Synthesis and Lum inescence Property of A Novel Blue-em itting BaKBP2O8:Eu2+Phosphor
2015-09-12HUANGJunSHAOZhimengRENYinbaoZHAOQingerHONGJiadanWANGQianDENGDegangYUHuaXUShiqing
HUANG Jun,SHAO Zhi-meng,REN Yin-bao,ZHAO Qing-er, HONG Jia-dan,WANG Qian,DENG De-gang*,YU Hua,XU Shi-qing*
(1.College ofMaterials Science and Engineering,China Jiliang University,Hangzhou 310018,China; 2.College ofMaterials and Environmental Engineering,Hangzhou Dianzi University,Hangzhou 310018,China) *Corresponding Authors,E-mail:dengdegang@cjlu.edu.cn;sxucjlu@hotmail.com
Synthesis and Lum inescence Property of A Novel Blue-em itting BaKBP2O8:Eu2+Phosphor
HUANG Jun1,SHAO Zhi-meng1,REN Yin-bao1,ZHAO Qing-er1, HONG Jia-dan1,WANG Qian1,DENG De-gang1*,YU Hua2,XU Shi-qing1*
(1.College ofMaterials Science and Engineering,China Jiliang University,Hangzhou 310018,China; 2.College ofMaterials and Environmental Engineering,Hangzhou Dianzi University,Hangzhou 310018,China) *Corresponding Authors,E-mail:dengdegang@cjlu.edu.cn;sxucjlu@hotmail.com
Novel efficient blue phosphors Ba1-xKBP2O8:x Eu2+were successfully synthesized by high-temperature solid state reaction.The structure and luminescence propertieswere characterized systematically by XRD and fluorescence spectra,respectively.The results show that Eu2+doping does not produce significant changes for the crystal structure which remains pure tetragonal.Excitation spectrum(monitoring wavelength is 443 nm)is composed of the acromion at307 nm due to host absorption,and themain peak at346 nm due to 4f7-4f65d transition Eu2+ions.Under violet light excitation,the samples emit blue light.When themole fraction of Eu2+is 0.03,the emission spectrum has amaximum intensity.However,with further increase of the concentration of Eu2+,the emission spectrum intensity decreases,as a result of concentration quenching.At temperatures over 370 K,the intensity of the phosphors is stillmore than 50%.The CIE chromaticity coordinate of BaKBP2O8:Eu2+is(0.176 6,0.168 1).
phosphors;BaKBP2O8:Eu2+;blue-emitting
1 Introduction
Since 1997,Nichia chemical company successfully developed a blue light-emitting diode(LED), the semiconductor solid-state lighting technology has been rapid development.Because of their high efficiency,compactness,good material stability,long operational lifetime,energy saving and environmental protection,white-emitting diodes(LED)are increasingly widely used in backlight,general lighting,etc.and will be fourth-generation lighting following the incandescent,fluorescent and HID lamps[1-7].Currently,the most common approach for manufacturing white LEDs is using yellow-emitting Y3Al5O12:Ce3+(YAG:Ce3+)phosphors with a blue InGaN/GaN LED chip.However,the white light obtained in this way has bad color reproduction,and the luminous intensity and colors easily affected by environmental conditions[8].To solve these problems,people try to use the near-ultraviolet (UV)radiation(350-410 nm)to excite trichromatic phosphor for achieving the white light.Because of the human eye less sensitive to 350-410 nm wavelength,the type ofwhite LED is only determined by the color of the phosphor[9].
At present,the blue phosphor excited by near ultraviolet study focused onaluminosilicate[10],silicates[11-16]and nitrogen oxide matrix[17-21].However,these materials generally have a higher sintering temperature,and easy deliquescent.In this way, Boron phosphate substrate which has lower sintering temperature and higher quantum efficiency is a potential three-color fluorescentmaterial[19].As early as 100 years ago,boron phosphate group phosphor was synthesized[21],recent studies have focused on the application in the high-pressuremercury of these materials,aswell as the impactof structural changes in the law luminescent properties[21].
