉中提取醇溶蛋白,采用回生-酶解法分离得到小麦直、支链淀粉。通过可见光谱、红外光谱、X-射线衍射、差热扫描等方法分析研究醇溶蛋白对小麦直、支链淀粉回生的影响。结果表明,在凝胶化及回生过程中醇溶蛋白与淀粉相互作用,导致淀粉回生率增加。红外光谱研究表明,直链淀粉与醇溶蛋白在高压糊化后干燥或回生的条件下,醇溶蛋白的酰胺II键伸缩振动从1 546 cm−1降低至1 539 cm−1,即直链淀粉与醇溶蛋白通过氢键结合。X-射线衍射图谱显示在2衍射角为17°,19°,22°等的衍射峰没有发生明显变化,表明添加醇溶蛋白后,直、支链淀粉的晶型未发生明显改变。DSC结果显示直链淀粉与醇溶蛋白之间的氢键是在共同回生过程中产生的,样品中多晶结构和双螺旋结构共存。研究结果表明,淀粉中空间位阻小的6位碳原子上的羟基与醇溶蛋白中的脯氨酸和谷氨酰胺通过氢键结合,这种类型的氢键阻碍了淀粉酶对淀粉的解离,即醇溶蛋白通过与淀粉形成新型氢键而促进了淀粉的回生。该研究提供了一种提高小麦淀粉的回生率的新技术,为进一步深入研究醇溶蛋白促进淀粉回生的机理提供理论支撑。
淀粉;农产品;品质控制;醇溶蛋白;回生机理;表征
0 引 言
淀粉是植物储备的碳水化合物,小麦中含有65%~70%的淀粉,11%~13%的蛋白以其他营养组分[1]。回生抗性淀粉[2](resistant starch 3, RS3)不被健康人体的小肠消化吸收,但能在大肠中发酵降解,该类淀粉具有广泛的应用:可以显著提高食品的膨胀系数、增加耐泡性、耐煮性、保持食品松脆[3];黏度稳定、持水力低、流变性高,常用作食品增稠剂,并可提高乳制品的益生菌活力,延长货架期[4];在体内代谢较慢,持续提供身体能量,从而增强人体的工作耐力[5];能与一些膳食纤维组分相互作用,促进类脂代谢、矿物质吸收,抑制肥胖[6-7];可以延缓血糖升高、激活肠道免疫系统、控制总胆固醇量,对于糖尿病、肠道疾病及心脑血管疾病等均具有良好预防效果[8-10]。因此每日摄入适量抗性淀粉对人体健康尤为重要[11]。
回生抗性淀粉主要通过回生法制备,淀粉回生的本质是糊化淀粉分子由高能无序态向有序态转化的过程,即糊化后的淀粉分子借助氢键相互吸引,排列成序、形成高度致密的、结晶化的不溶性分子微束[12]。目前制备回生淀粉的方法主要有酸解、挤压、压热处理、酶解和晶种促进等几种方法。其中酸解法可以显著降低淀粉的粘度,增加结晶性,从而提高回生率[13];挤压法和压热处理均使得淀粉颗粒完全破裂,直链淀粉分子更易于相互作用形成氢键,从而明显增加回生淀粉量[14];酶解法处理淀粉会生成很多游离的直链淀粉,直链淀粉分子在冷却老化过程中重新缠绕形成新的晶体,从而提高回生淀粉量,酶解法也是目前研究回生淀粉较多的一种方法[15]。但这些方法均不能使淀粉的回生率超过50%,低回生率也降低了该类产品的竞争优势,限制了其产业化和市场化,至今中国尚未有该类产品面世。
