氮杂环丙烷[3+2]环加成反应研究进展
2015-11-24任鸿胡宝祥
任鸿,胡宝祥
(浙江工业大学化学工程学院,浙江杭州310014)
氮杂环丙烷[3+2]环加成反应研究进展
任鸿,胡宝祥
(浙江工业大学化学工程学院,浙江杭州310014)
介绍了最近几年在不同催化体系下,氮杂环丙烷与含双键化合物的[3+2]环加成反应研究进展。
氮杂环丙烷;[3+2]环加成;综述
氮杂环丙烷是一类含氮原子的三元杂环化合物,由于该三元环的张力极高,导致其具有较强的反应活性,因而在有机合成中,基于氮杂环丙烷开环反应的应用非常广泛[1]。
如式1所示,氮杂环丙烷在加热或光辐射等条件下,易发生C-C键的断裂,将环打开,形成亚甲胺内钅翁盐,然后参与1,3-偶极环加成反应,生成含氮五元杂环。另一方面,在路易斯酸作用下,氮杂环丙烷则发生C-N键的断裂,与烯烃、炔烃、醛、腈类和联烯等含重键的化合物发生[3+2]环加成反应,生成另一种构型的含氮五元杂环[2]。

式1氮杂环丙烷参与的[3+2]开环加成反应
1 氮杂环丙烷与烯烃类化合物的反应
Yadav等人[3]报导了Sc(OTf)3可以作为路易斯酸催化N-对甲苯磺酰基芳基氮杂环丙烷得到1,3-二偶极体。当与环烯醚反应时,可以得到相应的吡咯烷,并且具有较高的收率和区域选择性(式2)。

式2 Sc(OTf)3催化氮杂环丙烷与烯烃的反应
2011年,Aggarwal等人[4]报导了应用于合成红藻氨酸的具有手性中心的吡咯烷类化合物的手性合成,如式3。

式3钯催化氮杂环丙烷的环加成反应
2 氮杂环丙烷与炔烃类化合物的反应
在与炔烃的[3+2]反应中,许多路易斯酸都能催化该反应的进行[5]。一系列的芳基炔烃(式4)都能在FeCl3催化下与芳基氮杂环丙烷反应,得到官能团化的吡咯类化合物。
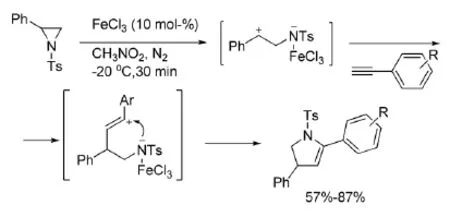
式4 FeCl3催化氮杂环丙烷与炔烃的反应
3 氮杂环丙烷与腈类化合物的反应
N-对甲苯磺酰基氮杂环丙烷与腈类化合物的[3+2]环合反应通常涉及到C-N键的断裂。在对这类反应的研究中,主要是关于催化剂的探索,研究发现,一系列的路易斯酸,比如BF3·OEt2[6-11,13],ZnX2(X=Cl,Br,I)[12],Zn(OTf)2[13],Sc(OTf)3[14],Bi(OTf)3[15]和Cu(OTf)2[16]都能应用于该反应。
2005年,Yadav等人[10]利用TBDPS取代的氮杂环丙烷,在BF3·OEt2催化下与氰基发生[3+2]环加成反应(式5)。
2011年,Wei等人[15]以Bi(OTf)3为催化剂,催化N-对甲苯磺酰基氮杂环丙烷和一系列腈类化合物之间的[3+2]环加成反应,得到相应的咪唑啉,如式6。该方法同样适用于稠环芳烃氮杂环丙烷。
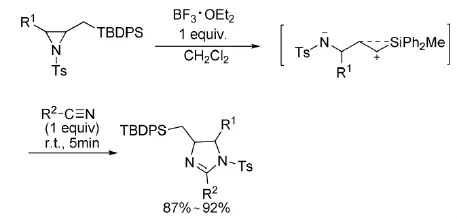
式5 BF3·OEt2催化氮杂环丙烷与氰基的反应

