溶菌酶触发的芽孢杆菌芽孢萌发及其异质性研究*
2016-06-27王桂文张鹏飞王晓春陈欢君PeterSetlowLiYongqing
王桂文,张鹏飞,王晓春,陈欢君,Peter Setlow,Li Yongqing
(1.广西科学院,广西南宁 530007;2.东卡罗来纳大学物理系,美国北卡罗来纳州格林维尔市,NC 27858;3.广西大学生命科学与技术学院,广西南宁 530004;4.康涅狄格大学健康中心分子、微生物与结构生物学系,美国康涅狄格州法明顿,CT 06030)
溶菌酶触发的芽孢杆菌芽孢萌发及其异质性研究*
王桂文1**,张鹏飞2,王晓春1,陈欢君3,Peter Setlow4,Li Yongqing2
(1.广西科学院,广西南宁530007;2.东卡罗来纳大学物理系,美国北卡罗来纳州格林维尔市,NC 27858;3.广西大学生命科学与技术学院,广西南宁530004;4.康涅狄格大学健康中心分子、微生物与结构生物学系,美国康涅狄格州法明顿,CT 06030)
摘要:【目的】了解溶菌酶触发芽孢杆菌孢子萌发的异质性及其机制,为认识芽孢(Spore)萌发机制和杀灭芽孢提供参考。【方法】应用拉曼光谱和微分干涉差(DIC)显微镜成像技术高通量分析大量单个Bacillus subtilis(Bs)和B.megaterium(Bm)芽孢经溶菌酶触发的萌发动态。【结果】溶菌酶浓度和温度越高,芽孢萌发越快,孢内CaDPA开始快速释放时间(Tlag)、快速释放所需时间(ΔTrelease)和芽孢皮层水解所需时间(ΔTlys)越短;低于20℃,Bs芽孢萌发的ΔTrelease值是25℃时的4倍以上。SpoVA蛋白高表达菌株的ΔTrelease值和普通菌株基本相同,而缺少皮层水解酶的菌株ΔTrelease值高于普通菌株。95℃处理15 min的孢子,其Tlag、ΔTrelease和ΔTlys值是对照的2倍以上。Bs芽孢萌发的异质性明显,不仅表现在芽孢间,也表现在菌株间。Bm芽孢对溶菌酶更敏感,芽孢间的异质性显著低于Bs。【结论】溶菌酶触发的芽孢萌发在物种、菌株和单细胞层面都存在显著的异质性;溶菌酶浓度和温度对芽孢萌发有重大影响;皮层水解酶也可能参与溶菌酶触发的芽孢萌发进程。
关键词:拉曼光谱微分干涉差成像芽孢杆菌芽孢萌发溶菌酶单细胞分析
0引言
【研究意义】部分杆菌属细菌会通过在细胞内形成休眠体芽孢(Spore)的方式来应对环境胁迫。由于富含2,6-吡啶二羧酸(Dipicolinic acid,DPA)与钙离子(Ca2+)的络合物CaDPA,且含水量极低,芽孢对逆境有极强的抵抗能力[1-3]。芽孢的休眠期可以很长,但对外界环境却极其敏感,只要一感受到适宜的条件就开始萌发,恢复到营养细胞状态。而一旦萌发,芽孢就丧失原有的抵抗力,这是杀灭芽孢类细菌的好时机[4]。因此,了解芽孢萌发机理和规律,对抑制其致病性、维护人类健康及生命安全具有极其重要的理论意义与应用价值。【前人研究进展】已知的芽孢萌发机制是营养性萌发剂(Germinants)与芽孢内膜上的受体蛋白结合,触发芽孢启动系列萌发事件[5-6]。除营养性萌发剂以外,溶菌酶、盐类、高压、CaDPA和阳离子表面活性剂(如月桂胺Dodecylamine)等其它非营养性化学试剂或物理因素,也可以触发芽孢萌发[6-8]。其中,溶菌酶触发的芽孢萌发是比较特殊的非营养萌发方式。已有研究显示,溶菌酶通过水解芽孢的肽聚糖皮层引起孢内CaDPA释放进而完成芽孢萌发[6]。但芽孢衣对芽孢有保护作用,溶菌酶无法越过这道屏障水解芽孢皮层,因而对溶菌酶触发的芽孢萌发研究较少,所研究的对象主要是食品工业上的污染菌[9-11],对芽孢杆菌属芽孢的研究极少[12]。芽孢的萌发机制已有大量报道[5-6,13-14],创新的技术方法在该领域的应用加速其研究进程,特别是近年来拉曼光谱、微分干涉差(DIC)显微术、荧光成像和拉曼成像等技术并结合单细胞分析在芽孢萌发研究中的应用与发展,对认识芽孢萌发机理及其异质性发挥极其重要的作用[15],并大大地拓展了研究对象[16-18]。另外,细菌芽孢经营养萌发剂触发的萌发存在显著的异质性,即在相同的萌发条件下,有的芽孢萌发极快,有的芽孢萌发则可能需要几小时甚至几天时间[14,16-18]。而单细胞分析显示,芽孢间的萌发差异主要体现在芽孢接受外源萌发剂刺激到孢内DPA开始释放的时间(Tlag),而胞内DPA大量释放阶段则具有较强的同质性[19]。【本研究切入点】对芽孢萌发的异质性研究已经有不少报道[7,19-21],但是对溶菌酶触发的芽孢萌发异质性研究还未见报道,因此本文从该方面入手研究芽孢萌发的异质性。【拟解决的关键问题】应用拉曼光谱与DIC成像技术,从单细胞水平上分析溶菌酶触发的芽孢萌发方式,了解细菌芽孢的非营养萌发规律、机制及其异质性。