据报道[16-17],小麦蛋白中的醇溶蛋白促进小麦淀粉的回生,谷蛋白却对淀粉回生产生抑制作用,而醇溶蛋白与淀粉作用的机理非常复杂[18]。通过现代表征方法探究淀粉回生的机理一直是近年研究的热点[19-21]。本文通过可见光谱、红外光谱、X-射线衍射和差热扫描量热法等手段研究和表征了小麦直、支链淀粉与醇溶蛋白混合前后的结构变化,探索醇溶蛋白促进小麦淀粉回生的可能机制。
1 材料与方法
1.1 材料与仪器
小麦粉为市售;NaCl,NaOH,I2,KI和CH3CH2OH均为分析纯,由天津富裕化学品公司提供;高温-淀粉酶、酸性、碱性和中性蛋白酶由天津市诺奥科技发展有限公司提供。
YXQG02手提式电热压力蒸汽消毒器,山东安德医疗科技有限公司;DH-101-3BS电热恒温鼓风干燥箱,天津市中环实验电炉有限公司;BCD-229KB海尔冰箱,青岛海尔股份有限公司;L535-1离心沉淀机,湖南湘仪离心机仪器有限公司;Bio-Rad FES135红外分光光度计,美国Bio-Rad公司;岛津UV-2450/2550紫外可见分光光度计;D/max-2500 X-射线衍射分析仪,日本理学公司;Q20 差热扫描量热器,美国TA公司。
1.2 试验方法
1.2.1 小麦面筋的制备
参照Lian等[16]的试验方法,将小麦面粉和水按质量比(1:3)充分混合揉搓成团,将面团在30℃下发酵30 min(添加0.5%的酵母),揉搓5 min后,再次于30℃发酵30 min,然后再揉搓10 min,静置10 min。最后用水充分冲洗除去面团中的淀粉,得到浅黄灰色面筋。
1.2.2 醇溶蛋白的提取
面筋蛋白冷冻干燥后粉碎,用95%的乙醇浸泡3 d,充分搅拌,将上清液中加5倍体积的水,析出醇溶蛋白,充分水洗后离心分离、冷冻干燥后粉碎备用。
1.2.3 小麦直、支链淀粉的分离
将水洗法[22]制备的小麦淀粉按照质量分数10%~15%,用水分散后,于95 ℃水浴锅中搅拌糊化2 h至透明,120 ℃进一步高压(0.2 MPa)糊化40 min。冷却至常温后放冰箱中老化2 d,高温淀粉酶酶解、水洗后得到回生淀粉。将得到的回生淀粉中加入3倍体积的正丁醇,充分搅拌后离心分离得小麦直链淀粉。小麦支链淀粉制备采用盐洗法,用0.15 mol/L NaOH分散400 g小麦淀粉,手动搅拌40 min后,加入质量分数为5%的NaCl溶液3 600 mL,充分搅拌后静置5 min,用HCl调pH值至7.0、静置24 h,离心分离(4 000 r/min,5 min)、盐洗(碘液测量变为紫红色),离心分离后烘干(65 ℃,3 d)。
1.2.4 小麦直、支链淀粉与醇溶蛋白的共同回生
将得到的小麦直、支链淀粉用脂肪酶和碱性蛋白酶先后酶解纯化后,按1.2.3法回生得回生后的小麦直、支链淀粉。
