离子液体1-乙基-3-丁基磺酸咪唑对甲苯磺酸盐催化合成氧杂蒽
2016-05-09田枭悦张永红孙亚栋王多志刘晨江
田枭悦, 张永红, 孙亚栋, 王多志, 刘晨江*
(1. 新疆大学 a. 石油化工天然气精细化工教育部&自治区重点实验室;
b. 化学化工学院,新疆 乌鲁木齐 830046)
·快递论文·
离子液体1-乙基-3-丁基磺酸咪唑对甲苯磺酸盐催化合成氧杂蒽
田枭悦1a, 张永红1a, 孙亚栋1a, 王多志1b*, 刘晨江1a*
(1. 新疆大学 a. 石油化工天然气精细化工教育部&自治区重点实验室;
b. 化学化工学院,新疆 乌鲁木齐 830046)
以1-乙基咪唑为原料,合成了布朗斯特酸性离子液体——1-乙基-3-丁基磺酸咪唑对甲苯磺酸盐(IL1),并将其用于催化芳香醛与5,5-二甲基-1,3-环己二酮或1,3-环己二酮在无溶剂条件下合成了系列氧杂蒽类化合物,其结构经1H NMR,13C NMR和ESI-MS表征。研究结果表明:当IL1用量为5 mol%,于80 ℃反应40 min,收率78%~95%; IL1循环使用3次,收率86%~89%,催化活性无明显变化。
布朗斯特酸性离子液体; 芳香醛; 环己二酮; 氧杂蒽; 催化; 无溶剂; 合成
氧杂蒽类化合物具有抗癌[1]、消炎[2]、抗病毒[3]和抗菌[4]等多种生物和药理活性,同时作为一类重要的有机中间体被用于药物合成领域[5]。此外,该类化合物由于具有优异的分光特性被应用于激光技术[6];也可用其制成对pH敏感的荧光材料而用于生物分子组装过程的标识[7]。因此,有关氧杂蒽化合物的合成研究受到了研究人员的广泛关注。
氧杂蒽化合物的合成方法由Nagarajan等[8]于1992年首次报道。该方法利用盐酸酸化双达米酮而合成目标产物。自此,许多催化体系如脯氨酸三氟甲磺酸盐[9]、三氟甲磺酸锶[10],P2O5/Al2O3[11], BF3/SiO2[12],氧化锌[13]、碘[14],NaHSO4[15], CAN/PEG-400体系[16]及Amberlyst-15[17]等均被用于该类化合物的合成,然而这些方法存在使用有机溶剂或反应时间较长等缺点。
离子液体因其具有极低的蒸汽压而不会挥发出有害气体,无溶剂反应因不需要使用有机溶剂作为反应介质,具有污染小、成本低和后处理简单等优点,符合绿色化学的发展方向。目前,离子液体已在氢化[18]、傅克[19],D-A[20], Heck[21],氧化脱氢偶联[22],Biginelli[23]等反应中得到了广泛应用,有关离子液体在无溶剂条件下催化合成氧杂蒽的研究也见诸报道[24-25]。
为了丰富氧杂蒽类化合物的绿色合成方法,在本课题组前期成功开展氨基磺酸锂催化合成氧杂蒽化合物的基础上[26],本文以1-乙基咪唑为起始原料,分别与1,4-丁烷磺酸内酯或正丁基溴发生亲核加成反应制得内盐,再与对甲苯磺酸、三氟乙酸或硫酸反应,合成了布朗斯特酸性离子液体——1-乙基-3-丁基磺酸咪唑对甲苯磺酸盐离子液体(IL1), 1-乙基-3-丁基磺酸咪唑硫酸氢盐离子液体(IL2), 1-乙基-3-丁基磺酸咪唑三氟乙酸盐离子液体(IL3)和1-乙基-3-丁基咪唑三氟乙酸盐(IL4)(Scheme 1),其中IL3为新化合物;研究了无溶剂条件下IL1催化芳香醛和5,5-二甲基-1,3-环己二酮(1a)或1,3-环己二酮(1k)反应合成了系列氧杂蒽化合物(3a~3m, Scheme 2),其结构经1H NMR,13C NMR和ESI-MS表征。

Scheme 1
1 实验部分
1.1 仪器与试剂
Buchi B-540型熔点仪(温度未校正);Varian Inova-400型核磁共振仪(DMSO-d6为溶剂,TMS为内标);Bruker Equinox 55型红外光谱仪(KBr压片);Agilent HP 1100型高效液相色谱质谱联用仪(ESI源)。