1材料与方法
1.1菌株
枯草芽孢杆菌(Bacillus subtilis,Bs),巨大芽孢杆菌(B.megaterium,Bm),相关菌株信息见表1。
1.2芽孢制备
Bm芽孢的培养方法[22]:菌株接入LB固体培养基,30℃条件下活化24~36 h。挑取单菌落,转接到LB液体培养基,200 r/min,过夜培养;按1%(V/V) 的比例转入产孢培养基(CCY),30℃,200 r/min培养48~60 h,镜检芽孢率达到99%后收集孢子。
Bs芽孢的培养方法:活化的菌株涂板在无抗生素的2×SG培养基琼脂平板,37℃条件下培养2~3 d,转到23℃下再培养数天[23]。用无菌水洗取菌苔,用于收集孢子。
芽孢收集:5 000 r/min离心收集、纯化芽孢,无菌水洗涤10次,4℃避光保存备用。
芽孢采用脱衣液70℃处理30 min去掉芽孢衣,无菌水洗10次。脱衣液组成为0.1 mol/L NaOH,0.1 mol/L NaCl,0.1 mol/L二硫苏糖醇,1%(W/V)十二烷基硫酸钠[29]。
表1实验所用菌株及其性质
Table 1Strains used and their properties
1.3芽孢萌发
芽孢基本萌发温度为25℃,在不同温度试验中选择16℃、20℃和25℃。将待分析的芽孢用无菌水稀释成每毫升芽孢液约含108个芽孢,吸取2 μL滴加在盖玻片上,真空干燥,将盖玻片固定在自制的恒温平台上。滴加预先恒温到实验所需温度的溶菌酶溶液。溶菌酶溶液浓度在不同浓度试验中选择1.00 μg/mL,2.50 μg/mL,3.75 μg/mL,5.00 μg/mL以及10.00 μg/mL。
1.4芽孢萌发监测
1.4.1拉曼光谱监测
应用单细胞激光拉曼光谱系统聚焦在单个芽孢上采集拉曼光谱[21]。激光功率为5 mW,连续采集拉曼光谱直到芽孢萌发后15~30 min,积分时间为15 s。
1.4.2微分干涉差(DIC)显微术监测
采用与文献[21]类似的DIC显微术监测大量单个芽孢的萌发动态,具体实验系统参见文献[30]。芽孢的核心是一个CaDPA的巨型仓库并且水的含量非常少,折射率高,DIC显微镜将其转换成一个振幅图像,可通过CCD将其记录下来。在萌发过程中,芽孢内部主要的大分子物质CaDPA大量释放并与周围水分子进行置换,使得折射率发生改变[31]。
将倒置DIC显微镜加以改造,并接入高性能CCD,实现同步输出芽孢萌发监测过程的拉曼光谱和DIC图像。通过自编VC程序,根据实验需要,每6 s采集一幅DIC图像。图像处理:用Matlab软件编写程序,识别芽孢图像,以单个芽孢为中心,读取芽孢范围内20个象素位点的灰度值,作为芽孢图像信号积分,依时间函数绘制成芽孢萌发过程的实时DIC信号强度变化曲线。通过统计不同时间段的芽孢萌发数来计算芽孢的群体萌发率。
芽孢的萌发监测时间一般为60~90 min,部分芽孢的萌发监测时间根据萌发进展有所调整。本文相关数据分析和讨论均限于实验监测的时间范围内。
本实验设定芽孢与溶菌酶开始接触的时间为0,孢内CaDPA开始快速释放的时间为迟滞时间Tlag,CaDPA释放完毕的时间为Trelease,芽孢皮层完全溶解的时间为Tlys。定义CaDPA释放需要的时间为△Trelease=Trelease-Tlag、皮层溶解所需时间为△Tlys=Tlys-Trelease。其中Tlag值反映的是芽孢启动萌发的速度,△Trelease和△Tlys值分别反映CaDPA释放和皮层溶解的快慢,是反映单个芽孢萌发进程的主要参数。参数Ilag和Irelease分别是Tlag和Trelease时芽孢的DIC亮度值(以0 min时的亮度为1,芽孢萌发后实验记录终点的亮度为0),前者间接反映Tlag时芽孢的CaDPA含量,后者间接反映Tlys时芽孢皮层的水解程度和芽孢的吸水程度。
2结果与分析
2.1溶菌酶浓度对芽孢萌发的影响
在较低的溶菌酶浓度下,芽孢需要经过一定的时间才开始萌发。溶菌酶浓度越高,芽孢萌发的迟滞期越短。在1.00 μg/mL浓度下,90 min观察期内仅有约7%的芽孢萌发,而在10.00 μg/mL浓度时,98%以上的芽孢在10 min内萌发(图1a)。读取单个芽孢的DIC亮度,以时间为函数,建立单个芽孢的萌发动态曲线(图1b、c),结果显示,芽孢的亮度在芽孢与溶菌酶接触后缓慢降低,紧接着是快速降低;溶菌酶浓度越高,芽孢的亮度变化越快。
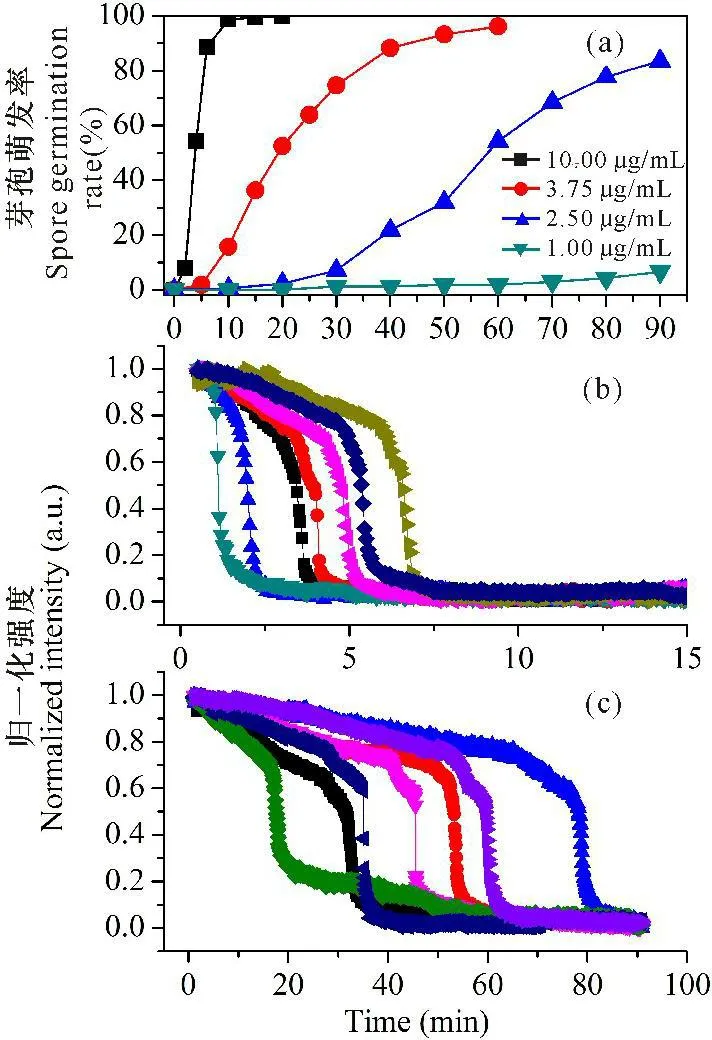
溶菌酶浓度Concentrations of lysozyme:(b)10.00 μg/mL;(c)2.50 μg/mL
