Modifi cation of tenascin-R expression following unilateral labyrinthectomy in rats indicates its possible role in neural plasticity of the vestibular neural circuit
2015-12-15BotondGaalEinarrnhannessonAmitDattaniAgnesMagyarIldikberClaraMatesz
Botond Gaal, Einar Örn Jóhannesson, Amit Dattani, Agnes Magyar, Ildikó Wéber, Clara Matesz,
1 Department of Anatomy, Histology and Embryology, Faculty of Medicine and Faculty of Dentistry, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
2 Department of Pediatric Surgery, Faculty of Medicine, Clinical Center, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
3 MTA-DE Neuroscience Research Group, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
Modifi cation of tenascin-R expression following unilateral labyrinthectomy in rats indicates its possible role in neural plasticity of the vestibular neural circuit
Botond Gaal1, Einar Örn Jóhannesson1, Amit Dattani1, Agnes Magyar2, Ildikó Wéber1, Clara Matesz1,3,*
1 Department of Anatomy, Histology and Embryology, Faculty of Medicine and Faculty of Dentistry, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
2 Department of Pediatric Surgery, Faculty of Medicine, Clinical Center, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
3 MTA-DE Neuroscience Research Group, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
We have previously found that unilateral labyrinthectomy is accompanied by modifi cation of hyaluronan and chondroitin sulfate proteoglycan staining in the lateral vestibular nucleus of rats and the time course of subsequent reorganization of extracellular matrix assembly correlates to the restoration of impaired vestibular function. The tenascin-R has repelling eff ect on pathfi nding during axonal growth/regrowth, and thus inhibits neural circuit repair. By using immunohistochemical method, we studied the modifi cation of tenascin-R expression in the superior, medial, lateral, and descending vestibular nuclei of the rat following unilateral labyrinthectomy. On postoperative day 1, tenascin-R reaction in the perineuronal nets disappeared on the side of labyrinthectomy in the superior, lateral, medial, and rostral part of the descending vestibular nuclei. On survival day 3, the staining intensity of tenascin-R reaction in perineuronal nets recovered on the operated side of the medial vestibular nucleus, whereas it was restored by the time of postoperative day 7 in the superior, lateral and rostral part of the descending vestibular nuclei. The staining intensity of tenascin-R reaction remained unchanged in the caudal part of the descending vestibular nucleus bilaterally. Regional diff erences in the modifi cation of tenascin-R expression presented here may be associated with diff erent roles of individual vestibular nuclei in the compensatory processes. The decreased expression of the tenascin-R may suggest the extracellular facilitation of plastic modifi cations in the vestibular neural circuit after lesion of the labyrinthine receptors.
nerve regeneration; extracellular matrix; brainstem; vestibular system; vestibular lesion; vestibular compensation; perineuronal net; neural plasticity; neural regeneration
Funding: This work was supported by a grant from the Hungarian Academy of Sciences (MTA-TKI 11008); a grant from the European Union and the State of Hungary; and the European Social Fund in the framework of TÁMOP-4.2.4.A/ 2-11/1-2012-0001 ‘National Excellence Program’.
Gaal B, Jóhannesson EÖ, Dattani A, Magyar A, Wéber I, Matesz C (2015) Modifi cation of tenascin-R expression following unilateral labyrinthectomy in rats indicates its possible role in neural plasticity of the vestibular neural circuit. Neural Regen Res 10(9):1463-1470.
Introduction
The vestibular system, or the system of balance, provides information about the motion, equilibrium, and spatial orientation of the body. Lesions of the vestibular system result in postural and visual defi cits accompanied by dizziness, vertigo, and changes in cardiorespiratory and gastrointestinal functions. Signs of the disorder are classifi ed as static and dynamic symptoms and many, but not all are regained spontaneously in the process of vestibular compensation (Dieringer, 1995; Vidal et al., 1998; Hitier et al., 2010). The vestibular compensation incorporates modifi cations in a number of processes, like changes in discharge properties of bilateral vestibular neurons (Dieringer, 1995, 2003; Vibert et al., 1999b; Straka et al., 2005; Dutia, 2010), in the effi cacy of synaptic inputs from the existing non-labyrinthine pathways to the deaff erented vestibular neurons and in remodeling of synaptic connections through axonal sprouting and synaptogenesis (Dieringer, 1995, 2003; Vibert et al., 1999a, b, 2000; Beraneck et al., 2004; Straka et al., 2005). Based on previous results suggesting the modifi cations of extracellular matrix components in other parts of the nervous system after injuries, we suppose that these interrelated events of vestibular compensation have infl uence on the molecular assembly of the extracellular matrix also in the vestibular nuclear complex.