所用试剂均为化学纯或分析纯。
1.2 合成
(1) IL1~IL4的合成(以IL1为例)
在反应瓶中依次加入1-乙基咪唑 5.00 g(52 mmol) 和1,4-丁烷磺酸内酯7.07 g(52 mmol),搅拌下于80 ℃反应24 h。冷却至室温,用乙醚洗涤,真空干燥得1-乙基-3-丁基磺酸咪唑内盐。
在反应瓶中加入1-乙基-3-丁基磺酸咪唑内盐4.87 g(21 mmol) 和对甲苯磺酸3.61 g(21 mmol),搅拌下于110 ℃反应72 h。用乙醚洗涤,减压蒸除乙醚,真空干燥得IL1。
用类似的方法合成IL2, IL3和IL4(两步反应分别为:于50 ℃反应12 h;于90 ℃反应72 h)。
IL1[27]:亮黄色液体,收率87%;1H NMR(D2O)δ: 1.34(t,J=7.2 Hz, 3H, CH3), 1.59~1.61(m, 2H, CH2), 1.85~1.86(m, 2H, CH2), 2.24(s, 3H, CH3), 2.79(m, 2H, CH2), 4.03~4.06(m, 4H, CH2), 7.21~7.54(m, 6H, ArH), 8.62(s, 1H, ArH); ESI-MSm/z: 234.1{[M+H]+}, 171.0[M-]。

Scheme 2
IL2[27]: 亮黄色液体,收率81%;1H NMR(D2O)δ: 1.29(t,J=7.6 Hz, 3H, CH3), 1.49~1.57(m, 2H, CH2), 1.78~1.85(m, 2H, CH2), 2.73(q,J=7.6 Hz, 2H, CH2), 3.99~4.05(m, 4H, CH2), 7.30(s, 2H, ArH), 8.58(s, 1H, ArH); ESI-MSm/z: 234.1{[M+H]+}, 97.0[M-]。
IL3: 亮黄色液体,收率85%;1H NMR(D2O)δ: 1.43(t,J=7.6 Hz, 3H, CH3), 1.64~1.72(m, 2H, CH2), 1.92~2.00(m, 2H, CH2), 2.87(m, 2H, CH2), 4.13~4.20(m, 4H, CH2), 7.43~7.45(m, 2H, ArH), 8.73(s, 1H, ArH);13C NMR(D2O)δ: 163.20(q,JC-F=35.7 Hz), 135.58, 122.92, 122.81, 116.83(q,JC-F=290.0 Hz), 50.74, 49.61, 45.52, 28.76, 21.63, 15.04; IRv: 3 447, 3 145, 3 103, 2 974, 2 878, 2 417, 1 767, 1 566, 1 457, 1 416, 1 331, 1 169, 1 038, 788, 704, 646, 602, 524 cm-1; ESI-MSm/z: 234.1{[M+H]+}, 113.0[M-]。
IL4[28]: 棕黄色离子液体,收率85%;1H NMR(D2O)δ: 0.77(t,J=7.2 Hz, 3H, CH3), 1.14~1.20(m, 2H, CH2), 1.36(t,J=7.2 Hz, 3H, CH3), 1.67~1.75(m, 2H, CH2), 4.05~4.13(m, 4H, CH2), 7.36~7.39(m, 2H, ArH),8.68(s, 1H, ArH);13C NMR(D2O)δ: 162.71(q,JC-F=35.6 Hz), 135.41, 123.00, 122.85, 116.71(q,JC-F=289.2 Hz), 50.00, 45.52, 31.95, 19.47, 15.24, 13.37; ESI-MSm/z: 154.2{[M+H]+}, 113.0[M-]。