图1Bs芽孢在不同溶菌酶浓度下的群体萌发曲线(a)及7个不同芽孢的萌发动态(b,c)
Fig.1Germination of wild-type B.subtilis spores triggered by 1.00 μg/mL,2.50 μg/mL,3.75 μg/mL and 10.00 μg/mL lysozyme (a) and germination kinetics of 7 individual spores(b,c)
应用拉曼光谱对芽孢的溶菌酶萌发过程进行验证发现,与营养萌发类似[24],溶菌酶触发的萌发动态是典型的三相态,DIC图像亮度变化与孢内CaDPA含量变化一致(图2),所得结果与文献[21,24,30]类似。因此DIC成像分析方法适用于实时监测溶菌酶触发的芽孢萌发动态。
图2a是5个芽孢的CaDPA拉曼光谱特征峰1 017 cm-1的信号强度(反映的是芽孢内CaDPA的含量)变化动态曲线,图2b是DIC成像同时记录的同一芽孢的亮度变化。结果显示,溶菌酶萌发过程单个芽孢的DIC图像亮度与孢内CaDPA含量变化对应,芽孢的亮度急速降低的过程就是孢内CaDPA快速释放的过程,孢内CaDPA完全释放,1 017 cm-1峰则完全消失;之后DIC图像亮度随着芽孢皮层溶解缓慢下降,并逐渐保持基本不变。
萌发参数(表2)显示,溶菌酶浓度越低,芽孢萌发越慢,孢内CaDPA的快速释放时间(ΔTrelease值)和芽孢皮层水解的时间(ΔTlys值)越长。2.50 μg/mL时的Tlag值分别是3.75 μg/mL、10.00 μg/mL的4倍和20倍左右,ΔTrelease值和ΔTlys值分别是后两者的2.50倍和1.5~3.0倍左右。当溶菌酶浓度达到1 000 μg/mL时,芽孢几乎是接触到溶菌酶溶液就开始水解皮层,并快速释放CaDPA,而且几乎没有观察到皮层水解的过程(Tlys)。参数Ilag显示,溶菌酶浓度越低,在Tlag前孢内已经缓慢释放的CaDPA越多。上述结果说明高浓度溶菌酶触发芽孢快速释放孢内CaDPA并水解芽孢皮层,但当溶菌酶的浓度高于3.75 μg/mL时,ΔTrelease值基本相同。

图2拉曼光谱(a)与DIC成像(b)实时同步监测同一芽孢的萌发过程
Fig.2Simultaneous monitoring of the lysozyme-triggered germination of individual Bacillus spores followed by Raman spectroscopy (a) and DIC microscopy imaging (b)
2.2温度对芽孢萌发的影响
如图3所示,在5.00 μg/mL溶菌酶溶液作用下,温度降低,PS832芽孢的萌发速度明显降低;芽孢在25℃、20℃和16℃下,90 min内的萌发率依次是99.9%、96.2%和86.7%。萌发参数(表3)显示,温度主要影响Tlag、Trelease和Tlys值;相应地,DPA完全释放和皮层水解结束所需的时间随温度降低而升高,特别是低于20℃(包括20℃)后,ΔTrelease值是25℃的4倍以上。
2.3湿热处理对芽孢萌发的影响
PS832芽孢经90℃水浴处理15 min后,40 min内的萌发率与对照相当(图4,5.00 μg/mL溶菌酶),但芽孢萌发明显推迟,其Tlag值约为对照的2倍,孢内CaDPA快速释放所需的时间(ΔTrelease)和皮层水解的时间(ΔTlys)是对照的2.5倍左右(表4)。CaDPA开始快速释放时芽孢的DIC亮度(Ilag)低于对照,而CaDPA完全释放时的亮度(Irelease)则高于对照。Bm孢子在85℃水浴处理15 min,所得结果类似(数据没有给出)。
表225℃时不同溶菌酶浓度下芽孢杆菌孢子的萌发参数
Table 2Mean values and standard deviations of germination parameters of decoated Bacillus spores triggered by various levels of lysozyme at 25℃

