基于不同毒性终点的双酚A(BPA)预测无效应浓度(PNEC)研究
2015-06-27冯承莲汪浩王颖吴丰昌
冯承莲,汪浩,王颖,2,吴丰昌,*
1. 中国环境科学研究院 环境基准与风险评估国家重点实验室,北京 100012 2. 北京师范大学 水科学研究院,北京 100875
基于不同毒性终点的双酚A(BPA)预测无效应浓度(PNEC)研究
冯承莲1,汪浩1,王颖1,2,吴丰昌1,*
1. 中国环境科学研究院 环境基准与风险评估国家重点实验室,北京 100012 2. 北京师范大学 水科学研究院,北京 100875
双酚A(BPA)已被证实是一种类雌激素类物质。本研究根据BPA对水生生物毒性效应的特点,按照不同的毒性终点将BPA的毒性数据进行归类,采用物种敏感度分布法(species sensitivity distribution, SSD)推导了BPA对水生生物的预测无效应浓度(predicted no effect concentration, PNEC)。结果表明:以雌激素效应为暴露终点的急、慢性PNEC分别为25.11 μg·L-1、1.075 μg·L-1;而以所有数据的急、慢性毒性效应为暴露终点推导的PNEC值分别为355.7 μg·L-1、7.549 μg·L-1。BPA对水生生物的雌激素效应更为敏感,建议在推导BPA这类内分泌干扰物的PNEC值时,应依据其毒性终点分别推导,从而得到更加合理的基准值。研究成果以期为我国地表水环境质量标准的制修订提供数据支持。
双酚A;淡水生物;雌激素效应;内分泌干扰;预测无效应浓度(PNEC)
双酚A(Bisphenol A, BPA)是重要的化工原料,被广泛应用于众多行业的环氧树脂、聚碳酸酯和聚酚氧树脂等材料的合成过程。据统计,每年约有270万吨BPA被生产使用,其中超过100吨进入大气中[1]。研究发现BPA具有雌激素效应,它对雌激素受体的亲和性是雌二醇的万分之一,过量摄入BPA能导致机体类分泌活动紊乱,从而影响生殖功能,破坏生殖系统,导致细胞癌变和器官发育畸形[2-3]。BPA能诱导生物体卵黄蛋白原(vitellogenin, VTG)含量增加,干扰生殖系统的功能和破坏生殖器官组织,以及对生物的生殖过程产生影响。环境中的BPA主要来源于工业废水、污水处理厂和垃圾填埋场渗滤液[4-5]。许多研究都提出塑料奶瓶以及可循环使用水桶等材料均含有BPA,由于BPA制品的大量使用,导致河流、湖泊以及沿海水域等都不同程度地有BPA存在[6-7]。国外如欧洲莱茵河、新加坡海岸周边水域、韩国西瓦湖及其周边河流、日本东京湾等部分水体中均有不同浓度的BPA被检测出来,浓度水平范围从数ng·L-1到数μg·L-1[8-11]。国内关于BPA在水体中的含量水平也有很多报道,如珠江流域、长江流域、辽河流域等地区,浓度范围在数ng·L-1到数十μg·L-1之间,跟国外部分水体的浓度水平相差不大[12-17]。
预测无效应浓度(predicted no effect concentration, PNEC)研究的基础是污染物的毒性数据。也是水质基准研究的重要依据。根据污染物毒性效应的不同,水质基准一般可分为急性和慢性基准。急性水质基准是为了应对环境污染的突发事件,而慢性水质基准主要是应对水生生态环境的日常维护和管理。目前关于BPA的PNEC研究相对较少。Staples等[18-19]比较深入地开展了BPA的PNEC研究,推导出BPA的PNEC为64 μg·L-1,随后在原有工作的基础上,用美国、荷兰、加拿大的推导方法再次推导BPA的PNEC的范围是11~71 μg·L-1。Wright-Walters等[20]用数据权重校正法推导出BPA在水体中的PNEC值为0.06 μg·L-1。日本、欧盟和加拿大等国家和组织都曾开展了BPA的PNEC相关研究工作,公布的BPA的PNEC分别为1.6 μg·L-1、1.5 μg·L-1、0.175 μg·L-1[21-23]。我国只有《食品容器及包装材料用聚碳酸酯树脂卫生标准》(GB14942—1994)中对酚类的浓度有相关规定[24],规定每升蒸馏水中含有酚类须≤0.05 mg。而关于BPA的地表水环境质量标准和其他行业标准,目前还没有基准限值。