(2) IL1催化合成3a~3m
在圆底烧瓶中加入芳香醛1 mmol, 1a或1k 2 mmol和0.05 mmol IL1,搅拌下于80 ℃反应40 min。冷却至室温,加入适量碎冰水,用刮刀将固体捣碎,抽滤,滤饼用适量蒸馏水洗涤,用乙醇重结晶得3a~3m。
3a: m.p.204~206 ℃(204~206 ℃[15]);1H NMRδ: 0.99(s, 6H, CH3), 1.10(s, 6H, CH3), 2.14~2.46(m, 8H, CH2), 4.75(s, 1H, CH), 7.10~7.30(m, 5H, ArH)。
3b: m.p.248~249 ℃(240~242 ℃[15]);1H NMRδ: 0.99(s, 6H, CH3), 1.10(s, 6H, CH3), 2.14~2.45(m, 8H, CH2), 3.73(s, 3H, CH3), 4.70(s, 1H, CH), 6.74~7.26(m, 4H, ArH)。
3c: m.p.224~226 ℃(222~224 ℃[15]);1H NMRδ: 1.02(s, 6H, CH3), 1.10(s, 6H, CH3), 2.13~2.45(m, 8H, CH2), 5.00(s, 1H, CH), 7.04~7.44(m, 4H, ArH)。
3d: m.p.189~190 ℃(186~187 ℃[25]);1H NMRδ: 1.01(s, 6H, CH3), 1.11(s, 6H, CH3), 2.16~2.48(m, 8H, CH2),4.73(s, 1H, CH), 7.09~7.26(m, 4H, ArH)。
3e: m.p.227 ℃(224 ℃[29]);1H NMRδ: 0.99(s, 6H, CH3), 1.11(s, 6H, CH3), 2.14~2.53(m, 8H, CH2), 4.83(s, 1H, CH), 7.46~8.11(m, 4H, ArH)。
3f: m.p.202~203 ℃(200 ℃[29]);1H NMRδ: 0.99(s, 6H, CH3), 1.12(s, 6H, CH3), 2.23~2.62(m, 8H, CH2), 4.67(s, 1H, CH), 7.00~7.26(m, 4H, ArH)。
3g: m.p.247~249 ℃(248~250 ℃[15]);1H NMRδ: 1.00(s, 6H, CH3), 1.10(s, 6H, CH3), 2.15~2.4(m, 8H, CH2), 4.68(s, 1H, CH), 6.59~7.26(m, 4H, ArH)。
3h: m.p.188~190 ℃(185~186 ℃[30]);1H NMRδ: 0.95(s, 6H, CH3), 1.09(s, 6H, CH3), 2.10~2.48(m, 8H, CH2), 3.77(s, 3H, CH3), 4.86(s, 1H, CH), 6.74~7.42(m, 4H, ArH)。
3i: m.p.209~210 ℃(209~211 ℃[31]);1H NMRδ: 1.00(s, 6H, CH3), 1.10(s, 6H, CH3), 2.15~2.46(m, 8H, CH2), 2.28(s, 3H, CH3), 4.71(s, 1H), 6.90~7.14(m, 4H, ArH)。
3j: m.p.223~224 ℃(226~229 ℃[32]);1H NMRδ: 1.03(s, 6H, CH3), 1.10(s, 6H, CH3), 2.13~2.45(m, 8H, CH2), 5.02(s, 1H, CH), 6.95~7.46(m, 4H, ArH)。