物种Species菌株Strains溶菌酶浓度Lysozymeconcentration(μg/mL)Tlag(min)Trelease(min)Tlys(min)ΔTrelease(min)ΔTlys(min)Ilag(%)Irelease(%)萌发率*Germinationrate(%)BsPS8321.00///////7.0(90min)2.5047.2±17.749.2±17.252.5±18.12.1±2.13.3±2.366.2±10.722.9±6.183.6(90min)3.7512.1±4.812.8±4.715.1±5.50.8±0.92.3±1.573.2±10.120.9±7.496.2(60min)10.002.5±1.43.1±1.53.8±1.80.8±0.70.8±0.680.7±9.118.3±10.799.9(20min)1000.000.9±1.01.6±1.2/0.6±0.2/87.1±10.66.2±9.099.9(15min)FB1103.7544.4±10.049.2±11.056.5±11.24.8±2.17.3±3.367.8±6.327.8±6.781.2(90min)FB1113.7537.9±18.941.2±17.944.6±18.13.3±2.93.4±1.660.8±12.917.1±6.569.8(90min)FB1133.7520.7±11.424.1±12.128.5±12.93.3±1.54.4±4.669.9±9.120.1±6.391.1(90min)PS34113.756.2±2.57.4±2.59.4±3.11.2±0.72.0±1.066.0±10.615.9±4.399.8(35min)PS36403.7523.0±11.524.2±11.426.5±11.61.2±0.82.3±1.058.6±10.420.4±5.591.2(60min)BmQMB15511.0027.5±3.728.4±3.931.6±3.50.9±0.83.2±1.974.5±7.524.5±8.699.9(50min)5.0011.4±3.712.2±3.614.7±3.90.8±0.73.3±1.877.4±10.126.2±8.7100.0(50min)100.001.5±0.71.8±0.82.2±0.90.3±0.10.4±0.491.5±4.911.5±8.8100.0(10min)
注:*括号内为观察时间
Note:*the observation periods were listed in brackets

图3Bs芽孢在不同温度下的群体萌发曲线(a)及8个不同芽孢的萌发动态(b,c)
Fig.3Germination of B.subtilis spores triggered by 5.00 μg/mL lysozyme at various temperatures(a) and the germination kinetics of 8 individual spores under 20℃(b) or 16℃(c)
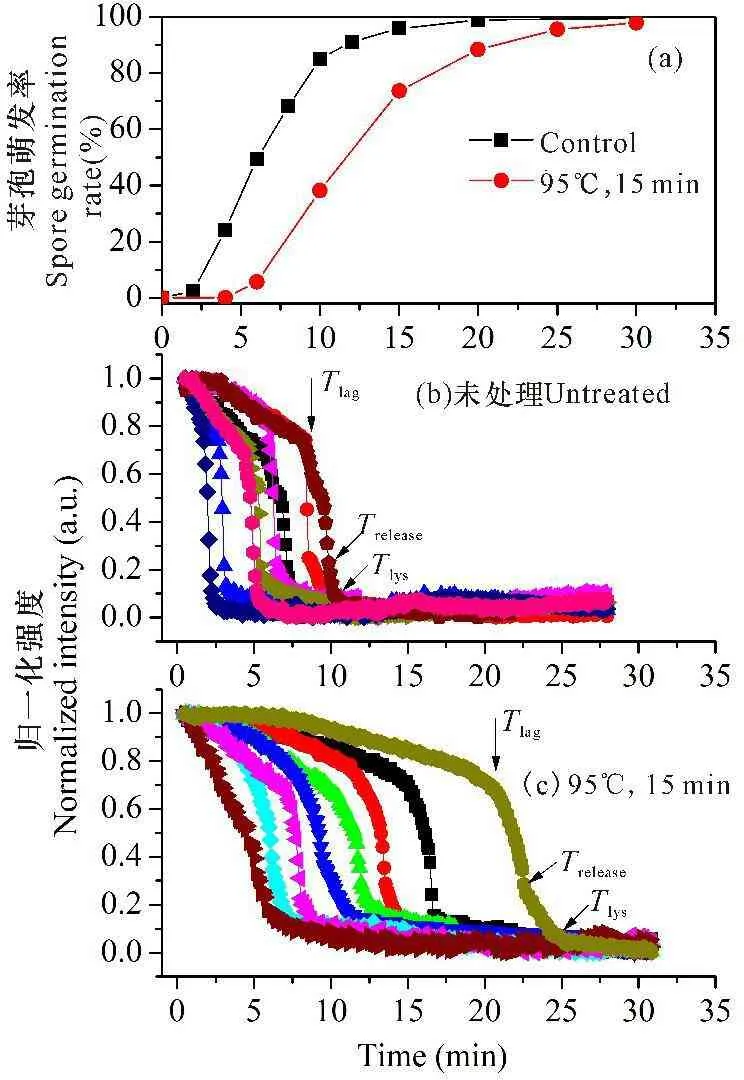
图4湿热处理后芽孢的群体萌发曲线(a)及8个不同芽孢的萌发动态(b,c)
Fig.4Spore germination of wet-heated spores with triggering of 5.00 μg/mL lysozyme (a) and the germination kinetic of 8 individual spores untreated(b) or treated at 95℃ for 15 min(c)
表3PS832芽孢在不同温度下的萌发参数
Table 3Mean values and standard deviations of germination parameters of B.sutilis PS832 spores at various temperatures

温度TemperatureTlag(min)Trelease(min)Tlys(min)ΔTrelease(min)ΔTlys(min)Ilag(%)Irelease(%)萌发率Germinationrate(%)25℃5.1±2.95.8±2.97.0±3.20.7±0.71.1±0.771.5±10.412.4±4.499.920℃12.6±6.315.5±7.017.5±7.22.9±1.62.0±1.276.6±8.912.6±4.196.216℃26.9±10.730.1±10.932.1±10.83.2±0.81.9±2.363.4±11.411.2±6.286.7
注:5.00 μg/mL溶菌酶,观察时间为60 min
Note:The germination was triggered by 5.00 μg/mL lysozyme and followed in 60 min
表4经湿热处理后的PS832芽孢萌发参数
Table 4Mean values and standard deviations of germination parameters of wet-heated B.sutilis PS832 spores