不同的物种对同一污染物的敏感度不同,生物区系的差异会最终导致基准值的差异[25-26]。我国地理环境、气候以及生态环境要素、污染类型及分布模式较国外有很大差异性,而且经济发展阶段、文化多元化、人民生活方式和消费习惯与国外也有很大不同,因此水质基准不能直接拿国外的基准直接使用,而应该根据我国的区域特征进行基准的研究。目前我国水质基准研究近几年已经陆续开展,对常规污染物如重金属、有机物的基准研究相对较多[27-30]。而对于内分泌干扰物水质基准的研究相对较少[31-32]。内分泌干扰物由于在较低的浓度下就可能对水生生物产生不可逆转的毒性效应,影响生物的繁殖、发育等性状,因此不能简单地用污染物的致死效应进行PNEC的研究,需要结合其他敏感毒性终点开展PNEC的研究。基于此,本研究以BPA为例,对这类物质的PNEC开展探索性研究,以期为我国水质基准的研究提供重要的数据支持。
1 研究方法(Methodology)
1.1 数据收集及筛选
本研究收集整理了BPA毒性数据,数据主要来自美国环保局ECOTOX数据库和已经发表的文献。所选淡水物种为中国本土物种和已经在中国普遍存在的引进物种,暴露方式为流水暴露,急性毒性效应终点主要选取48 h~96 h内的半数效应浓度(EC50)、半数致死浓度(LC50)值;慢性毒性效应终点主要选取最低可见效应浓度(LOEC)、无可见效应浓度(NOEC)值。如果同一物种有多个符合条件的毒性数据时,则采用其几何均值作为最后的毒性数据[33-36]。如果同一物种的毒性值相差10倍以上,或者某一个物种的毒性值相对所有的毒性数据离散度过高,则视为异常值,将其去除。有些学者也建议采用10%效应浓度EC10、25%效应浓度EC25推导PNEC[37],本研究优先采用受试生物的LOEC、EC50、LC50值。把符合上述条件的实验数据按照急性、慢性分为2类,再分别把毒性效应终点按照雌激素效应以及其他毒性效应进行归类,然后对比分析。
1.2 物种敏感度分布法
物种敏感度分布(species sensitivity distribution, SSD)法是基于不同的物种对同一污染物的敏感度不同的理论,认为不同物种对同一污染物的敏感性差异遵循一定的概率分布模型,由不同物种毒性数据的频数分布拟合出某种概率分布函数,即敏感度分布曲线[38]。SSD法的具体步骤:(1)确定毒性数据的累积概率p,即把毒性数据由低到高排列并分配等级(1,2,…,N),并除以N+1可得到p值;(2)将毒性效应值按照不同的模型拟合成SSD曲线,选择最优模型确定最终的SSD曲线;(3)根据SSD曲线计算出危险浓度HCp,一般取p值为5,即保护95%以上的生物对应的浓度。本研究采用Origin 8软件构建SSD曲线,分别对各组毒性数据进行拟合,推导出各组的PNEC,对比分析各组结果。
2 结果(Results)
2.1 BPA毒性数据汇总
对收集的BPA毒性数据进行筛选,最终获得52个毒性数据,包括26个急性毒性数据和26个慢性毒性数据;急性毒性数据中有10个雌激素效应数据,慢性毒性数据中有16个雌激素效应数据。表1、表2为BPA对水生生物的毒性数据统计表。

表1 BPA急性毒性效应统计表
续表1

泥鳅MudfishMisgurnusanguillicadatusLC50966430鱼鳃结构破坏Damageofgillstructural[54]中国林蛙ChinesefrogRanachensinensisLC50727305死亡Death[55]大型蚤DaphniaDaphniamagnaEC50487750死亡Death[56]大扁藻TetraselmisPlatmonashelgolanidicaEC50969320细胞密度受抑制作用Inhibitionofcelldensity[57]微型裸腹溞MoinaMoinamicruraLC50489630死亡Death[58]隆线溞DaphniacarinataLC504811640死亡Death[58]轮虫RotiferaBrachionuscalyciflorusLC502413760死亡Death[59]日本沼虾JapanesecrayfishMacrobrachiumnipponenseLC504863900死亡Death[60]
注:LOEC为最低可见效应浓度,NOEC为无可见效应浓度;EC50为半数效应浓度,LC50为半数致死浓度值。
Note: LOEC stands for lowest observed effect concentration; NOEC stands for no observed effect concentration; EC50stands for 50% effect concentration; LC50stands for 50% lethal concentration.