式6 Bi(OTf)3催化氮杂环丙烷与腈类化合物的反应
4 氮杂环丙烷与含羰基类化合物的反应
传统的合成1,3-恶唑烷衍生物的方法都是从1,2-氨基醇和含羰基类化合物出发,在较高的温度下进行。并且该方法对底物限制较大,同时副反应较多。因而越来越多的人开始对由路易斯酸催化氮杂环丙烷与含羰基化合物反应的研究产生了兴趣。但到目前为止,对于催化该反应的路易斯酸催化剂报导还比较少,只有BF3·OEt2[10],Cu(OTf)2[16],Zn(OTf)2[13],Sc(OTf)3[17]和AgSbF6[18]。
叔丁基二苯基硅烷基取代的氮杂环丙烷在BF3·OEt2催化下,也可以与醛发生[3+2]环加成反应[10]。该反应在温和的条件能快速进行,并且适用于芳香族和脂肪族醛,反应都有较高的收率,如式7。

式7 BF3·OEt2催化氮杂环丙烷与醛的反应
2009年,Nguyen等[17]以Sc(OTf)3为催化剂,将2-烷基-N-对甲苯磺酰基氮杂环丙烷与醛或者酮反应,得到了5-烷基-1,3-恶唑烷,如式8。
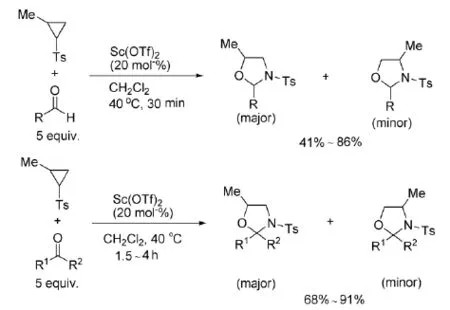
式8 Sc(OTf)3催化氮杂环丙烷与醛或酮的反应
2011年,Hanamoto等[18]开发了AgSbF6作为氮杂环丙烷与醛的[3+2]环加成反应催化剂,如式9。

式9 AgSbF6催化氮杂环丙烷与醛的反应
5 氮杂环丙烷与CO2的反应
恶唑酮作为经典的杂环化合物,广泛应用于有机合成及具有生物活性化合物的合成上面。而过去十年,CO2作为碳源研究的快速发展,吸引了越来越多来自有机合成及化工行业的兴趣。到目前为止,报导的催化体系有碱金属卤化物[19-24],四烷基季铵盐卤化物[20],DBN[33],碘[24-28],自然存在的氨基酸[29],Cr(III)/DMAP[30],氯氧化锆[31],聚乙二醇修饰的季铵盐[32],氨基酸修饰的聚苯乙烯[33],聚乙二醇修饰的季盐[34]和离子液体[35-36]等。
2011年,Liu等[24]以DBN作催化剂,Li为助催化剂,成功合成了N-官能团化2-恶唑酮,并推测了如式10所示反应机理:首先DBN固定CO2,得到中间体a,再与氮杂环丙烷反应得到中间体b;然后在LiI催化下环打开,并经由分子内环化得到目标产物,并有较高的收率。

式10 DBN催化氮杂环丙烷与CO2的反应及其机理
2010年,Jiang等[29]也报道了在无溶剂、催化剂的条件下,通过天然的α-氨基酸催化将CO2成功插入到氮杂环丙烷中,如式11。对于这类反应,几乎所有自然存在的α-氨基酸(例如,L-组氨酸)都是有效的催化剂。