In the phosphor material system,it was found Eu2+ions can substitute for Ba2+ions into thematrix lattice because Ba2+ions and Eu2+ions with the same ion valence,and the ionic radius of Ba2+ion being larger radius than Eu2+ions[22].With violet excitation,BaKBP2O8matrix doped Eu2+emit visible light.Moreover,such materials have advantage of high luminous efficiency,long life,good stability and aging resistance,strong UV absorption,etc.To the best of our knowledge,the blue-emitting phosphate phosphors of BaKBP2O8:Eu2+have not been reported in the literature.
In this paper,a series of BaKBP2O8:Eu2+sampleswere synthesized by conventional high-temperature solid-phasemethod,which can be effectively excited by near 443 nm violet and blue fluorescence emitted.We studied BaKBP2O8:Eu2+phosphor structure,emission spectra,excitation spectra, doping concentration of Eu2+affect on the emission spectrum and the excitation spectrum,and its CIE color coordinates.
2 Experiments
Ba1-xKBP2O8:x Eu2+phosphorswere prepared by solid-state reaction method under reductive atmosphere.The raw materials were BaCO3(A.R., 99.9%),K2CO3(A.R.,99.9%),H3BO3(A. R.,99.9%),NH4H2PO4(A.R.,99.9%)and Eu2O3(A.R.,99.99%).Stoichiometric amounts of staringmaterialswere thoroughly mixed in an agatemortar by grinding and sintered at 1 000℃in reductive atmosphere(5%H2/95%N2)for 3 h.
X-ray diffraction(XRD)pattern was collected by an X-ray diffractometer(Bruker Axs D2 PHASER diffractometer)with Cu Kαradiation(λ=0.154 06 nm)over the angular range 10°≤2θ≤80°,operating at30 kV and 10 mA.The excitation and emission spectrum of phosphorswere recorded by a PL3-211-P spectrometer(HORIBA JOBIN YVON, America)and a 450 W xenon lamp was used as the excitation source.All tests were performed at room temperature.
3 Results and Discussion
XRD patterns of Ba1-xKBP2O8:x Eu2+phosphors and BaKBP2O8ICSD standard pattern are shown in Fig.1.A single phase of Ba2Ca(PO4)2without any impuritieswas obtained and all of the peaks were in good agreement with the JCPDS card[BaKBP2O8,ICSD 249930],indicated that the doped Eu2+ions have no obvious influence on the structure of the host of BaKBP2O8,even at various Eu2+doping concentrations.The crystal space group is I-42d(122),with unit cell parameters a=0.720 2(2)nm,b/c= 0.503 6,c/a=1.985 6,cell volume V=0.741 72 (43)nm3,and no generate impurity phase.Compared with the BaKBP2O8standard card,the peak positions of the diffraction patterns shift toward larger angle,which is due to the substitution of the lager Ba2+ions by the smaller Eu2+ions.
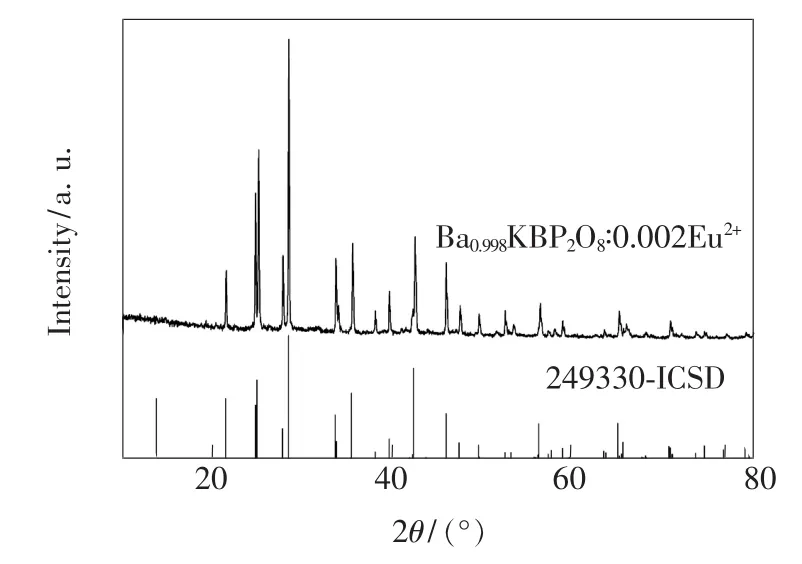
Fig.1 XRD patterns of BaKBP2O8:Eu2+phosphors and BaKBP2O8ICSD standard pattern
Researchers carried out a detailed study on the crystal structure of BaKBP2O8and the coordination of the centralmetal ion.The results showed that the crystal structure of BaKBP2O8belongs to tetragonal which has I-42d(122)space group,as shown in Fig.2(a).P-O and B-O are both tetrahedron polyhedron,and K/Ba-O is eight coordination polyhedron,as shown in Fig.2(b).The ionic radii of the eight-coordinated Ba2+is 0.142 nm.Similarly,the ionic radii of the eight-coordinated Eu2+is 0.139 nm,respectively.Because of the similarities of their ionic radii,Eu2+ions are expected to randomly occupy Ba2+ion sites in Ba1-xKBP2O8:x Eu2+crystal structure.