表2 BPA慢性毒性效应统计表
续表2

其他毒性效应Othertoxiceffect淡水鲑鱼SalmonSalmosalarLOEC4210肝脏BPA含量影响BPAconcentrationwasaffectedinliver[70]虾虎鱼GobyAcanthogobiusflavimanusNOEC2125mRNA表达受到抑制InhibitionofmRNAgeneexpression[71]鲤鱼CommoncarpCyprinuscarpioLOEC12708肝细胞酶活性升高Riseofenzymeactivityinliver[51]青鳉MedakaOryziaslatipesNOEC60803身长和体重受到抑制Inhibitionofbodylengthandweight[72]摇蚊ChironomidaeChironomusripariusLOEC201000幼虫湿重增加Increaseoflarvaewetweight[73]端足虫MillipedeHyalellaaztecaLOEC421100体重和长度受到影响Bodylengthandweightwereaffected[64]斑马鱼ZebrafishBrachydaniorerioLOEC212000基因片段表达显著提高Significantincreaseofgeneexpression[74]水螅PolypsHydraoligactisLOEC353000触手变短Shortertentacles[68]大型蚤DaphniaDaphniamagnaLOEC215000增加蜕皮速率Increaseofmoltingrate[75]膨胀浮萍DuckweedlemnagibbaLOEC722000生长较对照组有明显变化Significantgrowthdifferencebetweencontrolandtreatedgroups[64]
2.2 BPA的PNEC推导
本研究分别采用不同的函数模型对获得的毒性数据进行曲线拟合,主要的模型包括SGompertz、Slogistic1、DoseResp、SRichards1、Boltzmann等。发现Slogistic1模型较其他模型对毒性数据有较好的拟合效果,但是不同的毒性数据最佳拟合模型略有不同。主要根据模型的决定系数(R2)和残差平方和(residual sum of squares, RSS)来确定最终的拟合模型,R2越大,RSS越小,拟合结果越准确。不同模型的拟合结果如表3所示:

表3 不同模型拟合参数统计表
注:粗体为最终选择的最佳模型对应的相关参数。
Note: The boldface numbers are the parameters of the best fitted model.
将所有的急性毒性数据、所有的慢性毒性数据、急性雌激素效应数据、慢性雌激素效应数据、急性其他毒性效应数据和慢性其他毒性效应数据分别用最佳模型进行拟合,结果如图1所示。最终通过计算,得出以所有数据的急、慢性毒性效应为暴露终点推导的PNEC值分别为355.7 μg·L-1、7.549 μg·L-1;而雌激素效应为暴露终点的急、慢性预测无效应浓度PNEC分别为25.11 μg·L-1、1.075 μg·L-1,以其他毒性效应终点推导的BPA的急、慢性PNEC分别为:3 023 μg·L-1、235.2 μg·L-1。
3 讨论(Discussion)
3.1 实验条件对毒性效应的影响
温度、暴露时间对毒性效应值有一定的影响。Oehlmann等[76]用苹果螺作为受试生物,进行了150 d的BPA暴露实验,发现20 ℃和27 ℃的EC10分别为14.8 ng·L-1和998 ng·L-1,温度对苹果螺的暴露终点浓度影响很大;对鹦鹉螺的暴露实验的数据表明鹦鹉螺在产卵期和非产卵期对BPA的暴露终点效应值也差别很大。Jobling等[39]把斑马鱼、虹鳟以及泥螺等暴露于不同浓度的BPA溶液中,在暴露实验的不同阶段,受试生物对同一浓度的BPA的响应值变化很大。Staples等[19]在推导美国水域中BPA的PNEC时数据较少,未考虑温度变化的影响;同时,在暴露时间段的选择上,采用了4 d~164 d的数据。本研究所收集到的实验数据绝大多数也未考虑温度变化对实验数据的影响,暴露实验的温度基本在18 ℃~25 ℃之间;毒性数据中,涉及到不同实验条件的毒性数据相对较少,我们根据暴露时间的不同,将所有数据按照急性和慢性进行了分类。随着对BPA的PNEC研究的不断深入,温度、酸碱度等其他环境因子也应该逐渐被涉及。
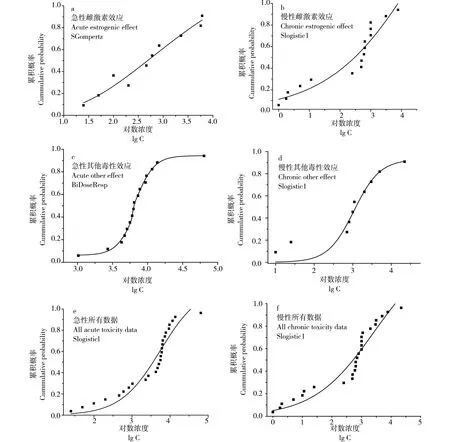
图1 BPA的物种敏感度分布曲线Fig. 1 Species sensitivity distribution of BPA
3.2 不同毒性终点获得的PNEC值比较
考虑到BPA的雌激素效应明显,因此本研究把BPA对水生生物较为敏感的雌激素效应终点单独分析,并与所有毒性数据得出的PNEC进行对比,结果发现:将所有的急性毒性数据不进行分类,得到的急性PNEC为355.7 μg·L-1,以所有急性毒性数据得出的PNEC值比单纯用雌激素效应得出的PNEC高出14倍。这一结果可能是由于急性暴露实验中受试生物对BPA的雌激素效应远高于致死等效应所致。以所有慢性毒性数据得出的PNEC值与单纯用雌激素效应数据得出的PNEC在同一数量级。当受试生物暴露于高剂量BPA流水环境中才会出现器官坏死、细胞变性、死亡等现象,在此之前BPA可能已经对受试生物产生雌激素效应,如对排卵量、精子活跃度、激素分泌等产生了影响。因此雌激素效应更能反映BPA对水生生物的危害,得出的PNEC值更能保护敏感水生生物。所以本研究根据雌激素效应暴露终点得出的BPA的急慢性PNEC分别为25.11 μg·L-1和1.075μg·L-1。由于类雌激素的化学物质对水生生物的毒性作用存在多种途径,因此建议在推导该类物质的PNEC时,应该依据暴露终点的不同分开研究,由最敏感的毒性终点确定最终的PNEC值,从而对水生生物进行更有利的保护。
3.3 与国外PNEC比较
将本研究获得的BPA的PNEC与其他国家推导的PNEC相比,发现相差不大。慢性PNEC与其他国家的保持在一个数量级范围(表4)。PNEC的差异很大程度上跟所采用的研究方法有关。从研究方法上来讲,其他国家大多采用评价因子法,即选用一个最敏感的物种,然后将其毒性值除以不确定性因子即得到最终的PNEC值。这种方法的优点在于它所需基础数据少、计算方法简单;但由于该法属于经验法,最终PNEC的值依赖于敏感生物的毒性值,所以不确定性很高。而本研究采用的物种敏感度分布法,充分利用了获得的所有物种的毒性数据,从某种程度上来说可以代表整个生态系统。当然这种方法的缺点就是由模型差异造成的最终基准值的差异很大。

表4 BPA的预测无效应浓度PNEC值比较
综上,本研究将BPA的毒性效应数据按照毒性终点进行分类后,得出的PNEC值差距较大,用雌激素效应终点推导出的PNEC更能保护敏感水生生物物种,得出的BPA急性和慢性PNEC分别为25.11 μg·L-1、1.075 μg·L-1。因此建议在推导这类内分泌干扰物的PNEC时,应基于我国淡水生物对该类物质的毒性终点不同分类推导,选择较为敏感的毒性终点,从而能够得到合理的PNEC。
[1] Vandenberg L N, Maffini M V, Sonnenschein C, et al. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption [J]. Endocrine Reviews, 2009, 30(1): 75-95
[2] Kuiper G G, Lemmen J G, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β [J]. Endocrinology, 1998, 139(10): 4252-4263
[3] Huang Y, Wong C, Zheng J, et al. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts [J]. Environment International, 2012, 42: 91-99