处理TreatmentTlag(min)Trelease(min)Tlys(min)ΔTrelease(min)ΔTlys(min)Ilag(%)Irelease(%)萌发率Germinationrate(%)Control5.0±2.35.8±2.96.3±2.90.8±0.70.5±0.678.0±11.88.6±7.099.495℃,15min9.9±4.711.9±4.613.1±4.72.0±0.81.3±0.972.5±9.320.8±7.397.7
注:萌发条件为5.00 μg/mL溶菌酶,25℃
Note:Germinations were triggered by 5.00 μg/mL lysozyme at 25℃
2.4不同菌株的萌发动态
SpoVA蛋白是处于芽孢内膜上控制CaDPA吸收与释放的关键蛋白,PS3411、PS3640是对SpoVA操控子进行改造的菌株[25-26]。在90 min的观察期内(图5,3.75 μg/mL溶菌酶),SpoVA蛋白高表达(4倍)的菌株PS3411的萌发很快,同样是spoVAC遗传改造菌株,PS3640菌株萌发也较快。但除萌发启动时间不同外,PS3411、PS3640菌株的萌发参数基本相同(表2)。
皮层水解酶CwlJ和SleB是参与芽孢萌发进程的两个重要酶,经遗传改造后的FB110菌株芽孢衣上无SleB酶,FB111菌株缺少CwlJ酶,FB113菌株则两种酶都缺乏。图5a显示,在同样的溶菌酶浓度下,FB113菌株萌发较快,FB110和FB111菌株的萌发过程基本相同。萌发参数(表2)显示,皮层水解酶基因改造的FB系列菌株的ΔTrelease和ΔTrelease值明显

图5不同的Bs芽孢菌株(a)和不同物种的芽孢萌发动态(b~d)
Fig.5Spore germination of various B.subtilis strains (a) and germination kinetics of individual spores triggered by 3.75 μg/mL lysozyme (b~d)
高于spoVA改造的PS3411、PS3640菌株和野生型菌株,特别是FB110菌株,其ΔTrelease、ΔTlys值分别是spoVA改造菌株和野生型菌株的4倍和3倍以上。Ilag值显示,PS3640菌株在Tlag前已经缓慢释放了较大量的CaDPA。
2.5巨大芽孢杆菌(Bm)芽孢的萌发动态
测试Bm芽孢在不同溶菌酶浓度下的萌发动态(图6),发现Bm芽孢对溶菌酶的敏感程度比Bs高,即使是仅1.00 μg/mL,95%以上的芽孢也在20 min内萌发。和Bs芽孢一样,溶菌酶浓度越低,Bm芽孢萌发越慢,ΔTrelease值和ΔTlys值越高。不同于Bs芽孢的是,Bm芽孢一旦开始萌发,90%以上的Bm芽孢在20 min内完成萌发,其Tlag、Trelease和Tlys值的标准差(SD值)比Bs芽孢小得多(表2)。除Tlag、Trelease和Tlys值外,Bm芽孢在1.00 μg/mL、5.00 μg/mL溶菌酶下的其他萌发参数基本相同。当溶菌酶浓度达到100.00 μg/mL 时,芽孢几乎是接触到溶菌酶溶液就开始水解皮层,并快速释放孢内的CaDPA。

图6Bm芽孢在不同溶菌酶浓度下的群体萌发曲线(a)及5.00 μg/mL溶菌酶下8个不同芽孢的萌发动态(b)
Fig.6Germination of wild-type B.megaterium QM B1551 spores triggered by various concentrations of lysozyme (a) and the kinetics of 8 individual spores under 5.00 μg/mL of lysozyme (b)
3讨论
溶菌酶触发的细菌芽孢萌发是一条比较特殊的萌发途径。与外源CaDPA触发的萌发类似,溶菌酶通过水解芽孢皮层的肽聚糖引起孢内CaDPA的释放过程,只不过前者是外源CaDPA激活芽孢衣上的皮层水解酶进而触发芽孢萌发,而溶菌酶是直接水解芽孢皮层触发芽孢萌发[6]。虽然芽孢杆菌属细菌的溶菌酶萌发不是主要的芽孢萌发方式[14],但对该方式的萌发规律和萌发机制进行研究有助于认识芽孢的抗性机制与萌发机制。
实验结果显示,在同样条件下,溶菌酶浓度和溶液温度是影响芽孢萌发的关键因素。浓度和温度越高,芽孢萌发越快,孢内CaDPA释放的时间和芽孢皮层水解的时间越短,这是因为较高的浓度和温度使得溶菌酶能更快地水解芽孢皮层。在高达100.00~1 000.00 μg/mL的浓度下,溶菌酶不仅会水解皮层,还会快速水解芽孢壁,引起芽孢内膜破裂,造成孢内物质直接泄漏,这可能是细菌芽孢在高浓度的溶菌酶下极速释放孢内CaDPA的原因(表2)。