In the central nervous system, the major form of extracellular matrix, the perineuronal net, emerges in condensed form around the perikarya, proximal dendrites and axon initial segment (Carulli et al., 2006; Bruckner et al., 2008; Dityatev et al., 2010; Frischknecht and Seidenbecher, 2012; Lendvai et al., 2012; Blosa et al., 2013). Principal molecular constituents
of perineuronal net are the hyaluronan, chondroitin sulfate proteoglycan lecticans, tenascin-R and various link proteins (Celio et al., 1998; Zimmermann and Dours-Zimmermann, 2008; Kwok et al., 2011). The constituents of perineuronal net is activity dependent and its molecular assembly, as the parts of the synaptic machinery (Dityatev and Rusakov, 2011), can modify the synaptic transmission (Bukalo et al., 2007; Dityatev et al., 2010).
Although the role of extracellular matrix in the lesion-induced plasticity and regeneration was confi rmed in various parts of the central nervous system (Dityatev and Schachner, 2003; Moon et al., 2003; Galtrey and Fawcett, 2007; Lin et al., 2009; Dityatev et al., 2010; Alilain et al., 2011; Dityatev and Rusakov, 2011; Michaluk et al., 2011), similar works on the vestibular system were published only from our lab (Halasi et al., 2007; Deak et al. 2012). We observed that unilateral labyrinthectomy is accompanied by the radical decrease of hyaluronan and chondroitin sulfate proteoglycans expression in the lateral vestibular nucleus during the compensatory period. The reorganization of extracellular matrix assembly in the perineuronal net and neuropil correlated with the time course of recovery from the postural defi cits and reappearance of normal resting discharge of vestibular neurons (Dieringer, 1995; Curthoys, 1996; Vidal et al., 1998; Curthoys and Halmagyi, 1999; Darlington and Smith, 2000), suggesting the involvement of hyaluronan and the lecticans in the process of vestibular plasticity (Deak et al., 2012). Here, we extend this study to the tenascin-R as the third major contributor of the perineuronal net integrity, forming the ternary network with the hyaluronan and lecticans (Koppe et al., 1997; Bruckner et al., 2000; Pesheva and Probstmeier, 2000; Dityatev and Schachner, 2003; Carulli et al., 2006; Deepa et al., 2006; Zimmermann and Dours-Zimmermann, 2008; Kwok et al., 2010; Wang and Fawcett, 2012). Other studies on various species indicated that tenascin-R is an important modulator of neural plasticity and repair processes in various parts of the central nervous system (Apostolova et al., 2006; Anlar and Gunel-Ozcan, 2012). Based on these fi ndings, we hypothesized that the tenascin-R expression in the superior, medial, lateral, and descending vestibular nuclei varied following unilateral vestibular lesion and subsequent compensation.
Materials and Methods
Animals and surgical procedures
The experiments were carried out in accordance with European Community guidelines and state regulations and with the approval of the University Animal Care Committee (DEMÁB, 11/2011/DE MAB). All eff orts were made to minimize animal discomfort and reduce the number of animals used. Adult female Wistar rats, aged 12–14 weeks old, weighing 250–300 g, were used in the experiments (n = 12). The animals were anesthetized using an intramuscular injection of 2% xylazine (10 mg/kg, CP Pharma Handels GmbH, Germany) and 10% ketamine (100 mg/kg, CP Pharma Handels GmbH). The surgical procedure was performed under operating microscope (Leica, Wild M3C). A 1.5 cm-long skin incision was made behind the left external acoustic meatus; the cervical muscles, posterior belly of the digastric muscle, the stylohyoid and their nerves were spared. The ventral wall of the tympanic bulla was carefully opened and the labyrinth containing the vestibular sensory organs was approached by breaking the promontory. The left vestibular sensory organs were mechanically destroyed with special care taken on keeping the stapedial artery and facial nerve intact. After a period of 1, 3, 7 or 14 days of survival, the rats (three animals at each time point) were re-anesthetized with intraperitoneal administration of 10% urethane (1.3 mg/100 g, Reanal, Budapest, Hungary) and perfused transcardially with physiological saline.
Tissue processing and immunohistochemical staining
The brainstem was removed and immersed into Sainte-Marie’s fixative (99% absolute ethanol and 1% glacial acetic acid) for 1 day at 4°C. The specimens were embedded in paraffi n and transverse sections of 8 μm thickness were made. The tenascin-R was detected by incubating the samples in polyclonal goat anti-tenascin-R antibody (R&D Systems, Minneapolis, MN, USA; AF 3867) diluted in 1% bovine serum albumin + 3% normal rabbit serum overnight at 4°C. The primary antibody incubation was followed by repeated rinse steps in PBS, and then biotinylated rabbit-anti-goat IgG (Vector Laboratories, Burlingame, CA, USA) was used as the secondary antibody. Visualization of labeling was performed by incubating the samples with ExtrAvidin Peroxidase complex (Sigma-Aldrich) diluted in PBS for 1 hour at room temperature, followed by 3,3′-diaminobenzidine-tetrahydrochloride (DAB; Sigma-Aldrich) with H2O2. After dehydration, sections were coverslipped with DPX mounting medium (Sigma-Aldrich). Specifi city of tenascin-R antibody was assessed previously in our lab and the details were already published (Gaál et al., 2014; Rácz et al., 2014). Images were recorded by using Nikon Eclipse E800 (Nikon Corporation, Tokyo, Japan) conventional light microscope and processed by Photoshop CS4 v11.0 (Adobe Systems Inc., San Jose, CA, USA) with minimal adjustments of contrast and background.