The excitation and emission spectra of Ba0.98-KBP2O8:0.02Eu2+phosphor are shown in Fig.3. Excitation spectrum(monitoring wavelength 443 nm) is coverage for a wide spectrum of 270-410 nm with the fullwidth at halfmaximum(FWHM)of100 nm, which is composed of the acromion at307 nm due to host absorption,and themain peak at346 nm due to 4f7-4f65d transition Eu2+ions.Such phosphors can be used to violet chip at the emission peak of 365 nm.The emission spectra cover a wide range of wavelength 400-600 nm with the FWHM of 90 nm under 346 nm excitation.And its peaks at443 nm, corresponding to Eu2+ions 4f65d-4f7transition.In addition to the emission intensity changes,the emission peak of the emission spectra do not change significantly.It is proved that there is only one luminous environment in the matrix.Eu2+ion luminescence covers all visible light emission from near UV to red.As the light-emitting ismore easily affected by the environment in which the crystal field,the energy emitted wave is determined by itsmatrix environment.It is consistent with the above mention crystal structure analysis.
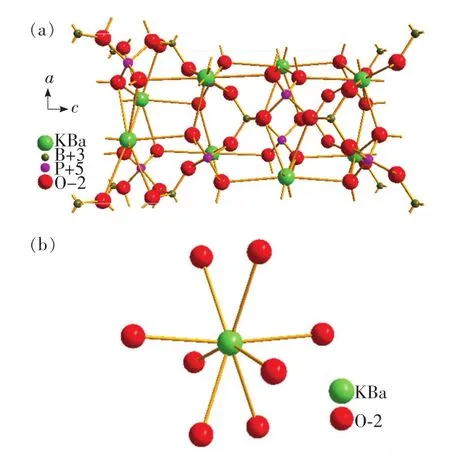
Fig.2 (a)Crystal structure pattern of BaKBP2O8.(b)Coordination pattern of K/Ba.
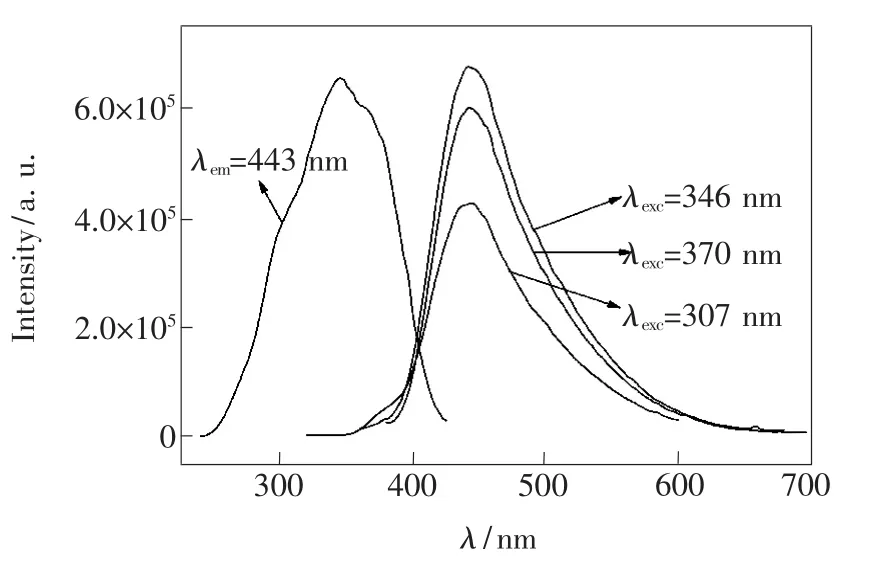
Fig.3 Excitation and emission spectra of Ba0.98KBP2O8: 0.02Eu2+
Excitation and emission spectra of Ba1-xKBP2O8: x Eu2+with different Eu2+mole fraction are shown inFig.4 and Fig.5,respectively.As can be seen in Fig.4,the peak shape of the excitation spectrum substantially unchanged with increasing the concentration of Eu2+ions.As shown in Fig.5,under 346 nm excitation,the position of emission peak happened red shift with the increase of Eu2+concentration,and the intensity of the emission spectra changed significantly.