直、支链淀粉与醇蛋白共同回生过程如下:分别将干燥后的小麦直链和支链淀粉10 g与0.4 g醇溶蛋白混合,加入20 mL 蒸馏水,95℃手动搅拌、糊化1.5 h,进一步高温高压(0.2 MPa,120℃)糊化30 min,干燥后得到醇溶蛋白与淀粉直接糊化后的样品。重复上述步骤,将所得糊化淀粉4℃老化24 h后干燥,得到醇溶蛋白与淀粉共同回生的样品。
1.2.5 测试方法
淀粉紫外可见吸收测定:将淀粉溶于4.0 mol/L的KOH溶液中,再用盐酸调节pH值至7.0,加1滴碘液[23],静置30 min后,用岛津UV-2450/2550紫外可见分光光度计测定最大吸收波长;红外分析:将样品用光谱纯KBr压片,在27℃下用红外分光光度计Bio-Rad FES135测定淀粉红外吸收,扫描范围4 000~400 cm-1;X-射线测试采用D/max-2 500 X-射线衍射分析仪,铜靶,测试范围2:4~50°,步长0.05°;DSC测试采用Q20 差热扫描量热器,扫描范围30~240℃,5 ℃/min。
2 结果与分析
2.1 淀粉-碘可见吸收测定
碘分子可嵌入淀粉的双螺旋结构内部而生成蓝紫色络合物[24-26],络合物的最大吸收波长取决于淀粉分子的重复单元数目[27]。在高温糊化过程中,小麦直链淀粉的碘吸收峰消失(图1a),表明其双螺旋结构被破坏,在小麦直链淀粉和醇溶蛋白之间形成了新的双螺旋结构。而在回生过程中,醇溶蛋白和小麦直链淀粉分离,淀粉双螺旋结构重新生成。小麦支链淀粉(图1b)与醇溶蛋白高温糊化及回生后,都没有碘吸收峰出现,表明小麦支链淀粉之间不能再形成双螺旋结构了。

图1 不同方式处理直链和支链淀粉的可见光谱图
2.2 红外光谱分析
图2为不同方式处理的小麦直、支链淀粉的红外光谱图。3 400 cm−1附近处的吸收峰为淀粉分子内和分子间O-H的伸缩振动。2 930 cm−1附近吸收峰代表淀粉中6位碳原子亚甲基C-H键的伸缩振动[28-29],直链及支链淀粉回生后,O-H和C-H的伸缩振动移向低场(如羟基的伸缩振动从3 417 cm−1移动到3 412 cm−1,3 431 cm−1移动到3 407 cm−1,亚甲基的吸收从2 927 cm−1移动到2 926 cm−1,2 930 cm−1移动到2 926 cm−1)。
小麦直链淀粉与醇溶蛋白在高压糊化后干燥或回生的条件下,酰胺II键从1 546 cm−1(醇溶蛋白的伸缩振动)降低至1 539 cm−1,表明小麦直链淀粉在高压糊化后干燥或回生的条件下都可以和醇溶蛋白结合。由于2 929 cm−1处的吸收未改变,这种结合不会是淀粉6位碳原子上的羟基与醇溶蛋白结合,应该是二者之间形成了双螺旋结构。小麦支链淀粉在1 546 cm−1在处的吸收表明,高压糊化后干燥或者回生,小麦支链淀粉均可以与醇溶蛋白结合。由于回生后的支链淀粉比直接干燥的吸收峰弱,表明回生过程会导致小麦支链淀粉与醇溶蛋白脱离。由于小麦支链淀粉的空间位阻,淀粉中所含的蛋白不能被完全被酶解,所以在小麦支链淀粉及回生的小麦支链淀粉的中1 547 cm−1处均有弱的吸收峰。