Semiquantitative assessment of histochemical and immunohistochemical reactions
For the semiquantitative assessment of tenascin-R reaction, pictures from identical cross sectional levels of each individual vestibular nucleus of three animals were captured using the same magnifi cation, contrast, and brightness. On the images, the staining intensity of tenascin-R reaction was evaluated on the same computer screen by using four-grade scaling: –: no staining, +: weak staining, ++: moderate staining, +++: strong staining (Table 1). The subjective grading was performed by two authors (BG and IW) and checked by the other (CM), independently. The optical density measurement would fail to detect the clear-cut distinction between the diffuse ECM and its condensed forms (Carulli et al., 2006, 2007; Costa et al., 2007; Galtrey et al., 2008; Gati et al., 2010; Lendvai et al., 2012; Rácz et al., 2013) and this method
was successfully applied in the inferior olive (Kecskes et al., 2014).
As the exact quantifi cation of our data was diffi cult because there are no objective “distances” between the grades of staining intensity established by semiquantitative assessment, we considered these data as ordinal variables allowing application of nonparametric statistical methods to confi rm our conclusions. Two diff erent hypotheses were tested. The fi rst statistical analysis was devoted to test the diff erences in staining intensities of perineuronal nets between the operated and unoperated sides in the vestibular nuclei of the same animal. The variables for this analysis were determined by calculating the median values of the intensity scales of tenascin-R provided by the three independent investigators. For the statistical analysis, the Wilcoxon signed rank test was applied.
In the second statistical analysis, we examined whether the staining intensity has been changed during the postoperative period in the individual vestibular nuclei by using Kruskal-Wallis analysis of variance. In those vestibular nuclei where changes were detected, the subsequent days were compared with Mann-Whitney U test. In each statistical analysis, P < 0.001 was considered statistically signifi cant.
The statistical analysis was performed using SPSS 21.0 software (SPSS, Chicago, IL, USA).
Results
In the unoperated animals, as shown in our previous work (Racz et al., 2014), the tenascin-R immunoreactivity was intense in the perineuronal nets of each vestibular nucleus (Figure 1A, C, E, G, I, K, M, O and Figure 2A, C, E, G, I, K, M, O; Table 1), except for the caudal part of the descending vestibular nucleus which showed weaker staining in the pericellular area (Figure 2Q, S, U, W). The neuropil showed a diff use, reticular appearance presenting strongly stained areas in the superior, medial and lateral nuclei, as well as in the rostral parts of the descending vestibular nucleus, whereas the staining intensity was weaker in the caudal part of the descending vestibular nucleus.
In the superior vestibular nucleus, the tenascin-R staining of perineuronal nets completely disappeared on the side of labyrinthectomy on the fi rst postoperative day, whereas the pericellular staining did not change on the intact side (Figure 1A, B; Table 1). In the neuropil, the intensity of reaction was similar to that of the control animals, bilaterally. On survival day 3, the tenascin-R staining pattern was similar to day 1 with the exception of the lighter staining of ipsilateral neuropil (Figure 1C, D). On survival days 7 and 14, perineuronal nets were recognizable at both sides, showing minor decrease of staining in the perineuronal nets of the operated side (Figure 1E–H; Table 1). The staining intensity of neuropil was stronger on the unoperated side compared to postoperative day 3 (Figure 1E–H).
In the magnocellular part of the medial vestibular nucleus, the staining pattern of perineuronal nets and neuropil showed similar appearance to that of superior vestibular nucleus bilaterally on postoperative day 1 (Figure 1I, J). On postoperative day 3, the staining of perineuronal nets no longer showed recognizable diff erence on the operated side and it was also similar on the following survival days (Figure 1K–P; Table 1). Neuropil remained the same in intensity on both sides.
In the lateral vestibular nucleus, the staining of perineuronal nets and the neuropil was the same as in case of the superior and medial vestibular nuclei on postoperative day 1 (Figure 2A, B). On postoperative day 3, the staining intensity of perineuronal nets occurred slightly weaker on the operated side, but was considerably increasing after postoperative day 1 on the side of labyrinthectomy (Figure 2C, D; Table 1). The perineuronal nets showed the same intensity bilaterally on postoperative day 7 and the staining of ipsilateral pericellular areas was lighter compared to that on postoperative day 3 (Figure 2E, F). By postoperative day 14, the staining of pericellular area showed no bilateral diff erences (Figure 2G, H; Table 1). The staining of neuropil remained unchanged during the postoperative periods.
In the rostral part of the descending vestibular nucleus, the perineuronal nets were not recognizable bilaterally on postoperative day 1, and the staining intensity of neuropil was weaker compared to the control animals at both sides (Figure 2I, J). On postoperative day 3, the staining intensity of perineuronal nets and neuropil appeared bilaterally at the level of control animals (Figure 2K, L; Table 1). The same pattern of tenascin-R immunoreactivity was shown on postoperative day 7 (Figure 2M, N). On postoperative day 14, the staining intensity decreased bilaterally both in the perineuronal nets and neuropil (Figure 2O, P). In the caudal part of the descending vestibular nucleus, moderately stained pericellular areas, similar to that of control animals, were observed bilaterally during the postoperative periods (Figure 2Q–X).