[4] Coors A, Jones P D, Giesy J P, et al. Removal of estrogenic activity from municipal waste landfill leachate assessed with a bioassay based on reporter gene expression [J]. Environmental Science & Technology, 2003, 37(15): 3430-3434
[5] Rodríguez-Mozaz S, Alda M L, Barcel D. Analysis of Bisphenol A in natural waters by means of an optical immunosensor [J]. Water Research, 2005, 39(20): 5071-5079
[6] Vandenberg L N, Hauser R, Marcus M, et al. Human exposure to Bisphenol A (BPA) [J]. Reproductive Toxicology, 2007, 24(2): 139-177
[7] Le H H, Carlson E M, Chua J P, et al. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons [J]. Toxicology Letter, 2008, 176(2): 149-156
[8] Hashimoto S, Horiuchi A, Yoshimoto T, et al. Horizontal and vertical distribution of estrogenic activities in sediments and waters from Tokyo Bay, Japan [J]. Archives of Environmental Contamination and Toxicology, 2005, 48(2): 209-216
[9] Lee H B, Peart T E. Organic contaminants in Canadian municipal sewage sludge. Part I. Toxic or endocrine-disrupting phenolic compounds [J]. Water Quality Research Journal of Canada, 2002, 37(4): 681-696
[10] Staples C, Dorn P B, Klecka G M, et al. Bisphenol A concentrations in receiving waters near US manufacturing and processing facilities [J]. Chemosphere, 2000, 40(5): 521-525
[11] Berkner S, Streck G, Herrmann R. Development and validation of a method for determination of trace levels of alkylphenols and Bisphenol A in atmospheric samples [J]. Chemosphere, 2004, 54(4): 575-584
[12] 龚剑, 冉勇, 杨余, 等. 珠江广州河段表层水中雌激素化合物的污染状况[J]. 环境化学, 2008, 27(2): 242-244
Gong J, Ran Y, Yang Y, et al. Contamination of estrogenic compounds in the surface water of Guangzhou reach of the Pearl River [J]. Environmental Chemistry, 2008, 27(2): 242-244 (in Chinese)
[13] 邵晓玲, 马军, 文刚. 松花江流域某自来水厂中内分泌干扰物的调查[J]. 环境科学, 2008, 29(10): 2723-2726
Shao X L, Ma J, Wen G. Investigation of endocrine disrupting chemicals in a drinking water work located in Songhua River Basin [J]. Environmental Science, 2008, 29(10): 2723-2726 (in Chinese)
[14] 李正炎, 傅明珠, 王馨平, 等. 冬季胶州湾及其周边河流中酚类环境激素的分布特征[J]. 中国海洋大学学报, 2006, 36(3): 451-455
Li Z Y, Fu M Z, Wang X, et al. Distribution of phenolic environmental hormones in Jiaozhou bay and its adjacent rivers in winter [J]. Periodical of Ocean University of China, 2006, 36(3): 451-455 (in Chinese)
[15] Bian H, Li Z, Liu P, et al. Spatial distribution and deposition history of nonylphenol and Bisphenol A in sediments from the Changjiang River (Yangtze River) Estuary and its adjacent East China Sea [J]. Acta Oceanologica Sinica, 2010, 29(5): 44-51
[16] Wang L, Ying G G, Zhao J L, et al. Assessing estrogenic activity in surface water and sediment of the Liao River system in Northeast China using combined chemical and biological tools [J]. Environmental Pollution, 2011, 159(1): 148-156
[17] Shi J H, Liu X W, Chen Q C, et al. Spatial and seasonal distributions of estrogens and Bisphenol A in the Yangtze River Estuary and the adjacent East China Sea [J]. Chemosphere, 2014, 111: 336-343
[18] Staples C, Dome P B, Klecka G M, et al. A review of the environmental fate, effects, and exposures of Bisphenol A [J]. Chemosphere, 1998, 36(10): 2149-2173
[19] Staples C, Woodburn K, Klecka G, et al. Comparison of four species sensitivity distribution methods to calculate predicted no effect concentrations for Bisphenol A [J]. Human and Ecological Risk Assessment, 2008, 14(3): 455-478