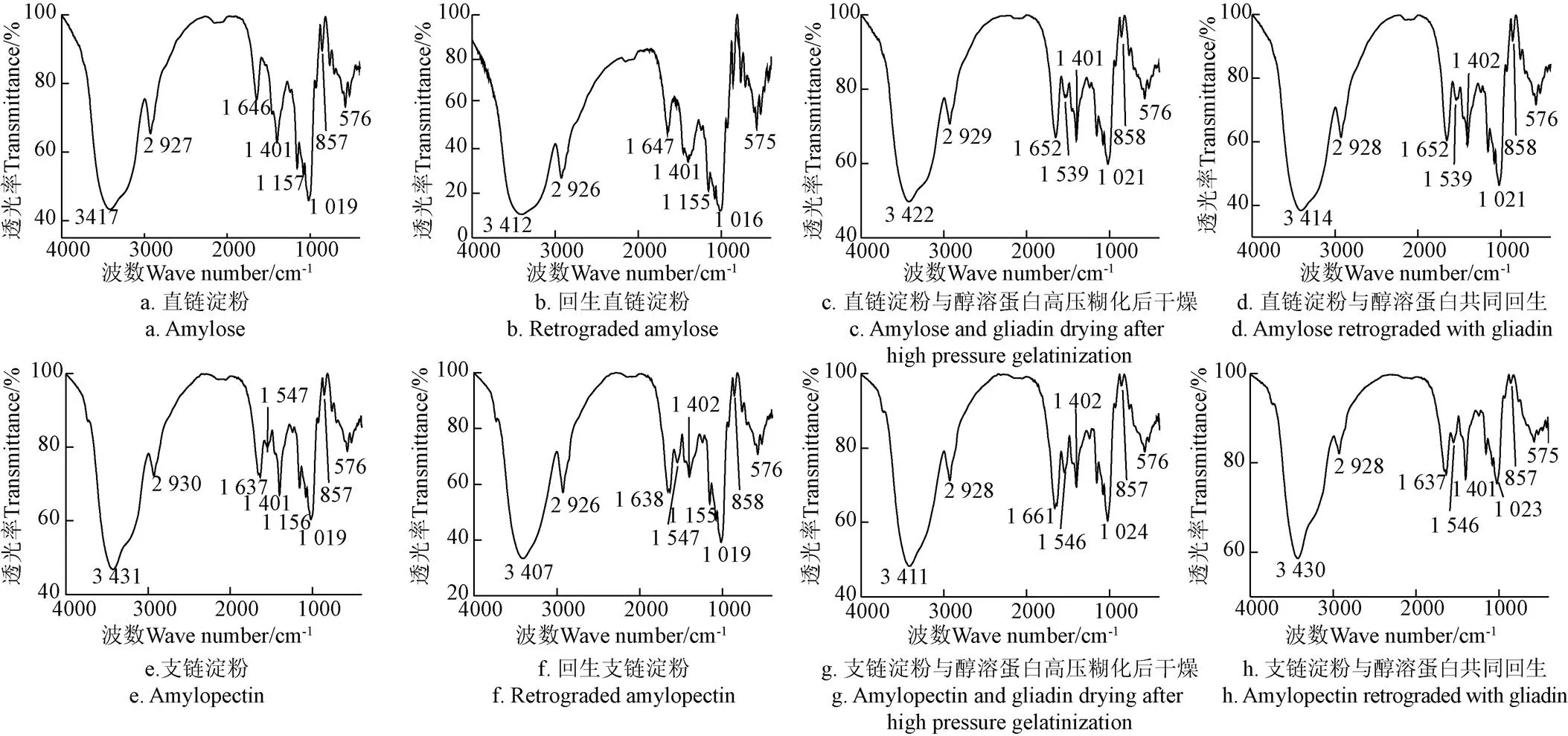
图2 不同方式处理小麦直链和支链淀粉的红外光谱图
2.3 X-射线分析
图3为不同方式处理的小麦直、支链淀粉的X-射线衍射图谱。一般来说,特定的淀粉晶型具有特定的X-射线衍射峰[30]。直链淀粉样品在2衍射角为17°处出现最强衍射峰,2衍射角为19°、22°一些小衍射峰以及5.6°的特征峰的出现说明直链淀粉是A型和B型的混合晶型。在回生过程中,2衍射角为19°的衍射峰几乎消失,表明回生使得有些晶面消失。与醇溶蛋白共同高温糊化后干燥或者回生,直链淀粉晶体形貌均未发生改变。
回生后的支链淀粉,没有明显的衍射峰,说明回生使得支链淀粉变成无定型结构。当与醇溶蛋白高温糊化并干燥后,支链淀粉的晶面增加,而回生后晶面反而减少了。2衍射角为5.2°衍射峰的出现,表明支链淀粉与醇溶蛋白共同回生后,变为B型结构。
2.4 DSC分析
图4和表1为不同方式处理小麦直链和支链淀粉的差热扫描图和热性能特征数据。小麦支链淀粉具有长于直链淀粉的分子链[31-32],所以支链淀粉的熔点(图4b)高于直链淀粉(图4a)。链长越长,分子间的氢键越多[33],熔点越高。
在小麦直链淀粉及回生小麦直链淀粉中(图4a),出现了代表双螺旋结构的低温峰,也出现了代表多晶结构的高温峰,说明杂多晶结构更加稳定。醇溶蛋白与小麦直链淀粉结合后,熔点降低,结合的蛋白越多,熔点降低的也越多。回生后的小麦支链淀粉(图4b)显示出3种微观形貌,A型多晶、双螺旋结构、体积稍大一些的多晶。与醇溶蛋白共同回生后,支链淀粉熔点升高,说明在回生过程中醇溶蛋白与支链淀粉发生了分离。混合醇溶蛋白后小麦回生支链淀粉没有了高温峰(晶体熔化峰),说明支链淀粉附近的醇溶蛋白抑制了较大结晶的形成。
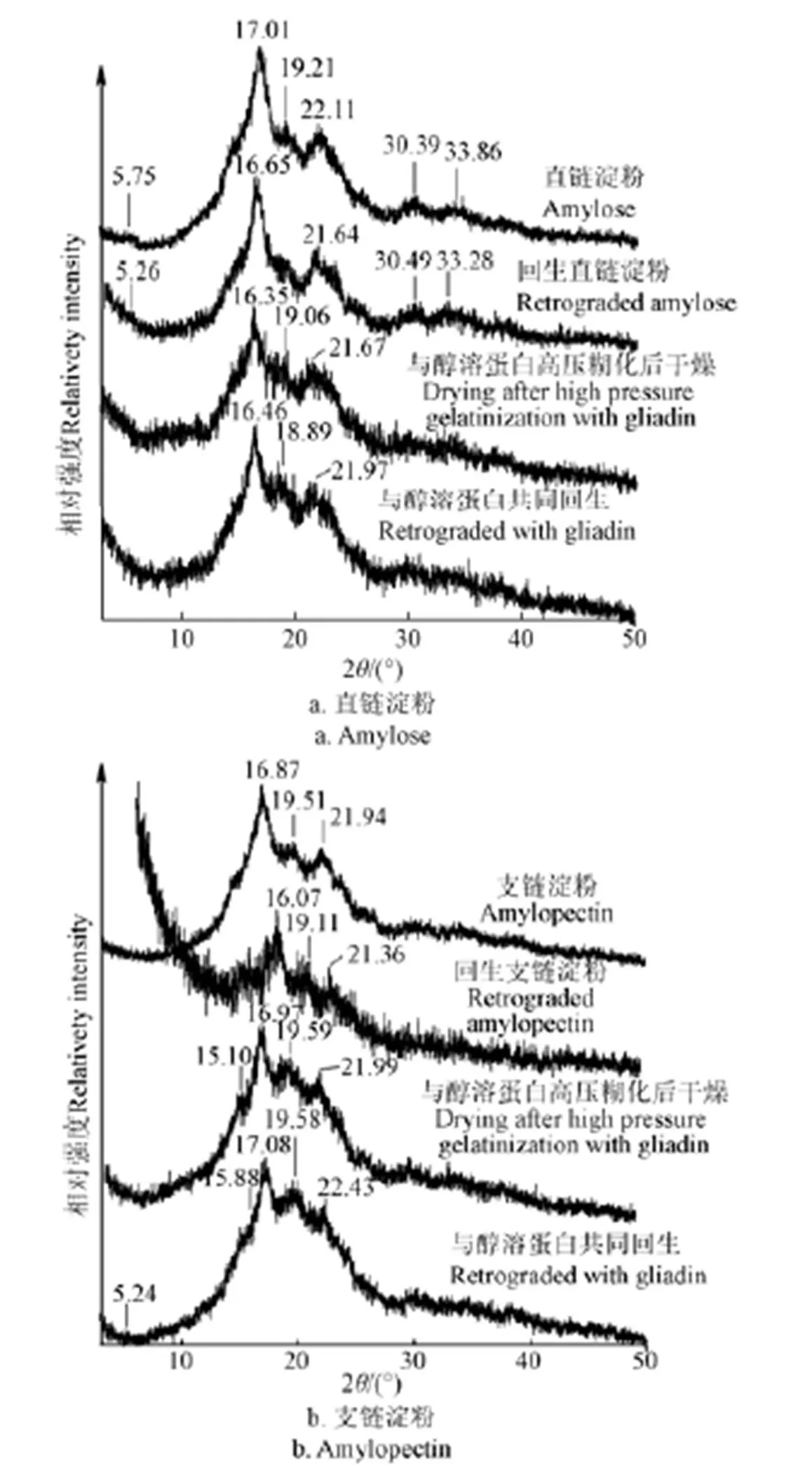
图3 不同方式处理直链和支链淀粉的X-射线衍射图

图4 不同方式处理小麦直链和支链淀粉的差热扫描图谱

表1 不同处理方式的小麦直、支链淀粉的热性能
2.5 淀粉与醇溶蛋白结合的机理
醇溶蛋白富含脯氨酸和谷氨酰胺,高温糊化会破坏淀粉之间的氢键并打开双螺旋结构。在回生过程中淀粉和蛋白表面重新形成氢键,生成双螺旋结构(图5),而导致淀粉回生率增加。淀粉6位碳原子空间位阻较小,羟基与富含酰胺键的脯氨酸和谷氨酸结合形成氢键,与红外研究结果一致。羟基与氨基酸这种相互作用抑制了淀粉酶酶解淀粉,即醇溶蛋白促进淀粉回生。而当醇溶蛋白与淀粉全部结合形成氢键后,就不会再促进淀粉回生了。
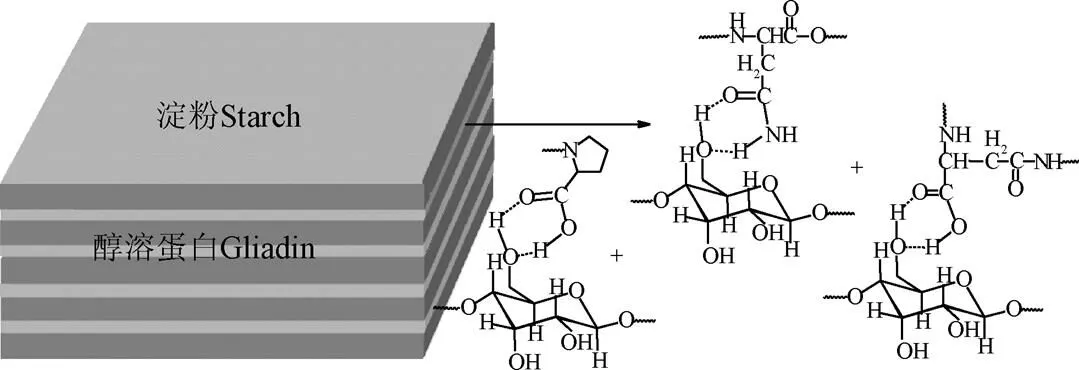
图5 醇溶蛋白促进小麦淀粉回生机理
3 结 论