Statistical analysis revealed that in the superior vestibular nucleus, the changes of staining intensity of tenascin-R reaction were non-signifi cant between days 1 and 3 and days 7 and 14, whereas they were statistically signifi cant between days 3 and 7 (P < 0.001). In the magnocellular part of the medial vestibular nucleus, the statistical analysis did not show any significant changes in the staining intensity of tenascin-R reaction during the postoperative period. In the lateral vestibular nucleus and the rostral part of the descending vestibular nucleus, the staining intensity was statistically signifi cant between days 1 and 3 (P < 0.001) and no signifi -cant changes were detected between the other survival days (Table 1).
Discussion
Unilateral labyrinthectomy results in elimination of sensory inputs from the vestibular receptors and the subsequent deaff erentation-induced plasticity contributes to the restoration of vestibular function. Our results demonstrated for the fi rst time that unilateral labyrinthectomy and subsequent compensation is accompanied by the modifi cation of tenascin-R staining pattern in the vestibular nuclei of the rat. The modifi cation of tenascin-R expression showed regional diff erences in the vestibular nuclear complex which may be
associated with the morphological and functional heterogeneity of the individual vestibular nuclei and with their different roles in the compensatory processes.

Figure 1 Distribution of tenascin-R staining intensity in the perineuronal nets of the superior (SVN) and medial vestibular nuclei (MVN) on the operated versus unoperated sides (A–P) on postoperative days 1, 3, 7 and 14.
The tenascin-R has versatile, sometimes opposite functions in the central nervous system depending on its location, the type of targeted cells, receptors, signaling pathways, the molecular composition of surrounding extracellular matrix as well as the embryonic and postembryonic periods of life (Pesheva and Probstmeier, 2000; Anlar and Gunel-Ozcan, 2012). Experimental studies showed that tenascin-R restricts functional recovery from spinal cord injury, and in agreement with this fi nding the tenascin-R-defi cient mice recovered better than wild-type controls after spinal cord compression (Apostolova et al., 2006). Therefore, it appears reasonable that the temporary decrease in the tenascin-R expression presented in our study plays a role in the recovery from the vestibular disorder. In the lack of data on the role of tenascin-R in the vestibular system, we can merely state, at present, that the tenascin-R expression is changing after unilateral labyrinthectomy and during the subsequent compensation. However, based on results of earlier experiments related to the role of tenascin-R in various parts of the central nervous system, we may suggest the following possible involvements of tenascin-R in the mechanisms of vestibular compensation (Dieringer, 1995; Dutia, 2010; Lacour and Tighilet, 2010).
First, the tenascin-R is known to activate the microglia cells which, in response, secrete cytokines and growth factors including brain-derived neurotrophic factor and nerve growth factor (Liao et al., 2005). In the deaff erented vestibular nuclei of the labyrinthectomized rat, intense microglial reaction was detectable as early as day 1 after lesion and it persisted several weeks afterwards (Campos Torres et al., 1999). This microglial reaction constitutes one of the signals responsible for astroglial reaction observed in the vestibular nuclei during the 1-3 postoperative days (de Waele et al., 1996). As suggested by Campos Torres et al. (2005), growth factors as well as pro- and anti-infl ammatory cytokines produced by activated astroglial cells could promote the survival of deaff erented vestibular neurons and contribute to recovery of their resting
discharge (Dieringer, 1995; Vibert et al., 1995; Li et al., 1999; Straka et al., 2005; Dutheil et al. 2013). To support these findings, Lacour and Tighilet (2010) confirmed bilateral up-regulation of brain-derived neurotrophic factors along with its TrkB receptor, both of which appeared as early as 1 day after unilateral labyrinthectomy and peaked at postoperative day 3 in the descending and lateral vestibular nucleus. As a result of glial reactions described above, the secreted growth factors facilitate the survival and recovery of deafferented vestibular neurons. The increased staining of tenascin-R in the neuropil from postoperative day 3 (sometimes from postoperative day 7) might be associated with the microglial activation thereby the tenascin-R may promote the plasticity of vestibular nucleus and contribute to the repair of vestibular disorders.