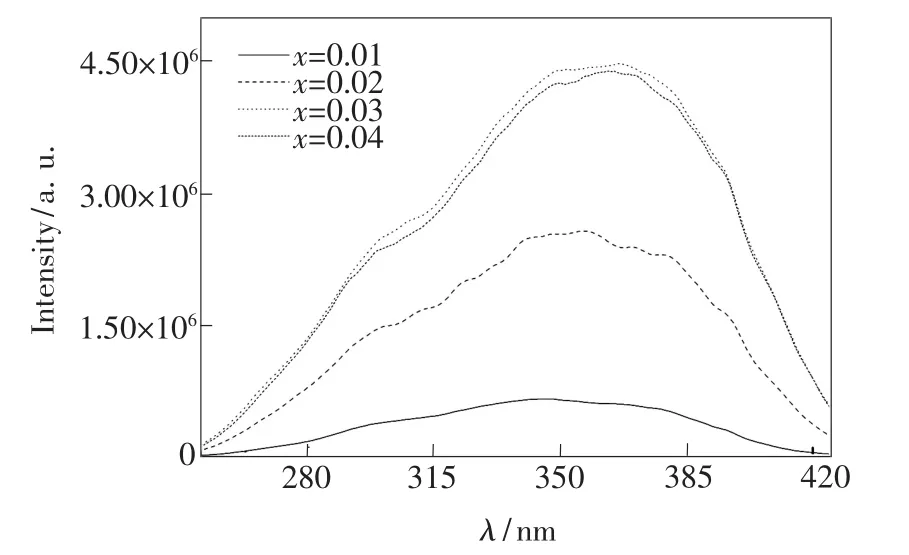
Fig.4 Excitation spectra of Ba1-xKBP2O8:x Eu2+with different Eu2+mole fraction
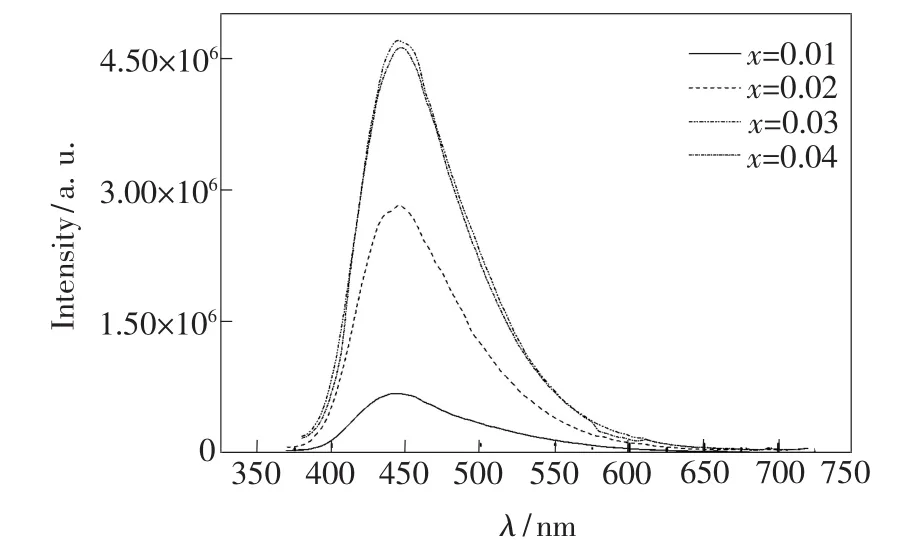
Fig.5 Emission spectra of Ba1-xKBP2O8:x Eu2+with different Eu2+mole fraction
The emission intensity of Ba1-xKBP2O8:x Eu2+with different Eu2+mole fraction is shown in Fig.6. The emission intensity increaseswith the increase of Eu2+mole fraction up to amaximum x value of about 0.03,after which it decreases,as shown in Fig.6. This is the result of concentration quenching.When the rate of energy transfer between Eu2+ions increases rapidly greater than the transmission rate,the excitation energy can be consumed by latticemigration.