3k: m.p.203~205 ℃(204~206 ℃[15]);1H NMRδ: 1.98~2.06(m, 4H, CH2), 2.26~2.41(m, 4H, CH2), 2.51~2.68(m, 4H, CH2), 3.73(s, 3H, CH3), 4.76(s, 1H, CH), 6.74~7.22(m, 4H, ArH)。
3l: m.p.237~239 ℃(248~250 ℃[15]);1H NMRδ: 1.92~2.05(m, 12H, CH2), 2.30~2.34(m, 4H, CH2), 2.55~2.64(m,4H, CH2), 5.01(s, 1H, CH), 7.04~7.48(m, 4H, ArH)。
3m: m.p.245~248 ℃(250~252 ℃[30]);1H NMRδ: 1.95~2.06(m, 12H, CH2), 2.31~2.35(m, 4H, CH2), 2.55~2.67(m,4H, CH2), 5.40(s, 1H, CH3), 7.23~7.65(m, 4H, ArH)。
2 结果与讨论
2.1 最佳反应条件的筛选
以1a和2a为模板底物,考察了IL1~IL4及其用量、反应温度和反应时间等因素对反应的影响,结果见表1。由表1可以看出,当四种离子液体用量均为5 mol%时,收率分别为88%, 85%, 84%和87%(No.1~4),其中IL1为催化剂时,收率最高。因此以IL1为催化剂,进一步考察催化剂用量对反应的影响(No.1, 5~7)。结果表明:IL1用量为5 mol%时催化效果最好,减少或者增加其用量均会导致收率降低;反应时间对反应的影响结果表明:最佳反应时间为40 min,收率89%(No.8);最后,考察了不同反应温度(80 ℃, 100 ℃和120 ℃)对收率的影响(No.8, No.10~11)。研究发现温度的变化对收率影响不大,从节能角度考虑,最佳反应温度为80 ℃。
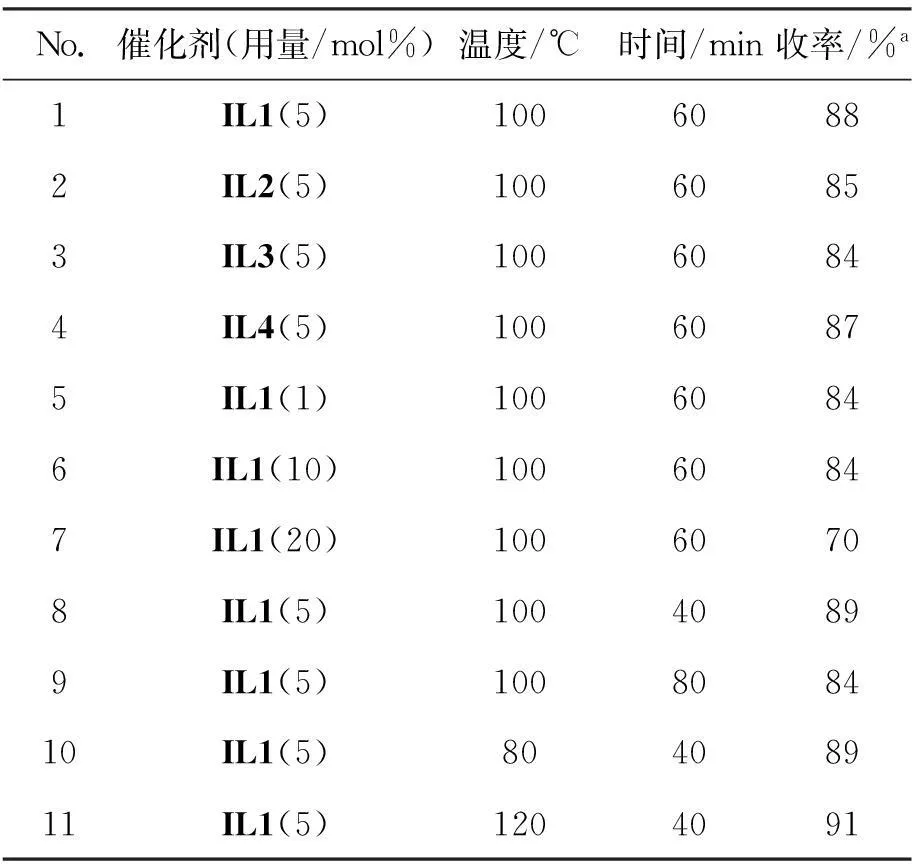
表1 不同反应条件对收率的影响*
*反应条件: 1a 2 mmol, 无溶剂;a分离产率。
综上所述,最佳反应条件为:5 mol% IL1为催化剂,反应温度为80 ℃,反应时间为40 min。
2.2 反应普适性
在最佳反应条件下,选取不同芳香醛分别和5,5-二甲基-1,3-环己二酮或1,3-环己二酮反应,考察IL1催化不同底物的普适性效果,结果见Scheme 1。由Scheme 1可见,芳香醛的取代基无论是吸电子基还是给电子基,或取代基无论在甲酰基的邻位、间位和对位,反应均能顺利进行,以78%~95%的收率获得目标化合物,表明该催化剂对于不同的底物均具有较好的催化性能。
2.3 离子液体的循环使用
为了研究IL1的循环使用效果,选取1a和2a的反应为研究对象,在反应后的混合物加入碎冰,待融化后抽滤。滤液减压蒸除溶剂,干燥回收离子液体,直接用于下次催化循环使用,结果见表2。由表2可见,IL1循环使用3次,收率无明显变化,说明IL1具有很好的循环使用效果。

表2 IL1的循环使用效果*
*反应条件: 1a 2 mmol, 5 mol% IL1,于80 ℃反应40 min。
3 结论
合成了一种布朗斯特酸性离子液体——1-乙基-3-丁基磺酸咪唑对甲苯磺酸盐,并将其用于催化芳香醛与5,5-二甲基-1,3-环己二酮或1,3-环己二酮在无溶剂条件下高收率地合成了系列氧杂蒽类化合物。该方法具有操作简便、收率高、对环境友好等优点,为该类化合物的合成提供参考。
[1] Silva D L, Terra B S, Lage M R,etal. Xanthenones: calixarenes-catalyzed syntheses, anticancer activity and QSAR studies [J].Org Biomol Chem,2015,13:3280-3287.