1)将小麦直、支链淀粉与小麦醇溶蛋白共混回生,研究回生前后其结构变化。可见光谱表明高温糊化过程中直链淀粉双螺旋结构被破坏,在直链淀粉和醇溶蛋白之间形成了新的双螺旋结构;红外光谱表明,在淀粉回生时添加醇溶蛋白使得直链淀粉之间的氢键减少;X-射线衍射图谱表明,与醇溶蛋白共同高温糊化后干燥或者回生,直链淀粉形貌均未发生改变;DSC结果显示直链淀粉与醇溶蛋白之间的氢键是在共同回生过程中产生的,样品中多晶结构和双螺旋结构共存。
2)醇溶蛋白对小麦直、支链淀粉回生的促进机理为醇溶蛋白与直、支链淀粉共同回生过程中,空间位阻小的6位碳原子上的羟基与醇溶蛋白中的脯氨酸和谷氨酰胺通过氢键结合,这种类型的氢键阻碍了淀粉酶辨别淀粉链的可水解部位,即醇溶蛋白通过与淀粉形成新型氢键而在它们表面结合成双螺旋结构,从而抑制了淀粉的酶水解,增加了淀粉的回生率。
[1] Rahman S, Kosar-Hashemi B, Samuel M S, et al. The major proteins of wheat endosperm starch granules[J]. Australian Journal of Plant Physiology, 1995, 22(5): 793-803.
[2] Birt D F, Boylston T, Hendrich S, et al. Resistant starch: Promise for improving human health[J]. Advances in Nutrition, 2013, 4(6): 587-601.
[3] Englyst H N, Wiggins H S, Cummings J H. Determination of the non starch polysaccharides in plant food by gas—liquid chromatography of constituent sugars as alditol acetates[J]. Analyst, 1982, 107(1272): 307-318.
[4] Klosterbuer A S, Hullar M A J, Li F, et al. Gastrointestinal effects of resistant starch, soluble maize fiber and pullulan in healthy adults[J]. British Journal of Nutrition, 2013, 110(6): 1068-1074.
[5] Hua X P, Xie Y Y, Jin Z Y, et al. Effect of single-, dual-, and triple-retrogradation treatments on in vitro digestibility and structural characteristics of waxy wheat starch[J]. Food Chemistry, 2014, 157: 373-379.