虽然溶菌酶能通过直接水解芽孢皮层触发细菌芽孢萌发,但本研究结果显示,芽孢的皮层水解酶也可能参与溶菌酶触发的芽孢萌发进程:(1)缺少皮层水解酶的FB系列菌株,其芽孢萌发的速度较慢,Tlag值高于其它菌株(图5a、表1~2),显示皮层水解酶可能促进溶菌酶触发的芽孢萌发,与其它萌发途径类似[13]。(2)ΔTlys值随着溶菌酶浓度的升高而降低,推测在较低的溶菌酶浓度时皮层水解酶也参与CaDPA释放后的芽孢皮层水解。(3)FB系列菌株的孢内CaDPA快速释放的时间和皮层水解的时间比其他菌株高;图5c显示,在Tlag后、CaDPA极速释放前,是孢内CaDPA慢速释放的过程,FB菌株的这个过程比较长,因而ΔTrelease值高于其他菌株,也显示芽孢的皮层水解酶参与了溶菌酶触发的芽孢萌发进程。对于SpoVA蛋白高表达(4倍)的PS3411菌株,其ΔTrelease值和野生型菌株类似,高表达的SpoVA蛋白并没有加快孢内DPA的释放,与表面活性剂十二胺触发的萌发不同[12]。
孢内丰富的CaDPA对孢内蛋白质和核酸起到较好的保护作用,但湿热处理仍会损伤芽孢内膜和皮层上与萌发相关的关键蛋白质和酶[24]。本文结果显示,95℃水浴处理15 min并不影响溶菌酶触发的整体萌发,但萌发启动推迟,孢内CaDPA释放所需的时间和皮层水解的时间均比对照高出1倍多,Tlag时的芽孢亮度(Ilag值)低于对照,说明CaDPA释放通道结构可能受损导致Tlag前已经有较多的CaDPA缓慢释放。这结果与营养萌发基本相同[24],从另一个角度证明湿热处理确实破坏CaDPA释放通道的蛋白质结构。
溶菌酶触发的细菌芽孢萌发有着显著的异质性。在物种水平上,Bm芽孢比Bs芽孢对溶菌酶更敏感,在1.00 μg/mL浓度下,99%的Bm芽孢在40 min内已经萌发,而Bs芽孢仅有极少数芽孢启动萌发(图1a)。而且,Bm物种的芽孢间异质性显著低于Bs物种的,不管溶菌酶的浓度大小,90%以上的Bm芽孢均在20 min的时间范围内完成萌发,因此其萌发参数的标准差较小(表2),说明芽孢间的差异较小。Bs芽孢萌发的异质性较大,不仅表现在芽孢间,也表现在菌株间。同样是SpoVA操控子改造菌株,在3.75 μg/mL浓度下,PS3411菌株的芽孢萌发速度比野生型菌株快,PS3640菌株萌发也较快。而缺少皮层水解酶的菌株,除FB113菌株萌发较快外,FB110和FB111菌株萌发较慢。上述结果显示菌株间的差异与其遗传背景没有必然的联系。
芽孢间的萌发异质性是芽孢萌发研究的兴趣点。已有研究表明,对于超级休眠芽孢,在营养萌发中即使是给予极好的萌发条件,启动萌发的时间可能需要数小时,也可能需要数天[32]。表2的数据显示,不同萌发条件、不同菌株和不同物种下,芽孢间萌发的异质性明显,主要表现在两个数据上:一是Tlag值,较高的Tlag值往往伴随着较高的标准差(SD值),也就是说越不容易萌发的芽孢群体,其芽孢间的萌发异质性越大,这可能与芽孢内部的信号传导差异有关;二是ΔTrelease值,观察图1~6中单个孢子的萌发动态,可以发现在孢内CaDPA释放的Tlag~Trelease期间,有一个CaDPA较慢释放和一个CaDPA极速释放过程,在不同的萌发条件下,极速释放的时间基本相同,约为0.3~0.5 min(统计数据表中没有给出),因而影响ΔTrelease值大小的主要因子就是孢子启动CaDPA释放后的较慢释放过程,结合前面的分析,可知这个过程与溶菌酶浓度、温度和菌株的皮层水解酶有关,与SpoVA蛋白没有关系。
4结论
本研究在群体水平和单个芽孢水平上,从温度、浓度、菌株、物种以及受损芽孢等多方面分析溶菌酶触发的芽孢杆菌的芽孢萌发动态。结果显示,芽孢需要一定浓度的溶菌酶或者适当的处理时间来触发,单个芽孢的动态与营养萌发剂触发的萌发基本相似;在同样条件下,溶菌酶浓度和温度是影响芽孢萌发的关键因素;溶菌酶直接水解芽孢皮层而触发芽孢萌发,但皮层水解酶也可能参与了溶菌酶触发的芽孢萌发进程。溶菌酶触发的细菌芽孢萌发在物种、菌株乃至单细胞水平都存在明显的异质性。研究结果同时也表明单细胞分析可以应用于芽孢萌发机制及其异质性。
参考文献:
[1]NICHOLSON W L,MUNAKATA N,HORNECK G,et al.Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments[J].Microbiology and Molecular Biology Reviews,2000,64(3):548-572.