[2] Hafez H N, Hegab M I, Ahmed-Farag I S,etal. A facile regioselective synthesis of novelspiro-thioxanthene andspiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inammatory agents[J].Bioorg Med Chem Lett,2008,18:4538-4543.
[3] Qin C, Lin X, Lu X,etal. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungusStachybotrysp.HH1 ZSDS1F1-2[J].J Antibiot,2015,68:121-125.
[4] Evangelinou O, Hatzidimitriou A G, Velali E,etal. Mixed-ligand copper(I) halide complexes bearing 4,5-bis(diphenylphosphano)-9,9-dimethyl-xanthene andN-methylbenzothiazole-2-thion:Synthesis,structures,luminescence and antibacterial activity mediated by DNA and membrane damage[J].Polyhedron,2014,72:122-129.
[5] Khurana J M, Lumb A, Chaudhary A,etal. Efficient and green syntheses of 12-aryl-2,3,4,12-tetrahydrobenzo[b]xanthene-1,6,11-triones in water and task-specific ionic liquid [J].Synth Commun,2013,43:2147-2154.
[6] Ahmad M, King T A, Ko D-K,etal. Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases [J].J Phys D:Appl Phys,2008,35:1473-1476.
[7] Kushida Y, Nagano T, Hanaoka K. Silicon-substituted xanthene dyes and their applications in bioimaging[J].Analyst,2015,140:685-695.
[8] Nagarajan K, Shenoy S J. Chemistry of dimedone: structures of aldehyde-dimedone adducts[J].Indian J Chem,1992,31:73-87.
[9] Li J, Lu L, Su W. A new strategy for the synthesis of benzoxanthenes catalyzed by proline triate in water[J].Tetrahedron Lett,2010,51:2434-2437.
[10] Li J, Tang W, Lu L,etal. Strontium triflate catalyzed one-pot condensation ofβ-naphthol, aldehydes and cyclic 1,3-dicarbonyl compounds[J].Tetrahedron Lett,2008,49:7117-7120.
[11] Zarei A, Hajipour A R, Khazdooz L. The one-pot synthesis of 14-aryl or alkyl-14H-dibenzo[a,j]xanthenes catalyzed by P2O5/Al2O3under microwave irradiation[J].Dyes and Pigments,2010,85:133-138.
[12] Mirjalili B B F, Bamoniri A, Akbari A. BF3·SiO2:An efficient alternative for the synthesis of 14-aryl or alkyl-14H-dibenzo[a,j]xanthenes[J].Tetrahedron Lett,2008,49:6454-6456.
[13] Ghomi S J, Ghasemzadeh M A. Zinc oxide nanoparticles:A highly efficient and readily recyclable catalyst for the synthesis of xanthenes[J].Chin Chem Lett,2012,23:1225-1229.
[14] Sun X J, Zhou J F, Zhi S J. Efficient one-pot synthesis of tetrahydrobenzo[c]xanthene-1,11-dione derivatives under microwave irradiation[J].Synth Commun,2012,42:1987-1994.
[15] 马晶军,李静慈,唐然肖,等. 离子液体中NaHSO4催化芳香醛与1,3-环己二酮的缩合反应 [J].有机化学,2007,27(5):640-642.
[16] 曹瑞伟,陈朝辉,吴春雷,等. CAN/PEG-400 体系催化氧杂蒽二酮类衍生物 [J].合成化学,2012,20(4):511-514.
[17] Piscopo C G, Bühler S, Sartori G,etal. Supported sulfonic acids:Reusable catalysts for more sustainable oxidative coupling of xanthene-like compounds with nucleophiles[J].Catal Sci Technol,2012,2:2449-2452.
[18] Wu Z F, Jiang, H Y. Effcient palladium and ruthenium nanocatalysts stabilized by phosphine functionalized ionic liquid for selective hydrogenation[J].RSC Adv,2015,5:34622-34629.
[19] Fekri L Z, Nikpassand M. Ultrasound-promoted Friedel-Crafts acylation of arenes and cyclic anhydrides catalyzed by ionic liquid of [bmim]Br/AlCl3[J].Russ J Gen Chem,2014,84:1825-1829.
[20] Do T D, Schmitzer A R. Intramolecular diels alder reactions in highly organized imidazolium salt-based ionic liquid crystals[J].RSC Adv,2015,5:635-639.