[6] Polakof S, Diaz-Rubio M E, Dardevet D, et al. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats[J]. The Journal of Nutritional Biochemistry, 2013, 24(11): 1920-1930.
[7] Klosterbuer A S, Hullar M A J, Li F, et al. Gastrointestinal effects of resistant starch, soluble maize fibre and pullulan in healthy adults[J]. British Journal of Nutrition, 2013, 110(6): 1068-1074.
[8] Leu R Le, Young G, Hu Y, et al. Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation[J].Digestive Diseases and Sciences, 2013, 58(12): 3475-3482.
[9] MacNeil S, Rebry R M, Tetlow I J, et al. Resistant starch intake at breakfast affects postprandial responses in type 2 diabetics and enhances the glucose-dependent insulinotropic polypeptide-insulin relationship following a second meal[J]. Applied Physiology Nutrition and Metabolism, 2013, 38(12): 1187-1195.
[10] Trautwein E A, Forgbert K, Rieckhoff D, et al. Impact of-cyclodextrin and resistant starch on bile acid metabolism and fecal steroid excretion in regard to their hypolipidemic action in hamsters[J]. Biochimica et Biophysica Acta, 1999, 1437(1): 1-12.
[11] Giuberti G, Gallo A, Masoero F, et al. Factors affecting starch utilization in large animal food production system: A review[J]. Starch, 2014, 66(1/2): 72-90.