According to Dexter's theory[23],we have calculated the critical distance between the Eu2+ions for energy transfer by using the relation given by Blasse and Grabmaie[24].
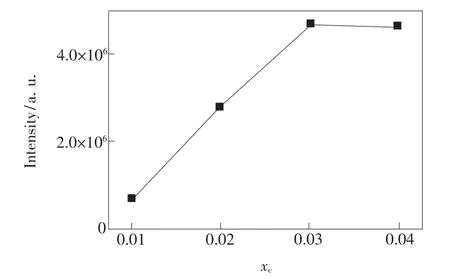
Fig.6 Emission intensity of Ba1-xKBP2O8:x Eu2+vs.Eu2+mole fraction
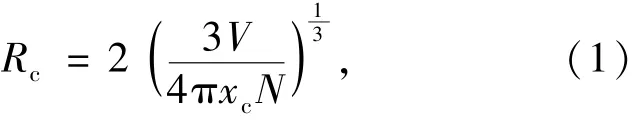
where V is the volume of the unit cell,xcis the critical concentration of activator,N is the number of formula units per unit cell.In BaKBP2O8phosphor activated by Eu2+,V=0.741 72 nm3,xc=0.03, N=4.The obtained Rcis 0.707 6 nm.
Fig.7 shows the temperature dependence of the relative emission intensity of Ba0.98KBP2O8:0.02Eu2+phosphor.We collect and processed the emission intensity of Ba0.98KBP2O8:0.02Eu2+phosphor monitored at346 nm under a continuously change of operating temperature(K)to explore the effect on the thermal stability of the phosphor.With the increase of the temperature,while the thermal stability of the phosphor decreased gradually.In excess of 370 K, the intensity of the phosphors is still more than 50%.
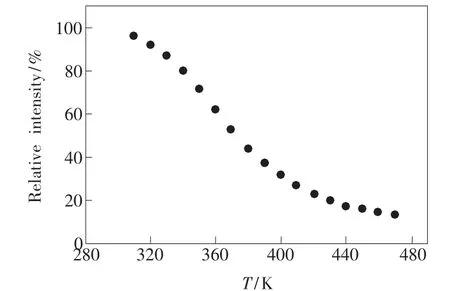
Fig.7 Relationship of the emission intensity of Ba0.98KBP2O8: 0.02Eu2+phosphor and operating temperature
The CIE chromaticity coordinates of Ba0.98KBP2O8: 0.02Eu2+is shown in Fig.8.The CIE chromaticity coordinates of BaKBP2O8:Eu2+is(0.176 6,0.168 1) in the blue region.The result reveals that BaKBP2O8:Eu2+is a potential blue-emitting phosphor for white LEDs.
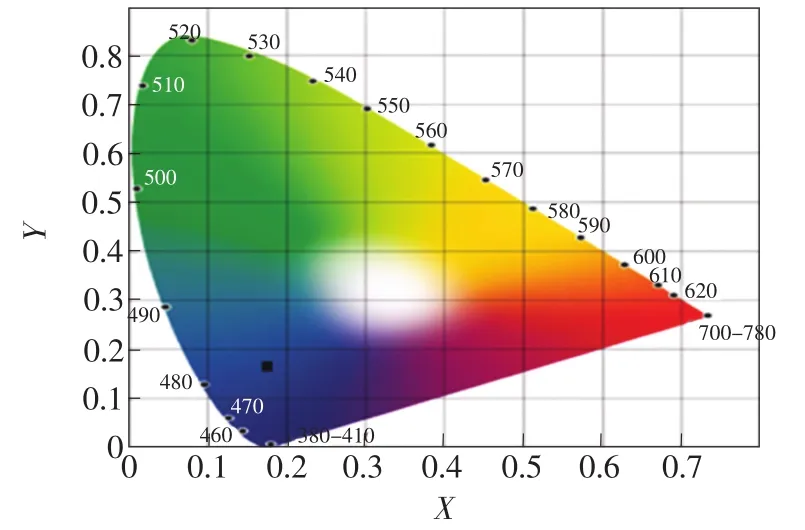
Fig.8 CIE chromaticity coordinates of BaKBP2O8:Eu2+
4 Conclusion
Ba1-xKBP2O8:x Eu2+can be obtained by using conventional solid-state reaction method at 1 000℃in a reductive atmosphere for 3 h and then cooled down slowly to room temperature.Excitation spectrum of this phosphor is composed of the acromion at 307 nm due to hostabsorption,and themain peak at 346 nm due to 4f7-4f65d transition Eu2+ions.Since there is only one light emitting environment in matrix,only one emission peak of the phosphor is located at 443 nm.The emission spectral intensity increases with the gradual increase of Eu2+ions concentration,however,concentration quenching occurs finally.And the emission peaks red shift.The optimum distance of energy transfer between Eu2+ions is 0.707 6 nm.At temperatures over 370 K,the intensity of the phosphors is stillmore than 50%.The CIE color coordinates indicate that the phosphors have higher efficiency in emitting blue light and better color purity than conventional phosphors at the near UV LED excitation.