式11α-氨基酸催化氮杂环丙烷与CO2的反应
2010年,He等[35]首次报道了在无溶剂下,Lewis碱的离子液体催化氮杂环丙烷与CO2的反应,合成5-芳基恶唑酮,如式12。
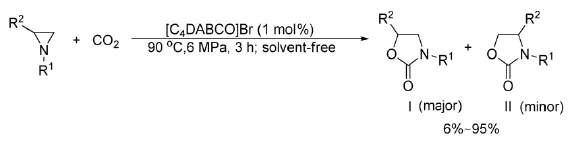
式12离子液体催化氮杂环丙烷与CO2的反应
2010年,Pinhas等[37]利用高速球磨技术(HSBM),在无溶剂和无催化剂条件下,在室温条件下就能使2-烷基或2-芳基氮杂环丙烷与CO2发生环加成反应,如式13。
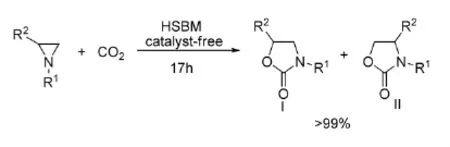
式13无溶剂条件下氮杂环丙烷与CO2的反应
6 氮杂环丙烷与杂联烯类化合物的反应
2008年,Hou等[38]以三丁基膦为催化剂,催化N-对甲苯磺酰基氮杂环丙烷与二硫化碳及异硫氰酸酯的环加成反应,得到1,3-四氢噻唑衍生物,该化合物被广泛应用于有机合成及药物化学领域,如式14。

式14三丁基膦催化氮杂环丙烷与CS2或异硫氰酸酯的反应
2013年,Tharmalingam等[39]以水为溶剂,硝酸铁为催化剂,催化氮杂环丙烷与异硫氰酸酯、异氰酸酯及异硒氰酸酯的环加成反应,如式15所示。