[20] Wright-Walters M, Volz C, Talbott E, et al. An updated weight of evidence approach to the aquatic hazard assessment of Bisphenol A and the derivation a new predicted no effect concentration (PNEC) using a non-parametric methodology [J]. Science of the Total Environment, 2011, 409(4): 676-685
[21] Nakanishi J, Miyamoto K, Kawasaki H. Bisphenol A Risk Assessment Document [R]. Tokyo: National Institute of Advanced Industrial Science and Technology, 2007
[22] Environment Canada, Health Canada. Updated risk assessment of 4,4'-isopropylidenediphenol (Bisphenol-A) [R]. Ottawa: Environment Canada, Health Canada, 2010
[23] Environment Canada, Health Canada. Screening Assessment for the Challenge Phenol, 4,4' (1-methylethylidene) bis- (Bisphenol A) [R]. Ottawa: Environment Canada, Health Canada, 2008
[24] 中华人民共和国卫生部. GB14942-94食品容器、包装材料用聚碳酸酯成型品卫生标准[S]. 北京: 中国标准出版社, 1994
[25] Wu F, Meng W, Zhao X, et al. China embarking on development of its own National Water Quality Criteria System [J]. Environmental Science & Technology, 2010, 44(21): 7992-7993
[26] Wu F, Feng C L, Zhang R Q, et al. Derivation of water quality criteria for representative water-body pollutants in China [J]. Science China: Earth Sciences, 2012, 55(6): 900-906
[27] 吴丰昌, 冯承莲, 曹宇静, 等. 锌对淡水生物的毒性特征与PNEC的研究[J]. 生态毒理学报, 2011, 6(4): 367-382
Wu F, Feng C L, Cao Y J, et al. Toxicity characteristic of zinc to freshwater biota and its water quality criteria [J]. Asian Journal of Ecotoxicology, 2011, 6(4): 367-382 (in Chinese)
[28] 吴丰昌, 冯承莲, 曹宇静, 等. 我国铜的淡水生物PNEC研究[J]. 生态毒理学报, 2011, 6(6): 619-630
Wu F, Feng C L, Cao Y J, et al. Aquatic life ambient freshwater quality criteria for copper in China [J]. Asian Journal of Ecotoxicology, 2011, 6(6): 619-630 (in Chinese)
[29] Feng C L, Wu F, Zhao X L, et al. Water quality criteria research and progress [J]. Science China: Earth Sciences, 2012, 55(6): 882-891
[30] 吴丰昌, 孟伟, 张瑞卿, 等. 保护淡水水生生物硝基苯PNEC研究[J]. 环境科学研究, 2011, 24(1): 1-10
Wu F, Meng W, Zhang R Q. Aquatic life water quality criteria for nitrobenzene in freshwater [J]. Research of Environmental Sciences, 2011, 24(1): 1-10 (in Chinese)
[31] Lei B L, Liu Q, Sun Y F, et al. Water quality criteria for 4-nonylphenol in protection of aquatic life [J]. Science China: Earth Sciences, 2012, 55(6): 892-899
[32] Wu F C, Fang Y X, Li Y S, et al. Predicted no-effect concentration and risk assessment for 17-[beta]-estradiol in waters of China [J]. Reviews of Environmental Contamination and Toxicology, 2014, 228: 31-56
[33] 孟伟, 吴丰昌. 水质基准的理论与方法学导论[M]. 北京: 科学出版社, 2010
[34] 吴丰昌等. 水质基准理论方法学及案例研究[M]. 北京: 科学出版社, 2012
[35] Hose G, Van den Brink P. Confirming the species-sensitivity distribution concept for endosulfan using laboratory, mesocosm, and field data [J]. Archives of Environmental Contamination and Toxicology, 2004, 47(4): 511-520
[36] Wheeler J, Grist E, Leung K, et al. Species sensitivity distributions: Data and model choice [J]. Marine Pollution Bulletin, 2002, 45(1): 192-202
[37] Chapman P M, Caldwell R S, Chapman P F. A warning: NOECs are inappropriate for regulatory use [J]. Environmental Toxicology and Chemistry, 1996, 15(2): 77-79
[38] Van Straalen N M, Denneman C A J. Ecotoxicological evaluation of soil quality criteria [J]. Ecotoxicology and Environmental Safety, 1989, 18(3): 241-251
[39] Jobling S, Casey D, Rodgers-Gray T, et al. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent [J]. Aquatic Toxicology, 2004, 66(2): 207-222