Figure 2 Distribution of tenascin-R staining intensity in the perineuronal nets of the lateral (LVN), rostral (DVN rostral) and caudal (DVN
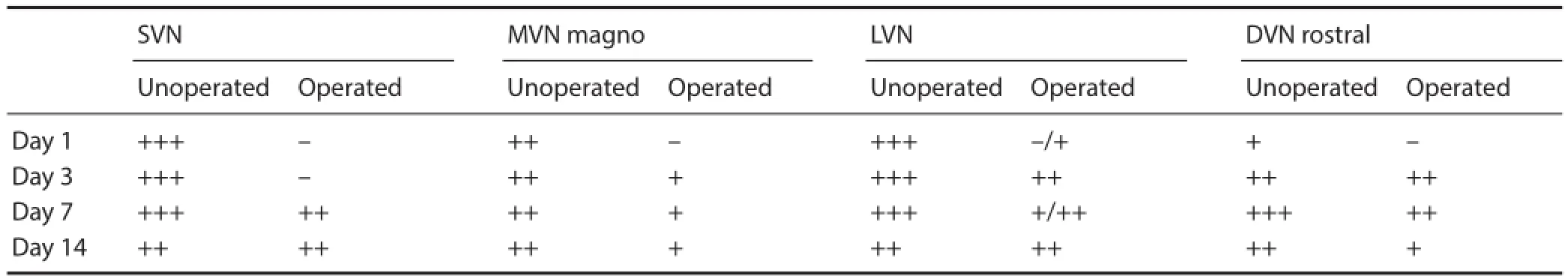
Table 1 Semiquantitative assessment of the staining intensity of tenascin-R in the perineuronal nets of individual vestibular nuclei on the operated versus unoperated sides on survival days 1, 3, 7, and 14 following unilateral labyrinthectomy
Second, the extracellular matrix in physiological conditions,
by stabilization of synapses, creates a barrier against the formation of new synaptic contacts and restricts the synaptic plasticity (Galtrey and Fawcett, 2007). In our present study, the common feature of the tenascin-R expression was the decrease or disappearance of tenascin-R immunoreactivity from the perineuronal nets in each vestibular nucleus. Decreased staining intensity of non-permissive tenascin-R may stimulate the formation of new synaptic contacts, as one of the possible mechanisms during the restoration of vestibular function (Dieringer, 1995; Li et al., 1999; de Waele et al., 2000; Lacour and Tighilet, 2010).
The third possible involvement of tenascin-R in the vestibular compensation might be related to inhibitory commissural pathways existing between the bilateral vestibular nuclei (Holstein et al., 1999; Bergquist et al., 2008; Malinvaud et al., 2010). Unilateral labyrinthectomy results in severe imbalance in the GABAergic commissural system and it is regarded as a key cause of the static oculomotor and postural symptoms. Similarly, the asymmetry in spontaneous resting activity between the intact and deaff erented vestibular neurons is due to the imbalance of GABAergic interaction between the ipsilateral and contralateral sides (Gliddon et al., 2004). The re-balancing of commissural inhibition occurs in parallel with the restoration of impaired resting activity and with the subsequent behavioral recovery during vestibular compensation (Gliddon et al., 2004; Straka et al., 2005; Tighilet et al., 2007; Bergquist et al., 2008). The tenascin-R regulates, via interactions of its HNK-1 (human natural killer cell) carbohydrate epitope, the GABABreceptor mediated perisomatic inhibition and thus infl uences synaptic transmission and plasticity in the hippocampus (Saghatelyan et al., 2000, 2001; Bukalo et al., 2001; Dityatev and Schachner, 2003; Brenneke et al., 2004). As the unilateral labyrinthectomy resulted in marked downregulation of the functional effi cacy of GABABreceptors in the cells of the ipsilesional medial vestibular nucleus (Yamanaka et al., 2000; Horii et al., 2003) similar regulatory function of tenascin-R is possible in the vestibular system.
Fourth, the tenascin-R is detectable around the nodes of Ranvier predominantly at the large, myelinated axons (Apostolova et al., 2006; Bekku et al., 2009). Here, the tenascin-R is binding to the voltage-gated sodium channels and initiates the clustering of channels and then stabilizes the clusters after they have formed thereby it is regarded as a functional modulator of sodium channel beta subunits (Srinivasan et al., 1998; Xiao et al., 1999). In the tenascin-R-defi cient mice, there was a signifi cant decrease in conduction velocity of myelinated axons (Weber et al., 1999). As the vestibular nuclei have large caliber myelinated axons (Sotelo and Palay, 1970), similar function of the tenascin-R might be suggested here, as well.
Although the individual vestibular nuclei are different from each other in their morphological, physiological and biochemical characteristics associated with their diff erent functions in balance, vestibulo-ocular refl exes, spatial cognition and automatic responses (Babalian and Vidal, 2000; Birinyi et al., 2001; Straka et al., 2005; Eugene et al., 2011; McCall and Yates, 2011; Kodama et al., 2012; Racz et al., 2014) their specifi c role in the compensatory processes is not yet determined. Most of the experiments on vestibular lesion and subsequent compensation have been performed on the medial vestibular nucleus. This nucleus is engaged mostly in the postural refl exes, and restoration of which is known as the initial event during the vestibular compensation (Yamanaka et al., 2000; Beraneck et al., 2003; Beraneck and Idoux, 2012). The earliest re-establishment of tenascin-R reaction in the perineuronal nets around the neurons of medial vestibular nucleus might support these fi ndings. The only part of the vestibular nuclear complex where the tenascin-R reaction remained almost unchanged following unilateral labyrinthectomy is the caudal part of descending vestibular nucleus. This subnucleus is involved in the rapid modulation of the cardiovascular, respiratory and digestive systems in response to locomotion and postural adjustments (Ruggiero et al., 1996; Matesz et al., 1997; Porter and Balaban, 1997; Holstein et al., 2011). In the light of our results, it is tempting to assume that the role of extracellular matrix is less important in the compensatory processes of impaired vestibulo-autonomic function.