式15 Fe(NO3)3催化氮杂环丙烷与异硒氰酸酯的反应
7 结语
氮杂环丙烷参与的[3+2]环加成反应在合成含氮五元杂环化合物中具有重要作用。随着人们对其研究的深入,开发了一系列催化剂并应用于该反应中,使其在有机合成、药物和生物等领域的应用越来越广泛。在今后的研究中,重点仍在开发出更多高效新型的催化剂,以在温和的反应条件中进行,并且具有更广的底物适用性。
[1]Liu P.Recent developments inregioselective ring opening of aziridines[J].Tetrahedron,2010,66:2549-2560.
[2]Dauban P,Malik G.Ein maskierter,aus Aziridinen freigesetzter 1,3-Dipol[J].Angew.Chem.2009,121(48),9188-9191;Dauban P,Malik G.A masked 1,3-dipole revealed from aziridines[J].Angew.Chem.Int.Ed.2009,48:9026-9029.
[3]Yadav J S,Reddy B V S,Pandey S K.Scandium triflatecatalyzed 1,3-dipolar cycloaddition of aziridines with alkenes[J].Tetrahedron Lett.2001,42:5399-5403.
[4]Lowe M A,Ostovar M,Aggarwal V K.Palladium-mediated annulation of vinyl aziridines with michael acceptors:stereocontrolled synthesis of substituted pyrrolidines and its application in a formal synthesis of(-)-alpha-Kainic acid [J].Angew.Chem.Int.Ed.2011,50:6370-6374.
[5]Wender P A,Strand D.Cyclocarboamination of alkynes with aziridines:synthesis of 2,3-dihydropyrroles by a catalyzed Formal[3+2]cycloaddition[J].J.Am.Chem.Soc. 2009,131:7528-7529.
[6]Hiyama T,Koide H,Fujita S,Nozaki H.Reaction of N-alkoxycarbonylaziridines with nitriles[J].Tetrahedron 1973, 29:3137-3139.
[7]Legters J,Willems J,Thijs L,Zwanenburg B.Synthesis of functionalized amino-acids by ring-opening reactions of aliphatically substituted aziridine-2-carboxylic esters[J]. Recl.Trav.Chim.Pays-Bas 1992,111:59-68.
[8]Papa C,Tomasini C.Synthesis and ring opening of methyl 2-alkyl-3-(alkyl/aryl)-1-benzo ylaziridine-2-carboxylates:Synthesis of polysubstituted amino acids[J].Eur.J. Org.Chem.2000,2000:1569-1576.
[9]Prasad B A B,Pandey G,Singh V K.Synthesis of substituted imidazolines via[3+2]-cycloaddition of aziridines with nitriles[J].Tetrahedron Lett.2004,45:1137-1141.
[10]Yadav V K,Sriramurthy V.Silylmethyl-substituted aziridine and azetidine as masked 1,3-and 1,4-dipoles for formal[3+2]and[4+2]cycloaddition reactions[J].J.Am. Chem.Soc.2005,127:16366-16367.
[11]Concellon J M,Riego E,Suarez J R.Synthesis of enantiopureimidazolines through a ritter reaction of 2-(1-aminoalkyl)aziridines with nitriles[J].Org.Lett.2004,6: 4499-4501
[12]Ghorai M K,Kumar A,Ghosh K,An efficient route to regioselective opening of N-tosylaziridines with zinc(II)halides[J].Tetrahedron Lett.2005,46:4103-4106.
[13]Gandhi S,Bisai A,Singh V K.Studies on the reaction of aziridines with nitriles and carbonyls:Synthesis of imidazolines and oxazolidines[J].J.Org.Chem.2007,72:2133-2142. [14]Wu J,Sun X,Xia H G.Sc(OTf)(3)-catalyzed[3+2]-cycloaddition of aziridines with nitriles under solvent-free conditions[J].Tetrahedron Lett.2006,47:1509-1512.
[15]Li X,Yang X,Chang H.A new and efficient procedure for Bi(OTf)(3)-promoted[3+2]cycloaddition of N-tosylaziridines to yield imidazolines[J].Eur.J.Org.Chem. 2011:3122-3125.
[16]Ghorai M K,Das K,Ghosh K.Copper(II)triflate promoted cycloaddition of alpha-alkyl or aryl substituted N-tosylaziridines with nitriles:a highly efficient synthesis of substituted imidazolines[J].Tetrahedron Lett.2006,47:5399-5403.
[17]Kang S B,Miller A W,Nguyen S T.Sc(OTf)(3)-catalyzed condensation of 2-alkyl-N-tosylaziridine with aldehydes or ketones:an efficient synthesis of 5-alkyl-1,3-oxazolidines [J].Chem.Commun.2009:3928-3930.
[18]Maeda R,Ishibashi R,Hanamoto T.AgSbF6-promoted cycloaddition reaction of 2-trifluoromethyl-N-tosylaziridine with aldehydes[J].Org.Lett.2011,13:6240-6243.
[19]Hancock M T,Pinhas A R.A convenient and inexpensive conversion of an aziridine to an oxazolidinone[J].Tetrahedron Lett.2003,44:5457-5460.
[20]Sudo A,Koizumi E,Senda F,Endo T.Highly efficientchemical fixations of carbon dioxide and carbon disulfide by cycloaddition to aziridine under atmospheric pressure[J]. Tetrahedron Lett.2003,44:7889-7891.
[21]Sudo A,Senda F,Endo T.N-tosylaziridine,a new substrate for chemical fixation of carbon dioxide via ring expansion reaction under atmospheric pressure[J].Tetrahedron Lett.2004,45:1363-1365.