[2]SETLOW P,JOHNSON E A.Spores and their significance[M]//DOYLE M P,BUCHANAN R(eds.).Food Microbiology,Fundamentals and Frontiers.4th ed.Washington,D C:ASM Press,2012:45-79.
[3]SETLOW P.Spore resistance properties [J].Microbiology Spectrum,2014,2(5):TBS-0003-2012.
[4]SETLOW P.Spores of Bacillus subtilis:Their resistan-
ce to and killing by radiation,heat and chemicals[J].Journal of Applied Microbiology,2006,101(3):514-525.
[5]MOIR A.How do spores germinate? [J].Journal of Applied Microbiology,2006,101(3):526-530.
[6]SETLOW P.Spore germination [J].Current Opinion of Microbiology,2003,6(6):550-556.
[7]KONG L,DOONA C J,SETLOW P,et al.Monitoring rates and heterogeneity of high-pressure germination of Bacillus spores by phase-contrast microscopy of individual spores[J].Applied and Environmental Microbiology,2014,80(1):345-353.
[8]SETLOW B,COWAN A E,SETLOW P.Germination of spores of Bacillus subtilis with dodecylamine [J].Journal of Applied Microbiology,2003,95(3):637-648.
[9]CASSIER M,SEBALD M.Lysozyme-dependent germination of spores of Clostridium perfringens ATCC 3624 after heat treatment[J].Annales de l’lnstitut Pasteur,1969,117(3):312-324.
[10]DUNCAN C L,LABBE R G,REICH R R.Germination of heat- and alkali-altered spores of Clostridium perfringens type A by lysozyme and an initiation protein[J].Journal of Bacteriology,1972,109(2):550-559.
[11]ADAMS D M.Sensitization by ethylenediaminetetraacetate of Clostridium perfringens type A spores to germination by lysozyme[J].Journal of Bacteriology,1973,116(1):500-502.
[12]VEPACHEDU V R,SETLOW P.Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme[J].Journal of Bacteriology,2007,189(5):1565-1572.
[13]SETLOW P.Summer meeting 2013-when the sleepers wake:The germination of spores of Bacillus species [J].Journal of Applied Microbiology,2013,115(6):1251-1268.
[14]SETLOW P.Germination of spores of Bacillus species:What we know and do not know[J].Journal of Bacteriology,2014,196(7):1297-1305.
[15]陈越,王璐玮,陶站华,等.光学技术的创新应用:细菌芽孢萌发机理研究[J].激光与光电子学进展,2015,52(10):100002.
CHEN Y,WANG L W,TAO Z H,et al.Novel application of optical techniques:Revealing the mechanism of germination of bacterial spores[J].Laser & Optoelectronics Progress,2015,52(10):100002.
[16]WANG G,ZHANG P,PAREDES-SABJA D,et al.Analysis of the germination of individual Clostridium perfringens spores and its heterogeneity[J].Journal of Applied Microbiology,2011,111(5):1212-1223.
[17]ZHOU T,DONG Z,SETLOW P,et al.Kinetics of germination of individual spores of Geobacillus stearothermophilus as measured by Raman spectroscopy and differential interference contrast microscopy[J].PloS One,2013,8(9):e74987.
[18]WANG S,SHEN A,SETLOW P,et al.Characterizati-
on of the dynamic germination of individual Clostridium difficile spores using Raman spectroscopy and differential interference contrast microscopy[J].Journal of Bacteriology,2015,197(14):2361-2373.
[19]CHEN D,HUANG S S,LI Y Q.Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy[J].Analytical Chemistry,2006,78(19):6936-6941.
[20]INDEST K J,BUCHHOLZ W G,FAEDER J R,et al.Workshop report:Modeling the molecular mechanism of bacterial spore germination and elucidating reasons for germination heterogeneity [J].Journal of Food Science,2009,74(6):R73-R78.
[21]ZHANG P,KONG L,WANG G,et al.Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores [J].Journal of Biomedical Optics,2010,15(5):056010.
[22]STEWART G S,JOHNSTONE K,HAGELBERG E,et al.Commitment of bacterial spores to germinate.A measure of the trigger reaction [J].The Biochemical Journal,1981,198(1):101-106.