Our results may provide new insights into the mechanisms of vestibular plasticity. The presented results contribute to our earlier fi ndings on the spatially and temporally specifi c alterations of hyaluronan and chondroitin sulfate proteoglycans during vestibular compensation (Deak et al., 2012). The reduction of the immunostaining of tenascin-R also suggests the extracellular facilitation of plastic modifi cations of the vestibular circuit after lesion. The results may also assist in developing new therapeutic strategies for the treatment of symptoms of vestibular lesion.
Acknowledgments: We thank Timea Horvath (Department of Anatomy, Histology and Embryology, Faculty of Medicine, University of Debrecen, Hungary) for skillful technical assistance and Andras Birinyi (Department of Anatomy, Histology and Embryology, Faculty of Medicine, University of Debrecen, Hungary) for assistance in statistical analysis.
Author contributions: CM and BG conceived and designed this study, and wrote the paper; EÖJ, AD, AM and IW were responsible for data acquisition; EÖJ, AD, AM, IW, and BG analyzed the data; BG revised critically for the important intellectual content; CM supervised the study. All authors approved the fi nal version of this paper.
Confl icts of interest: None declared.
Alilain WJ, Horn KP, Hu HM, Dick TE, Silver J (2011) Functional regeneration of respiratory pathways after spinal cord injury. Nature 475:196-200.
Apostolova I, Irintchev A, Schachner M (2006) Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci 26:7849-7859.
Babalian AL, Vidal PP (2000) Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. J Neurophysiol 84:2514-2528.
Bekku Y, Rauch U, Ninomiya Y, Oohashi T (2009) Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem 108:1266-1276.
Beraneck M, Idoux E (2012) Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol 3:25.
Beraneck M, Idoux E, Uno A, Vidal PP, Moore LE, Vibert N (2004) Unilateral labyrinthectomy modifi es the membrane properties of contralesional vestibular neurons. J Neurophysiol 92:1668-1684.
Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N (2003) Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol 90:184-203.
Bergquist F, Ludwig M, Dutia MB (2008) Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol 586:4441-4452.
Birinyi A, Straka H, Matesz C, Dieringer N (2001) Location of dye-coupled second order and of eff erent vestibular neurons labeled from individual semicircular canal or otolith organs in the frog. Brain Res 921:44-59.
Blosa M, Sonntag M, Bruckner G, Jager C, Seeger G, Matthews RT, Rubsamen R, Arendt T, Morawski M (2013) Unique features of extracellular matrix in the mouse medial nucleus of trapezoid body--implications for physiological functions. Neuroscience 228:215-234.
在机载无线射频识别技术的实现中,主要使用了固定翼无人机、读写器、电子标签、高清摄像机,实现了视频信息的远距离传输。其中,固定翼无人机的飞行半径为100公里、电子标签与读写器的传输距离为300米,能够对航标巡检的需求进行满足。通过使用机载无线射频识别技术,能够在不需要人员出海、或是船舶运行距离更近的情况下,完成航标巡检工作,除了能够对巡检的效率进行提升、对巡检成本进行控制,还降低了航标巡检的危险性,提升了安全管理的效果。
Brenneke F, Bukalo O, Dityatev A, Lie AA (2004) Mice defi cient for the extracellular matrix glycoprotein tenascin-R structural hallmarks show physiological and of increased hippocampal excitability, but no increased susceptibility to seizures in the pilocarpine model of epilepsy. Neuroscience 124:841-855.
Bruckner G, Morawski M, Arendt T (2008) Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 151:489-504.
Bruckner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M (2000) Postnatal development of perineuronal nets in wild-type mice and in a mutant defi cient in tenascin-R. J Comp Neurol 428:616-629.
Bukalo O, Schachner M, Dityatev A (2001) Modifi cation of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of Tenascin-R diff erentially aff ects several forms of synaptic plasticity in the hippocampus. Neuroscience 104:359-369.
Bukalo O, Schachner M, Dityatev A (2007) Hippocampal metaplasticity induced by defi ciency in the extracellular matrix glycoprotein tenascin-R. J Neurosci 27:6019-6028.
Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW (2006) Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol 494:559-577.
Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L (1998) Perineuronal nets: past and present. Trends Neurosci 21:510-515.
Curthoys IS (1996) The role of ocular torsion in visual measures of vestibular function. Brain Res Bull 40:399-403; discussion 403-395.
Curthoys IS, Halmagyi GM (1999) Vestibular compensation. Adv Otorhinolaryngol 55:82-110.