[40] Muncke J, Junghans M, Eggen R I L. Testing estrogenicity of known and novel (xeno-)estrogens in the MolDarT using developing zebrafish (Danio rerio) [J]. Environmental Toxicology, 2007, 22(2): 185-193
[41] Yang F X, Xu Y, Wen S. Endocrine disrupting effects of nonylphenol, Bisphenol A, and p,p′-dde on Rana nigromaculata tadpoles [J]. Bulletin of Environmental Contamination and Toxicology, 2005, 75(6): 1168-1175
[42] Kashiwada S, Ishikawa H, Miyamot N, et al. Fish test for endocrine-disruption and estimation of water qualityof Japanese rivers [J]. Water Research, 2002, 36(8): 2161-2166
[43] Pascoe D, Carroll K, Karntanut W, et al. Toxicity of 17-ethinylestradiol and Bisphenol A to the freshwater cnidarian Hydra vulgaris [J]. Archives of Environmental Contamination Toxicology, 2002, 43(1): 56-63
[44] Rhee J S, Raisuddin S, Hwang D S, et al. Differential expression of metallothionein (MT) gene by trace metals and endocrine-disrupting chemicals in the hermaphroditic mangrove killifish, Kryptolebias marmoratus [J]. Ecotoxicology and Environmental Safety, 2009, 72(1): 206-212
[45] Watts M M, Pascoe D, Carroll K. Survival and precopulatory behaviour of gammarus pulex (l.) exposed to two xenoestrogens [J]. Water Research, 2001, 35(10): 2347-2352
[46] Rankouhi T R, Sanderson J T, Holsteijn V, et al. Effects of natural and synthetic estrogens and various environmental contaminants on vitellogenesis in fish primary hepatocytes: Comparison of bream (Abramis brama) and carp (Cyprinus carpio) [J]. Toxicological Sciences, 2004, 81(1): 90-102
[47] Peter T, Jennifer S. Interference by atrazine and Bisphenol-A with progestin binding to the ovarian progestin membrane receptor and induction of oocyte maturation in Atlantic croaker [J]. Marine Environmental Research, 2008, 66(1): 1-2
[48] Segner H, Navas J M, Schäfers C, et al. Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo [J]. Ecotoxicology and Environmental Safety, 2003, 54(3): 315-322
[49] Hirano M, Ishibashi H, Matsumura N, et al. Acute toxicity responses of two crustaceans, Americamysis bahia and Daphnia magna, to endocrine disrupters [J]. Journal of Health Science, 2004, 50(1): 97-100
[50] Alexander H C, Dill D C, Smith L W, et al. Bisphenol A: Acute aquatic toxicity [J]. Environmental Toxicology and Chemistry, 1988, 1(1): 19-26
[51] 庄惠生, 杨光. 双酚A对鲤鱼急性和亚急性毒性的研究[J]. 环境化学, 2005, 24(6): 682-684
Zhuang H S, Yang G. Study on the acute and subacute toxicities of Bisphenol A on the carp [J]. Environmental Chemistry, 2005, 24(6): 682-684 (in Chinese)
[52] Lee S B, Choi J. Effects of Bisphenol A and ethynyl estradiol exposure on enzyme activities, growth and development in the fourth instar larvae of Chironomus riparius (Diptera Chironomidae) [J]. Ecotoxicology and Environmental Safety, 2007, 68(1): 84-90
[53] 曹娜, 魏华, 吴陵广, 等. 双酚A对斑马鱼肝脏和性腺的作用[J]. 生态学杂志,2010, 29(11): 2192-2198
Cao N, Wei H, Wu G L, et al. Effects of Bisphenol A on zebrafish (Danio rerio) liver and gonad [J]. Chinese Journal of Ecology, 2010, 29(11): 2192-2198 (in Chinese)
[54] 雷忻, 李宗强, 廉振民, 等. 双酚A和对硝基酚对泥鳅的急性毒性效应[J]. 生态学杂志, 2009, 28(11): 2257-2261
Lei X, Li Z Q, Lian Z M, et al. Acute toxicity effects of Bisphenol A and p-nitrophenol to Misgurnus anguillicadatus [J]. Chinese Journal of Ecology, 2009, 28(11): 2257-2261 (in Chinese)
[55] 牛海岗. 双酚A对中国林蛙蝌蚪的毒性和隔代效应[D]. 西安: 陕西师范大学, 2009: 13-24
[56] Brennan S J, Brougham C A, Roche J J, et al. Multi-generational effects of four selected environmental oestrogens on Daphnia magna [J]. Chemosphere, 2006, 64(1): 49-55