[21] Wang F R, Tang S S, Yu Y H,etal. Preparation of palladium nanoparticle catalyst in ionic liquidand its catalytic properties for Heck-Mizoroki reaction[J].Chineses J Catal,2014,35:1921-1926.
[22] Basle O, Borduas N, Dubois P,etal. Aerobic and electrochemical oxidative cross-dehydrogenative-coupling(CDC) reaction in an imidazolium-based ionic liquid[J].Chem Eur J,2010,16:8162-8166.
[23] Zhang Y H, Wang B, Zhang X M,etal. An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones catalyzed by a novel Brønsted acidic ionic liquid under solvent-free conditions[J].Molecules,2015,20:3811-3820.
[24] Fang D,Liu Z L. Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes catalyzed by acyclic acidic ionic liquids[J].J Heterocyclic Chem,2010,47:509-512.
[25] Hamid R S, Kobra A. Brønsted acidic ionic liquids catalyzed one-pot synthesis of benzoxanthene leuco-dye derivatives[J].Res Chem Intermed,2015,41:409-417.
[26] 王昭申,刘晨江. 无溶剂条件下氨基磺酸锂催化合成氧杂蒽化合物 [J].高等学校化学学报,2012,33(3):507-510.
[27] Tao F R, Song H L, Chou L J. Hydrolysis of cellulose in SO3H-functionalized ionic liquids [J].Bioresour Technol,2011,102:9000-9006.
[28] Bonhote P, Dias A P, Papageorgiou N, etal. Hydrophobic highly conductive ambient-temperature molten salts[J].Inorg Chem,1996,35:1168-1178.
[29] Sagar A D, Chamle S N, Yadav M V. Microwave assisted rapid synthesis of 1,8-dioxo-Octahydroxanthenes using lignin sulphonic acid [J].J Chem Pharm Res,2013,5(7):156-160.
[30] Shirini F, Moghadam P N, Moayedi S,etal. Introduction ofO-sulfonated poly(4-vinylpyrrolidonium) chloride as a polymeric and reusable catalyst for the synthesis of xanthene derivatives[J].RSC Adv,2014,4:38581-38588.
[31] Zare A, Mokelesi M, Hasaninejad A,etal. Solvent-free synthesis of 1,8-dioxooctahydroxanthenes and 14-aryl-14H-dibenzo[a,j]xanthenes using saccharin sulfonic acid as an efficient and green catalyst[J].E-J Chem,2012,9(4):1854-1863.
[32] Ilangovan A, Malayappasamy S, Muralidharan S,etal. A highly efficient green synthesis of 1,8-dioxo- octahydroxanthenes[J].Chem Cent J,2011,5:81-86.
Synthesis of Xanthenes Catalyzed by Ionic Liquid 1-Ethyl-3-(4-sulfobutyl)-1H-imidazol-3-iump-Toluenesulfonate
TIAN Xiao-yue1a, ZHANG Yong-hong1a, SUN Ya-dong1a,WANG Duo-zhi1b*, LIU Chen-jiang1a*
(a. The Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education & Xinjiang Uygur Autonomous Region;b. College of Chemistry and Chemical Engineering, 1. Xinjiang University, Urumqi 830046, China)
Brønsted acidic ionic liquid, 1-ethyl-3-(4-sulfobutyl)-1H-imidazol-3-iump-toluenesulfonate(IL1), was prepared using 1-ethylimidazole as raw material. A series of xanthenes were synthesized by condensation reaction of aromatic aldehydes with 5,5-dimethyl-1,3-cyclohexanedione or 1,3-cyclohexanedione catalyzed by IL1 under solvent-free conditions. The structures were characterized by1H NMR,13C NMR and ESI-MS. The results indicated that 5 mol% IL1 as catalyst, reaction at 80 ℃ for 40 min, the yields were 78%~95%. The catalytic activity of IL1 keep stable with the yields of 86%~89% after recycling of three times.
Brønsted acidic ionic liquid; aromatic aldehyde; cyclohexanedione; xanthene; catalysis; solvent-free; synthesis
2015-04-15;
2016-01-18
国家自然科学基金资助项目(21162025, 21262035)
田枭悦(1989-),男,汉族,湖北襄阳人,硕士研究生,主要从事有机合成研究。 E-mail: 852472476@qq.com
王多志,副教授, E-mail: wangdz@xju.edu.cn; 刘晨江,教授, Tel. 0991-8582901, E-mail: pxylcj@126.com
O626.3
A
10.15952/j.cnki.cjsc.1005-1511.2016.04.15153