Darlington CL, Smith PF (2000) Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol 62:313-325.
de Waele C, Campos Torres A, Josset P, Vidal PP (1996) Evidence for reactive astrocytes in rat vestibular and cochlear nuclei following unilateral inner ear lesion. Eur J Neurosci 8:2006-2018.
de Waele C, Loquet G, Campos Torres A, Vidal PP (2000) Lack of growth-associated protein-43 reemergence or of growth-associated protein-43 mRNA modulation in deafferented vestibular nuclei during the fi rst 6 weeks after unilateral inner ear lesion. Exp Brain Res 132:464-475.
Deak A, Bacskai T, Gaal B, Racz E, Matesz K (2012) Eff ect of unilateral labyrinthectomy on the molecular composition of perineuronal nets in the lateral vestibular nucleus of the rat. Neurosci Lett 513:1-5.
Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW (2006) Composition of perineuronal net extracellular matrix in rat brain: a diff erent disaccharide composition for the net-associated proteoglycans. J Biol Chem 281:17789-17800.
Dieringer N (1995) ‘Vestibular compensation’: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol 46:97-129.
Dieringer N (2003) Activity-related postlesional vestibular reorganization. Ann N Y Acad Sci 1004:50-60.
Dityatev A, Schachner M (2003) Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4:456-468.
Dityatev A, Rusakov DA (2011) Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21:353-359.
Dityatev A, Schachner M, Sonderegger P (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11:735-746.
Dutheil S, Escoffi er G, Gharbi A, Watabe I, Tighilet B (2013) GABA(A) receptor agonist and antagonist alter vestibular compensation and diff erent steps of reactive neurogenesis in deaff erented vestibular nuclei of adult cats. J Neurosci 33:15555-15566.
Dutia MB (2010) Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol Head Neck Surg 18:420-424.
Eugene D, Idoux E, Beraneck M, Moore LE, Vidal PP (2011) Intrinsic membrane properties of central vestibular neurons in rodents. Exp Brain Res 210:423-436.
Frischknecht R, Seidenbecher CI (2012) Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol 44:1051-1054.
Gaál B, Rácz É, Juhász T, Holló K, Matesz C (2014) Distribution of extracellular matrix macromolecules in the vestibular nuclei and cerebellum of the frog, Rana esculenta. Neurosci 258:162-173.
Galtrey CM, Fawcett JW (2007) The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54:1-18.
Gliddon CM, Darlington CL, Smith PF (2004) Rapid vestibular compensation in guinea pig even with prolonged anesthesia. Neurosci Lett 371:138-141.
Halasi G, Wolf E, Bacskai T, Szekely G, Modis L, Szigeti ZM, Meszar Z, Felszeghy S, Matesz C (2007) The eff ect of vestibular nerve section on the expression of the hyaluronan in the frog, Rana esculenta. Brain Struct Funct 212:321-334.
Hitier M, Besnard S, Vignaux G, Denise P, Moreau S (2010) The ventrolateral surgical approach to labyrinthectomy in rats: anatomical description and clinical consequences. Surg Radiol Anat 32:835-842. Holstein GR, Martinelli GP, Wearne S, Cohen B (1999) Ultrastructure of vestibular commissural neurons related to velocity storage in the monkey. Neuroscience 93:155-170.
Holstein GR, Friedrich VL, Kang T, Kukielka E, Martinelli GP (2011) Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience 175:104-117.
Horii A, Kitahara T, Smith PF, Darlington CL, Masumura C, Kubo T (2003) Effects of unilateral labyrinthectomy on GAD, GATI and GABA receptor gene expression in the rat vestibular nucleus. Neuroreport 14:2359-2363.
Kodama T, Guerrero S, Shin M, Moghadam S, Faulstich M, du Lac S (2012) Neuronal classifi cation and marker gene identifi cation via single-cell expression profi ling of brainstem vestibular neurons subserving cerebellar learning. J Neurosci 32:7819-7831.
Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V (1997) Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res 288:33-41.
Kwok JC, Carulli D, Fawcett JW (2010) In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J Neurochem 114:1447-1459.
Kwok JC, Dick G, Wang DF, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71:1073-1089.
Lacour M, Tighilet B (2010) Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a “deafferentation” code. Restor Neurol Neurosci 28:19-35.
Lendvai D, Morawski M, Bruckner G, Negyessy L, Baksa G, Glasz T, Patonay L, Matthews RT, Arendt T, Alpar A (2012) Perisynaptic aggrecan-based extracellular matrix coats in the human lateral geniculate body devoid of perineuronal nets. J Neurosci Res 90:376-387.
Li H, Godfrey DA, Rubin AM (1999) Astrocyte reaction in the rat vestibular nuclei after unilateral removal of Scarpa’s ganglion. Ann Otol Rhinol Laryngol 108:181-188.
Liao H, Bu WY, Wang TH, Ahmed S, Xiao ZC (2005) Tenascin-R plays a role in neuroprotection via its distinct domains that coordinate to modulate the microglia function. J Biol Chem 280:8316-8323.