[57] 王晶晶. 双酚A和壬基酚对青岛大扁藻的复合干扰效应研究[D]. 广州: 暨南大学, 2013
Wang J J. The joint effects of Bisphenol A and nonylphenol on Platmonas helgolanidica [D]. Guangzhou: Jinan University, 2013 (in Chinese)
[58] 郭匿春, 谢平. 双酚A和壬基酚对隆线溞和微型裸腹溞的毒性[J]. 水生生物学报, 2009, 33(3): 492-497
Guo N C, Xie P. The toxic effects of BPA and NP on D. Carinata and M. Nicrura [J]. Acta Hydrobiologica Sinica, 2009, 33(3): 492-497 (in Chinese)
[59] 陆正和, 赵宝坤, 杨家新. 双酚A对萼花臂尾轮虫毒性及生活史的影响[J]. 生态学报, 2012, 32(21): 6828-6835
Lu Z H, Zhao B K, Yang J X. Effects of Bisphenol A on the toxicity and life history of the rotifer Brachionus calyciflorus [J]. Acta Ecologica Sinica, 2012, 32(21): 6828-6835 (in Chinese)
[60] 赵卫红. 日本沼虾(Macrobrachium nipponense)卵黄蛋白原代谢相关基因的克隆和表达研究[D].上海: 华东师范大学, 2011: 55-65
Zhao W H. Cloning and expression of the genes involving in the vitellogenin metabolism in oriental river prawn, Macrobrachium nipponense [D]. Shanghai: East China Normal University, 2011: 55-65 (in Chinese)
[61] Lahnsteiner F, Berger B, Kletzl M, et al. Effect of Bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario [J]. Aquatic Toxicology, 2005, 75(3): 213-224
[62] 杨君, 张育辉, 王宏元, 等. 双酚A对中国林蛙精巢芳香化酶和雌激素受体的影响[J]. 陕西师范大学学报: 自然科学版, 2011, 39(4): 69-74
Yang J, Zhang Y H, Wang H Y, et al. Effects of Bisphenol A on aromatase and estrogen receptor in the testis of frog Rana chensinensis [J]. Journal of Shaanxi Normal University: Natural Science Edition, 2011, 39(4): 69-74 (in Chinese)
[63] 张晖, 孔繁翔, 王世和, 等. 双酚A与内源性雌激素联合作用的探讨[J]. 安全与环境工程, 2008, 15(2): 13-17
Zhang H, Kong F Y, Wang S H, et al. Combined estrogenic effects of Bisphenol A on the action of steroidal estrogen [J]. Safety and Environmental Engineering, 2008, 15(2): 13-17 (in Chinese)
[64] Mihaich E M, Friederich U, Caspers N, et al. Acute and chronic toxicity testing of Bisphenol A with aquatic invertebrates and plants [J]. Ecotoxicology and Environmental Safety, 2009, 72(5): 1392-1399
[65] Brian J V, Harris C A, Scholze M, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals [J]. Environmental Health Perspectives, 2005, 113(6): 721-728
[66] Schirling M, Bohlen A, Triebskorn R, et al. An invertebrate embryo test with the apple snail Marisa cornuarietis to assess effects of potential developmental and endocrine disruptors [J]. Chemosphere, 2006, 64(10): 1730-1738
[67] Belt K V D, Verheyen R, Witters H. Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens [J]. Ecotoxicology and Environmental Safety, 2003, 56(2): 271-281
[68] Fukuhori N, Kitano M, Kimura H. Toxic effects of Bisphenol A on sexual and asexual reproduction in Hydra oligactis [J]. Archives of Environmental Contamination and Toxicology, 2005, 48(4): 495-500
[69] 周群芳, 江桂斌. 双酚A对Medaka的类雌激素效应研究[J]. 环境科学学报, 2005, 25(11): 1550-1554
Zhou Q F, Jiang G B. Estrogenic effect of Bisphenol-A on Medaka [J]. Acta Scientiae Circumstantiae, 2005, 25(11): 1550-1554 (in Chinese)
[70] Honkanen J O, Holopainen I J, Kukkonen J V. Bisphenol A induces yolk-sac oedema and other adverse effects in landlocked salmon (Salmo salar m. sebago) yolk-sac fry [J]. Chemosphere, 2004, 55(2): 187-196
[71] Mochida K, Ohkubo N, Matsubara T, et al. Effects of endocrine-disrupting chemicals on expression of ubiquitin C-terminal hydrolase mRNA in testis and brain of the Japanese common goby [J]. Aquatic Toxicology, 2004, 70(2): 123-136
[72] Yokota H, Tsuruda Y, Maeda M, et al. Effect of Bisphenol A on the early life stage in Japanese medaka (Oryzias latipes) [J]. Environmental Toxicology and Chemistry, 2000, 19(7): 1925-1930