[12] Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review[J]. Carbohydrate Polymers, 2001, 45(3): 253-267.
[13] Hirashima M, Takahashi R, Nishinari K. The gelatinization and retrogradation of cornstarch gels in the presence of citric acid[J]. Food Hydrocolloids, 2012, 27(2): 390-393.
[14] Guo Z B, Zeng S X, Zhang Y, et al. The effects of ultra-high pressure on the structural, rheological and retrogradation properties of lotus seed starch[J]. Food Hydrocolloids, 2015, 44(1): 285-291.
[15] Shi M M, Chen Y, Yu S J, et al. Preparation and properties of RS III from waxy maize starch with pullulanase[J]. Food Hydrocolloids, 2013, 33(1): 19-25.
[16] Lian X J, Guo J J, Wang D L, et al. Effects of protein in wheat flour on retrogradation of wheat starch[J]. Journal of Food Science, 2014, 79(8): 1505-1511.
[17] 郭俊杰,康海岐,孙海波,等. 抑制玉米淀粉回生的面粉蛋白酶解液筛选[J]. 农业工程学报,2014,30(12):265-269.
Guo Junjie, Kang Haiqi, Sun Haibo, et al.The influence of four kinds of hydrolysis of wheat protein by protease on retrogradation of corn starch[J]. Transactions of the Chinese Society for Agricultural Engineering (Transactions of the CSAE), 2014, 30(12): 265-269. (in Chinese with English abstract)
[18] Lian X J, Liu L Z, Guo J J, et al. Screening of seeds prepared from retrograded potato starch to increase retrogradation rate of maize starch[J]. International Journal of Biological Macromolecules, 2013, 60(9): 181-185.
[19] Guo Y B, Cai W R, Tu K, et al. Infrared and raman spectroscopic characterization of structural changes in albumin, globulin, glutelin, and prolamin during rice aging[J]. Journal of Agricultural Food Chemistry, 2013, 61(1/2): 185-192.
[20] 张雅媛,洪雁,顾正彪,等. 玉米淀粉与黄原胶复配体系流变和凝胶特性分析[J]. 农业工程学报,2011,27(9):357-362.
Zhang Yayuan, Hong Yan, Gu Zhengbiao, et al. Rheological and gel properties of corn starch-xanthan mixed systems[J]. Transactions of the Chinese Society for Agricultural Engineering (Transactions of the CSAE), 2011, 27(9): 357-362. (in Chinese with English abstract)
[21] 谢涛,李英,易翠平,等.回生抗性淀粉种类对米淀粉凝胶形成的影响[J].农业工程学报,2017,33(4):309-314.
Xie Tao, Li Ying, Yi Cuiping, et al. Effect of retrograded resistant starch types on forming rice starch gel[J]. Transactions of the Chinese Society for Agricultural Engineering (Transactions of the CSAE),2017, 33(4): 309-314. (in Chinese with English abstract)
[22] Lian X J, Zhang K S, Luo Q F et al. A possible structure of retrograded maize starch speculated by UV and IR spectra of it and its components[J].International Journal of Biological Macromolecules, 2012, 50(1): 119-124.
[23] Lian X J, Wang C J, Zhang K S, et al. The retrogradation properties of glutinous rice and buck wheat starches as observed with FT-IR, 13C NMR and DSC[J]. International Journal of Biological Macromolecules, 2014, 64(3): 288-293.
[24] Jane J L, Kasemsuwan, T Leas S, et al. Anthology of starch granule morphology by scanning electron microscopy[J]. Starch-Stärke, 1994, 46(4): 121-129.
[25] Banks W, Greenwood, Khan K M. The interaction of linear, amylase oligomers with iodine[J]. Carbohydrate Polymer, 1971, 17(1): 25-33.
[26] Chauhan F, Seetharaman K. On the organization of chains in amylopectin[J]. Starch, 2013, 64(3): 191-199.
[27] Knutson C A. Evaluation of variations in amylase-iodine absorbance spectra[J]. Carbohydrate Polymer, 1999, 42(1): 65-72.
[28] Flores-Morales A, Jiménez-Estrada M, Mora-Escobedo R. Determination of the structural changes by FT-IR, Raman, and CP/MAS13C NMR spectroscopy on retrograded starch of maize tortillas[J]. Carbohydrate Polymer, 2012, 87(1): 61-68.