[23]NICHOLSON W L,SETLOW P.Sporulation,germination and outgrowth[M]//HARWOOD C R,CUTTING S M(eds.).Molecular Biological Methods for Bacillus.Chichester.England:John Wiley and Sons,1990:391-450.
[24]WANG G,ZHANG P,SETLOW P,et al.Kinetics of germination of wet-heat-treated individual spores of Bacillus species,monitored by Raman spectroscopy and differential interference contrast microscopy [J].Applied and Environmental Microbiology,2011,77(10):3368-3379.
[25]VEPACHEDU V R,SETLOW P.Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis [J].Journal of Bacteriology,2005,187(16):5677-5682.
[26]VEPACHEDU V R,SETLOW P.Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon [J].FEMS Microbiology Letters,2004,239(1):71-77.
[27]PAIDHUNGAT M,RAGKOUSI K,SETLOW P.Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate[J].Journal of Bacteriology,2001,183(16):4886-4893.
[28]KEYNAN A,HALVORSON H O.Calcium dipicolinic acid-induced germination of Bacillus cereus spores [J].Journal of Bacteriology,1962,83:100-105.
[29]VARY J C.Germination of Bacillus megaterium spor-
es after various extraction procedures[J].Journal of Bacteriology,1973,116(2):797-802.
[30]陈越,彭立新,王晓春,等.微分干涉差显微成像方法高通量分析单个苏金云芽孢杆菌HD-1芽孢萌发[J].分析化学,2015,43(12):1787-1794.
CHEN Y,PENG L X,WANG X C,et al.High-throughput investigation of germination of individual Bacillus thuringiensis HD-1 spores by differential interference contrast microscopy imaging[J].Chinese Journal of Analytical Chemistry,2015,43(12):1787-1793.
[31]ZHANG P,KONG L,WANG G,et al.Monitoring the wet-heat inactivation dynamics of single spores of Bacillus species by using Raman tweezers,differential interference contrast microscopy,and nucleic acid dye fluorescence microscopy[J].Applied and Environmental Microbiology,2011,77(14):4754-4769.
[32]ZHANG P,KONG L,WANG G,et al.Analysis of the slow germination of multiple individual superdormant Bacillus subtilis spores using multifocus Raman microspectroscopy and differential interference contrast microscopy [J].Journal of Applied Microbiology,2012,112(3):526-536.
(责任编辑:米慧芝)
Kinetics of Germination of Individual Bacillus Spores and its Heterogeneity Triggered by Lysozyme
WANG Guiwen1,ZHANG Pengfei2,WANG Xiaochun1,CHEN Huanjun3,Peter Setlow4,LI Yongqing2
(1.Guangxi Academy of Sciences,Nanning,Guangxi,530007,China;2.Department of Physics,East Carolina University,Greenville,NC 27858,USA;3.College of Life Sciences and Technology,Guangxi University,Nanning,Guangxi,530004,China;4.Department of Molecular,Microbial and Structural Biology,University of Connecticut Health Center,Farmington,CT 06030,USA)
Abstract:【Objective】The heterogeneous germination and its mechanism of Baillus spores triggered by lysozyme were studied in order to look insight into the mechanism of spore germination in general and provide new knowledge of inactivating bacterial spores.【Methods】The kinetic of germinations of multiple individual decoated spores of Bacillus subtilis (Bs) and B.megaterium (Bm) triggered by lysozyme were followed by Raman spectroscopy and differentail interference contrast (DIC) microscopy.【Results】Higher concentrations of lysozyme and temperatures significantly speeded the germination of Bs decaoted spores and reduced the time Tlag,at which spores began release of the great majority of spores’ 1∶1 chelate of Ca2+with dipicolinic acid (CaDPA),the period time of completing the release of CaDPA (named ΔTrelease) and the time period of hydrolysis of the spore’s peptidoglycan cortex (named ΔTlys).The ΔTreleasevalues of germination below 20℃ were 4 times more than that at 25℃.The ΔTreleasevalues of Bs spores with 4-fold-elevated level of SpoVA protein were basically similar to control strains,while spores lacking cortex-lytic enzymes exhibited notably higher ΔTreleasevalues than wild-type spores.The values of Tlag,ΔTreleaseand ΔTlysof spores treated by wet heat at 95℃ for 15 min were two times more than the untreated spores.Heterogeneous germinations were observed not only among individual decoated Bs spores,but also among Bs strains.The decoated Bm spores were more sensitive to lysozyme and its germinations of individual spores were less heterogeneous.【Conclusion】The germinations of decoated Bacillus spores triggered by lysozyme,on which the lysozyme concentration and temperature have a significant effect,are significantly heterogeneous at the levels of single spores and strains,as well as of species.The cortex-lytic enzymes may be involved in the initiation of germination.
Key words:Raman spectroscopy,DIC imaging,Baillus,spore germination,lysozyme,single-cell analysis
收稿日期:2016-02-19
作者简介:王桂文(1969-),男,研究员,主要从事生物物理与应用微生物研究。
中图分类号:Q935,Q937
文献标识码:A
文章编号:1005-9164(2016)02-0102-09
修回日期:2016-04-01
*国家自然科学基金项目(11264004,31460035)资助。
**通讯作者:E-mail:wguiwen@gxas.cn。
广西科学Guangxi Sciences 2016,23(2):102~110
网络优先数字出版时间:2016-05-12
网络优先数字出版地址:http://www.cnki.net/kcms/detail/45.1206.G3.20160512.0944.016.html