[73] Watts M M, Pascoe D, Carroll K. Exposure to 17α-ethinylestradiol and Bisphenol A——Effects on larval moulting and mouthpart structure of Chironomus riparius [J]. Ecotoxicology and Environmental Safety, 2003, 54(2): 207-215
[74] Kallivretakia E, Eggenb R, Neuhaussc S, et al. Aromatase in zebrafish: A potential target for endocrine disrupting chemicals [J]. Marine Environmental Research, 2006, 62: S187-S190
[75] Mu X, Rider C V, Hwang G S, et al. Covert signal disruption: Anti-ecdysteroidal activity of Bisphenol A involves cross talk between signaling pathways [J]. Environmental Toxicology and Chemistry, 2005, 24(1): 146-152
[76] Oehlmann J, Schulte-Oehlmann U, Bachmann J, et al. Bisphenol A induces superfeminization in the ramshorn snail Marisa cornuarietis (Gastropoda: Prosobranchia) at environmentally relevant concentrations [J]. Environmental Health Perspectives, 2006, 114(s1): 127-133
[77] EU. European Union Updated Risk Assessment Report. Bisphenol A, CAS No: 80-05-7 [R]. Luxembourg: Institute for Health and Consumer Protection, European Chemicals Bureau, European Commission Joint Research Centre, Office for Official Publications of the European Communities, 2008
[78] AIST (Japan's National Institute of Advanced Industrial Science and Technology). AIST Risk Assessment Document Series 4. Bisphenol A [R]. Tykyo: National Institute of Advanced Industrial Science and Technology, 2007
◆
Predicted No Effect Concentration of Bisphenol A (BPA) Based on Different Toxicological Endpoints
Feng Chenglian1, Wang Hao1, Wang Ying1,2, Wu Fengchang1,*
1. State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China 2. College of Water Science, Beijing Normal University, Beijing 100875, China
10 June 2014 accepted 7 August 2014
Bisphenol A (BPA) has been confirmed to be an endocrine disrupting chemical. In the present study, the BPA toxicity data were classified based on different toxicological endpoints. Then, the acute and chronic PNEC (predicted no effect concentration) for protecting aquatic life were derived by use of species sensitivity distribution approach. The results showed that the acute and chronic PNECs of BPA derived from the estrogen effect data were 25.11 μg·L-1and 1.075 μg·L-1, respectively; while for all the toxicity data, the corresponding PNECs were 355.7 μg·L-1and 7.549 μg·L-1, respectively. Therefore, estrogen effects of BPA to organism were more sensitive than other effects. It is recommended that the PNECs for endocrine disrupting chemicals should be derived based on different toxicological endpoints. The results in the present study could provide data support for the establishment and revision of water quality standard in China.
Bisphenol A (BPA); freshwater organism; estrogen effect; endocrine disruption; PNEC
环保公益性行业科研专项(201409037);环保公益性行业科研专项(201309060)
冯承莲(1981-),女,博士,副研究员,研究方向为水生态毒理和水质基准,E-mail: fengcl@craes.org.cn;
*通讯作者(Corresponding author), E-mail: wufengchang@vip.skleg.cn
10.7524/AJE.1673-5897.20140610002
2014-06-10 录用日期:2014-08-07
1673-5897(2015)1-119-11
X171.5
A
吴丰昌(1964—),男,研究员,博士生导师,环境基准与风险评估国家重点实验室主任,主要研究方向为环境基准与风险评估,天然有机质环境生物地球化学行为等。
冯承莲, 汪浩, 王颖, 等. 基于不同毒性终点的双酚A(BPA)预测无效应浓度(PNEC)研究[J]. 生态毒理学报, 2015, 10(1): 119-129
Feng C L, Wang H, Wang Y, et al. Predicted no effect concentration of Bisphenol A (BPA) based on different toxicological endpoints [J]. Asian Journal of Ecotoxicology, 2015, 10(1): 119-129 (in Chinese)