Lin CM, Lin JW, Chen YC, Shen HH, Wei L, Yeh YS, Chiang YH, Shih R, Chiu PL, Hung KS, Yang LY, Chiu WT (2009) Hyaluronic acid inhibits the glial scar formation after brain damage with tissue loss in rats. Surg Neurol 72 Suppl 2:S50-54.
Malinvaud D, Vassias I, Reichenberger I, Rossert C, Straka H (2010) Functional organization of vestibular commissural connections in frog. J Neurosci 30:3310-3325.
Matesz C, Nagy E, Kulik A, Tonkol A (1997) Projections of the medial and superior vestibular nuclei to the brainstem and spinal cord in the rat. Neurobiology (Bp) 5:489-493.
McCall AA, Yates BJ (2011) Compensation following bilateral vestibular damage. Front Neurol 2:88.
Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, Medvedev N, Wilczek E, De Roo M, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J (2011) Infl uence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci 124:3369-3380.
Moon LD, Asher RA, Fawcett JW (2003) Limited growth of severed CNS axons after treatment of adult rat brain with hyaluronidase. J Neurosci Res 71:23-37.
Pesheva P, Probstmeier R (2000) The yin and yang of tenascin-R in CNS development and pathology. Prog Neurobiol 61:465-493.
Porter JD, Balaban CD (1997) Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res 7:63-76.
Racz E, Gaal B, Kecskes S, Matesz C (2014) Molecular composition of extracellular matrix in the vestibular nuclei of the rat. Brain Struct Funct 219:1385-1403.
Ruggiero DA, Mtui EP, Otake K, Anwar M (1996) Vestibular aff erents to the dorsal vagal complex: substrate for vestibular-autonomic interactions in the rat. Brain Res 743:294-302.
Saghatelyan AK, Gorissen S, Albert M, Hertlein B, Schachner M, Dityatev A (2000) The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and longterm potentiation in the CA1 region of the hippocampus. Eur J Neurosci 12:3331-3342.
Saghatelyan AK, Dityatev A, Schmidt S, Schuster T, Bartsch U, Schachner M (2001) Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice defi cient for the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci 17:226-240.
Sotelo C, Palay SL (1970) The fi ne structure of the later vestibular nucleus in the rat. II. Synaptic organization. Brain Res 18:93-115.
Srinivasan J, Schachner M, Catterall WA (1998) Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc Natl Acad Sci U S A 95:15753-15757.
Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB (2005) Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76:349-392.
Tighilet B, Brezun JM, Sylvie GD, Gaubert C, Lacour M (2007) New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci 25:47-58.
Vibert D, Hausler R, Safran AB (1999a) Subjective visual vertical in peripheral unilateral vestibular diseases. J Vestib Res 9:145-152.
Vibert N, Beraneck M, Bantikyan A, Vidal PP (2000) Vestibular compensation modifi es the sensitivity of vestibular neurones to inhibitory amino acids. Neuroreport 11:1921-1927.
Vibert N, Serafi n M, Crambes O, Vidal PP, Muhlethaler M (1995) Dopaminergic agonists have both presynaptic and postsynaptic eff ects on the guinea-pig’s medial vestibular nucleus neurons. Eur J Neurosci 7:555-562.
Vibert N, Babalian A, Serafin M, Gasc JP, Muhlethaler M, Vidal PP (1999b) Plastic changes underlying vestibular compensation in the guinea-pig persist in isolated, in vitro whole brain preparations. Neuroscience 93:413-432.
Vidal PP, de Waele C, Vibert N, Muhlethaler M (1998) Vestibular compensation revisited. Otolaryngol Head Neck Surg 119:34-42.
Wang DF, Fawcett J (2012) The perineuronal net and the control of CNS plasticity. Cell Tissue Res 349:147-160.
Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, Schachner M (1999) Mice defi cient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci 19:4245-4262.
Xiao ZC, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL (1999) Tenascin-R is a functional modulator of sodium channel beta subunits. J Biol Chem 274:26511-26517.
Yamanaka T, Him A, Cameron SA, Dutia MB (2000) Rapid compensatory changes in GABA receptor effi cacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol 523 Pt 2:413-424.
Zimmermann DR, Dours-Zimmermann MT (2008) Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol 130:635-653.
Copyedited by Alpar A, Essa MM, Li CH, Song LP, Zhao M
*Correspondence to:
Clara Matesz, M.D., Ph.D.,
matesz@anat.med.unideb.hu.
orcid:
0000-0002-3091-0756 (Clara Matesz)
10.4103/1673-5374.165517
http://www.nrronline.org/
Accepted: 2015-07-20
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- PTEN inhibition and axon regeneration and neural repair
- Neural correlates of the Heidelberg Music Therapy: indicators for the regeneration of auditory cortex in tinnitus patients?
- The choline pathway as a strategy to promote central nervous system (CNS) remyelination
- Enhancing endogenous stem cells in the newborn via delayed umbilical cord clamping
- Elastic modulus aff ects the growth and diff erentiation of neural stem cells
- Non-steroidal anti-infl ammatory drugs (NSAIDs) and neuroprotection in the elderly: a view from the mitochondria