[1]Schubert E F,Kim JK.Solid-state light sources getting smart[J].Science,2005,308(5726):1274-1278.
[2]Narukawa Y,Narita J,Sakamoto T,et al.Recent progress of high efficiency white LEDs[J].Phys.Stat.Sol.(a), 2007,204(82):2078-2093.
[3]Sun L,Zhang Y,Hu X K,et al.Synthesis and luminescent properties of KZn4(BO3)3:Eu3+phosphors for LED[J]. Chin.J.Liq.Cryst.Disp.(液晶与显示),2014,29(6):893-900(in Chinese).
[4]Zhou Q C,Bai Z L,Lu L.Research progress and prospectof remote phosphor LED forwhite light[J].Chin.Opt.(中国光学),2015,8(3):313-328(in Chinese).
[5]Chen F,Yuan X M,Xie A,etal.Preparation and luminescence properties of Sr3(PO4)2:Tb3+,Li+forwhite LED[J]. Chin.J.Liq.Cryst.Disp.(液晶与显示),2011,26(4):448-454(in Chinese).
[6]Zhang JH,Lv W,Hao Z D,et al.Bymeans of energy transfer can be adjusted panchromatic single white BaMg2Al6Si9-O30:Eu2+Tb3+,Mn2+phosphor(guest)[J].Chin.Opt.(中国光学),2012,5(3):203-208(in Chinese).
[7]Xie Y,Wang T,Wang H B.Alkaline earth,rare earth doped on luminescence properties of Y(VP)O4:Eu3+phosphor [J].Chin.J.Liq.Cryst.Disp.(液晶与显示),2011,26(5):587-591(in Chinese).
[8]Setlur A A,Heward W J,Hannah M E,et al.Incorporation of Si4+-N3-into Ce3+-doped garnets for warm white LED phosphors[J].Chem.Mater.,2008,20(19):6277-6283.
[9]Steigerwald D A,Bhat JC,Collins D,et al.Illumination with solid stste lighting technology[J].IEEE J.Sel.Top. Quant.Elect.,2002,8(2):310-320.
[10]Sheu j k,Chang S J,Kuo C H,et al.White-light emission from near UV InGaN-GaN LED chip precoated with blue/ green/red phosphors[J].IEEE Photon.Technol.Lett.,2003,15(1):18-20.
[11]Yoo JS,Kim SH,Yoo W T,et al.Control of spectral properties of strontium-alkaline earth-silicate-europium phosphors for LED applications[J].J.Electrochem.Soc.,2005,152(5):G382-G385.
[12]Lakshminarasimhan N,Varadaraju U V.Influence of6s2lone pair electrons of Bi3+on its preferential site occupancy in fluorapatite,NaCa3Bi(PO4)3F—An insight from Eu3+luminescent probe[J].J.Electrochem.Soc.,2005,152(9):H152-H156.
[13]Liu J,Sun JY,Shi C S.A new luminescentmaterial:Li2CaSiO4:Eu2+[J].Mater.Lett.,2006,60(23):2830-2833.
[14]Saha S,Chowdhury PS,Patra A.Luminescence of Ce3+in Y2SiO5nanocrystals:Role of crystal structure and crystal size[J].Phys.Chem.B,2005,109(7):2699-2702.