[29] Holse M, Larsen F H, Hansen A, et al. Characterization of marama bean () by comparative spectroscopy: NMR, FT-Raman, FT-IR and NIR[J].Food Research International, 2011, 44(1): 373-384.
[30] Cheetham N W H, Tao L. Variation in crystalline type with content in maise starch granules: An X-ray powder diffraction study[J]. Carbohydrate Polymer, 1998, 36(4): 277-284.
[31] Gidley M J, Cooke D, Darke A H, et al. Molecular order and structure in enzyme-resistant retrograded starch[J]. Carbohydrate Polymer, 1995, 28(1): 23-31.
[32] Bail P Le, Rondeau C, Buléon A. Structural investigation of complexes with small ligands: Helical conformation, crystalline structure and thermostability[J]. International Journal of Biological Macromolecules, 2005, 35(1): 1-7.
[33] Hizukuri S, Kakelo T, Takeda Y. Measurement of the chain length of amylopectin and its relevance to the origin of crystalline polymorphism of starch granules[J]. Biochimica et Biophysica Acta, 1983, 760(1): 188-191.
Property analysis of resistant wheat amylose and amylopectin with wheat gliadin
Guo Junjie1, Ma Qiaozhi1, Kang Haiqi2, Lian Xijun1※
(1.300134; 2.610066)
Starch is the reserve carbohydrate in the plant kingdom. There is about 65 - 70% starch, 11 - 13% protein and some other components in wheat. Resistant starch (RS) has been investigated mainly with regard to colonic effects, glycemic index, cholesterol lowering capability, and losing weight effect. The daily intake of a certain amount of resistant starch is particularly important to human health. Retrogradation is the process of starch recrystallization which is one of the most important methods forthe preparation of RS. Gliadin, accounting for 40%-50% of wheat gluten, promotes the retrogradation of wheat starch while glutelin retards it. The objective of this research is to study the effects of external gliadin in gluten on deferent kinds of wheat starch after purified by lipaseand protease, which is a part of a large research program aimed at gaining an enhanced molecular understanding of the transformations occurring during the processing and storage of starch materials. Gliadin was isolated from wheat flour and its effect on retrogradation of wheat starch was investigated by visible absorbance (starch-iodine), IR, XRD, DSC respectively. The results showed that gliadin probably interacted with starch during the process of gelation and retrogradation, resulting in enhance of starch retrogradation. The IR spectra indicated that the addition of gliadin to wheat starch led to the reduction of hydrogen bonds between amylose. Addition of gliadin in crystal of retrograded wheat starch caused presence of two new lattice planes. The DSC results indicated that the hydrogen bond of amylose and gliadin was formed in the retrogradation progress. The polycrystal structure and the double helix reign were coexisting. The hydroxyl group of C-6 with less steric hindrance can form six-membered ring with carboxyl and acylaminogroup of prolines and glutamine by hydrogen bond respectively. Starch could combine with gliadin by hydrogen bond to form double helix during retrogradation, which resulted in the promotion of short term retrogradation of wheat starch. Gliadin and starch formed double helix in the interface of themselves that inhibit the enzymolysis of starch. This kind of hydrogen bonding might be an inhibitor for-amylase. The gliadin would not promote the retrogradation of starch anymore when all of the aminoacid formed hydrogen bond with starch. In a word, this study has provided some distinct insights into the understanding of the effects of gliadin on the retrogradation of wheat starch.
starch; agricultural products; quality control; gliadin; retrograded mechanism; characterization
2017-01-15
2018-01-31
国家自然科学基金项目(31571834);天津市应用基础与前沿技术研究计划项目(14JCYBJC30800);天津市自然科学基金企业科技特派员项目(17JCTPJC53800)资助
郭俊杰,博士,副教授,研究方向为食品科学。Email:gjjie@tjcu.edu.com
连喜军,博士,副教授,主要从事回生淀粉研究。Email:lianliu2002@163.com
10.11975/j.issn.1002-6819.2018.04.036
TS231
A
1002-6819(2018)-04-0293-06
郭俊杰,马乔治,康海岐,连喜军. 含醇溶蛋白小麦回生抗性直支链淀粉性质分析[J]. 农业工程学报,2018,34(4):293-298.doi:10.11975/j.issn.1002-6819.2018.04.036 http://www.tcsae.org
Guo Junjie, Ma Qiaozhi, Kang Haiqi, Lian Xijun. Property analysis of resistant wheat amylose and amylopectin with wheat gliadin[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(4): 293-298. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2018.04.036 http://www.tcsae.org