[22]Mu W H,Chasse G A,Fang D C.High level a initio exploration on the conversion of carbon dioxide into oxazolidinones:The mechanism and regioselectivity[J].J.Phys. Chem.A.2008,112:6708-6714.
[23]Phung C,Pinhas A R.The high yield and regioselective conversion of an unactivated aziridine to an oxazolidinone using carbon dioxide with ammonium iodide as the catalyst [J].Tetrahedron Lett.2010,51:4552-4554.
[24]Wu Y,Liu G.Organocatalyzed cycloaddition of carbon dioxide to aziridines[J].Tetrahedron Lett.2011,52:6450-6452.
[25]Shen Y M,Duan W L,Shi M.Chemical fixation of carbon dioxide co-catalyzed by a combination of Schiff bases or phenols and organic bases[J].Eur.J.Org.Chem.2004: 3080-3089.
[26]Soga K,Hosoda S,Ikeda S.A new synthetic route to 2-oxazolidones[J].J.Chem.Soc.Chem.Commun.1976:617-617. [27]Kawanami H,Ikushima Y.Regioselectivity and selective enhancement of carbon dioxide fixation of 2-substituted aziridines to 2-oxazolidinones under supercritical conditions[J].Tetrahedron Lett.2002,43:3841-3844.
[28]Kawanami H,Ikushima Y.Effective ScCO2-ionic liquid reaction system based on symmetric aliphatic ammonium salts for the rapid CO2fixation with aziridine to 2-oxazolidinone[J].Chem.Lett.2005,34:60-61.
[29]Jiang H F,Ye J W,Huang L B.Naturally occurring alphaamino acid:a simple and inexpensive catalyst for the selective synthesis of 5-aryl-2-oxazolidinones from CO2and aziridines under solvent-free conditions[J].Tetrahedron Lett.2010,51:928-932.
[30]Miller A W,Nguyen S T.(Salen)chromium(III)/DMAP: An efficient catalyst system for the selective synthesis of 5-substituted oxazolidinones from carbon dioxide and aziridines[J].Org.Lett.2004,6:2301-2304.
[31]Wu Y,Miao C X,Li W.Zirconyl chloride:an efficient recyclable catalyst for synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2under solvent-free conditions[J]. Tetrahedron.2009,65:6204-6210.
[32]Du Y,Wu Y,He L N.Quaternary ammonium bromide functionalized polyethylene glycol:A highly efficient and recyclable catalyst for selective synthesis of 5-aryl-2-oxazolidinones from carbon dioxide and aziridines under solvent-free conditions[J].J.Org.Chem.2008,73:4709-4712. [33]Chaorong Q,Jinwu Y,Wei Z,Huanfeng J.Polystyrenesupported amino acids as efficient catalyst for chemical fixation of carbon dioxide[J].Adv.Synth.Catal.2010,352: 1925-1933.
[34]Watile R A,Bagal D B,Bhanage B M.Regioselective synthesis of 5-aryl-2-oxazolidinones from carbon dioxide and aziridines using Br-Ph3+PPEG(600)P+Ph3Br-as an efficient,homogenous recyclable catalyst at ambient conditions.Tetrahedron Lett[J].2011,52:6383-6387.
[35]Yang Z Z,Peng S Y,He L N.Lewis basic ionic liquidscatalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2under solvent-free conditions[J].Green Chem.2010,12:1850-1854.
[36]Yang Z Z,He L N.He.Protic onium salts-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2under mild conditions[J].Green Chem.2011,13:2351-2353 [37]Dou X Y,He Z Z,Wang J L,He L N.Catalyst-free process for the synthesis of 5-aryl-2-oxazolidinones via cycloaddition reaction of aziridines and carbon dioxide[J]. synlett.2010,14:2159-2163.
[38]Wu J Y,Luo Z B,Dai L X,Hou X L.Tributylphosphinecatalyzed cycloaddition of aziridines with carbon disulfide and isothiocyanate[J].J.Org.Chem.2008,73:9137-9139
[39]Mani S,Tharmalingam P.“On Water”:efficient iron-catalyzed cycloaddition of aziridines with heterocumulenes[J]. Angew.Chem.Int.Ed.2013,52:572-575.
The Research Progress in the[3+2]Cycloaddition of Azirdines
REN Hong,HU Bao-Xiang
(College of Chemical Engineering,Zhejiang University of Technology,Hangzhou,Zhejiang 310014,China)
The research of the[3+2]cycloaddition of azirdines and compounds with double bond,for instance,alkene,alkyne,aldehyde and carbon dioxide,was reviewed.The developing prospects was also discussed.
azirdines;[3+2]cycloaddition;research progress
1006-4184(2015)1-0022-07
2014-04-16
国家自然科学基金资助项目(编号:20876149)。
任鸿(1989-),男,浙江东阳人,硕士研究生,主要从事有机合成研究。E-mail:renhong1004@163.com。