[15]Choi K J,Park JK,Kim K N,et al.Luminescence characteristics of Sr3MgSi2O8:Eu blue phosphor for light-emitting diodes[J].Electrochem.Solid-State Lett.,2004,7(10):H42-H43.
[16]Umetsu Y,Okamoto S J.Photoluminescence properties of Ba3MgSi2O8:Eu2+blue phosphor and Ba3MgSi2O8:Eu2+,Mn2+blue-red phosphor under near-ultraviolet-light excitation sensors and displays:Principles,materials,and processing[J]. Electrochem.Soc.,2008,155(7):J193-J197.
[17]Li Y Q,Delsing A C A,De With G,et al.Luminescence properties of Eu2+-activated alkaline-earth silicon-oxynitride M Si2O2-N2+2/3(M=Ca,Sr,Ba):A promising class of novel LED conversion phosphors[J].Chem.Mater,2005,17 (12):3242-3248.
[18]Li Y Q,With G D,Hintzen H T.Luminescence properties of Ce3+-activated alkaline earth silicon nitride M2Si5N8(M= Ca,Sr,Ba)materials[J].J.Lumin.,2006,116(1):107-116.
[19]Xu X R,Su M Z.Luminescence and Luminescent Material[M].Beijing:Chemical Industry Press,2004:293-330(in Chinese).
[20]Van Klooster H S.Uber das verhalten der metaborund der metaphosphorsaure in den schmelzen ihrer alkalisalze[J]. Anor.Allg.Chem.,1997,19(11):122-134.
[21]Kniep R.Borophosphates—A neglected class of compounds crystal structures of Mu(BPOs)(Mu=Ca,Sr)and Ba3(BP3O12)[J].Angew.Chem.Int.,1994,33(7):749-751.
[22]Zhang R,Maeda T,Maruta R.Luminescence enhancementof Eu2+,Ce3+co-doped Ba3Si5O13-N phosphors[J].J.Solid State Chem.,2010,183(3):620-623.
[23]Dexter D L.A theory of sensitized luminescence in solids[J].J.Chem.Phys.,1953,21(5):836-850.
[24]Blasse G.Energy Transfer in Oxidic Phosphors[R].Philips Res.Rep.,1969,24:131-136.

黄君(1990-),男,浙江杭州人,硕士研究生,2013年于中国计量学院获得学士学位,主要从事稀土发光及荧光粉方面的研究。
E-mail:410043564@qq.com

徐时清(1975-),男,四川绵阳人,博士,教授,2005年于中国科学院上海光学精密机械研究所获得博士学位,主要从事光电功能材料与器件的研究。
E-mail:sxucjlu@hotmail.com
一种新型蓝光BaKBP2O8:Eu2+荧光粉的合成与性能研究
黄 君1,邵智猛1,任印宝1,赵情儿1,洪佳丹1,王 倩1,邓德刚1*,余 华2,徐时清1*
(1.中国计量学院材料科学与工程学院,浙江杭州 310018; 2.浙江电子科技大学材料与环境工程学院,浙江杭州 310018)
通过高温固相法成功合成了一种新型蓝光荧光粉BaKBP2O8:Eu2+,分别用XRD和荧光光谱表征了其结构与光学性能。结果表明,Eu2+的引入并没有显著改变其四方相的晶体结构。样品的激发光谱(监测波长为443 nm)是由主晶格吸收的307 nm吸收肩与Eu2+离子4f7-4f65d跃迁的346 nm主峰组成。在紫光激发下,样品的发射光谱为蓝色。当Eu2+离子摩尔分数为0.03时,样品的发射强度达到最大值。然而,随着Eu2+浓度的进一步增加,由于浓度猝灭机制,其发射强度开始降低。在超过370 K的温度下,荧光粉样品的相对发光强度仍然超过50%。BaKBP2O8:Eu2+的色坐标为(0.176 6,0.168 1)。
荧光粉;BaKBP2O8:Eu2+;蓝光发射
1000-7032(2015)10-1126-06
2015-06-26;
2015-07-28
浙江省自然科学基金(LR14A040002,LQ13E020003,LZ14F050001);国家自然科学基金(61008042,51272243,61370049);浙江省大学生科技创新项目(2014R409035)资助
O482.31 Document code:ADOI:10.3788/fgxb20153610.1126
