1,3-双(2-(2,2-二氰乙烯基)苯氧基)-2-丙醇及其银(Ⅱ)配合物的合成、晶体结构及其荧光性质
2015-04-01张奇龙王焕宇胡鹏朱必学
张奇龙 王焕宇 胡鹏 朱必学
(1贵阳医学院化学教研室,贵阳550004)
(2贵州省大环化学及超分子化学重点实验室,贵阳550025)
1,3-双(2-(2,2-二氰乙烯基)苯氧基)-2-丙醇及其银(Ⅱ)配合物的合成、晶体结构及其荧光性质
张奇龙*,1王焕宇1胡鹏1朱必学2
(1贵阳医学院化学教研室,贵阳550004)
(2贵州省大环化学及超分子化学重点实验室,贵阳550025)
用1,3-双(2-甲酰基苯氧基)-2-丙醇和丙二腈进行反应得到1,3-双(2-(2,2-二氰乙烯基)苯氧基)-2-丙醇配体L,然后将配体与AgSbF6进行配位反应,得到配合物[AgLSbF6]n·nCHCl3(1),并用元素分析,FTIR和X-射线单晶衍射进行了表征。结果表明,配体L属于单斜晶系,空间群P21/n,晶体学参数:a=0.990 2(11)nm,b=2.181(2)nm,c=1.012 2(11)nm,β=109.374(10)°,V=2.062(4) nm3,Z=4,Dc=1.277 g·cm-3,Mr=396.40,μ=0.087 mm-1,F(000)=824,R1=0.064 2,wR2=0.117 4(I>2σ(I))。配合物1属于单斜晶系,空间群P21/n,晶体学参数:a=1.270 57(11)nm,b=1.456 44(13)nm,c=1.669 85(14)nm,β=105.643(3)°,V=2.975 6(4)nm3,Z=4,Dc= 1.918 g·cm-3,Mr=859.39,μ=1.907 mm-1,F(000)=1 664,R1=0.0417,wR2=0.1 032(I>2σ(I))。在配合物1中,配体L表现为四齿配体分别与4个银(Ⅱ)离子配位,同时,每一个银(Ⅱ)离子与4个相邻配体配位形成2D层状结构。同时,研究了配体和配合物的固体荧光性质。
1,3-双(2-(2,2-二氰乙烯基)苯氧基)-2-丙醇;晶体结构;Ag(Ⅰ)配合物;荧光性质
0 Introduction
The assembly of coordination polymers(CPs), consisting of metal ions and organic polymers,with specific network topologies has attracted significant researchinterestinrecentyearsowingtotheir tremendous potential applications as functional solidstate materials[1-4]in material science and engineering. However,the rational design and fabrication of CPs withspecificstructuresandtunablephysical properties is a challenging task.The diverse structures of such materials always depend on several factors, such as type of metal ion,template,metal-ligand ratio, pH value,counter anion,and number of coordination sites provided by organic ligands[5-6].Silver(I)-based CPs(AgCPs)have attracted remarkable attention becauseoftheirdistinctmolecularstructures. Moreover,theyactaspromisingcandidatesfor conductive,luminescent,andmagneticmaterials. Interestingly,Ag(I)exhibits strong preference toward coordination to nitrogen donor than to harder oxygencontaining ligands.Therefore,it is extremely important to explore and rationalize the effect of different ligands on the topology of AgCPs.
An outstanding aspect of the AgCPs is that they possessuniqueluminescencefeaturesthathave attractedsignificantinterestoftheresearchers. Therefore,extensiveresearcheffortshavebeen devoted to the AgCPs.Great affinity of Ag ions leads to dynamic coordination range varying from two to six, even to seven and eight,showing strong tendency to displayversatilecoordinationspheres,partly attributed to the d10electronic configuration of Ag[7-10]. Moreover,there are numerous ligands with rational design and reasonable use to compose functional CPs with excellent structures and properties[11-12].Based on the fact that flexible organic molecules adopt different conformations under different conditions[13-16],in this study,interaction of the flexible ligand 1,3-bis(2-(2,2-dicyanovinyl)phenoxy)-2-propanol(L,Scheme 1)with Ag(I)was investigated.Herein,we describe the synthesis,crystal structure,and fluorescent properties of Ag(I)complex coordinated to flexible,ether-bridgin g,tetradentate ligand L.
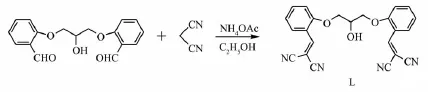
Scheme 1 Schematic representation of the formation of ligand L
1 Experimental
1.1 Materials and measurements
All chemicals from commercial sources were of reagent grade and used without purification.Elemental (C,H,N)analysis data were obtained with a Vario ELⅢelemental analyzer.IR spectra were recorded by KBr pellets on Bio-Rad FTIR spetrophotometer(in the 400~4 000 cm-1range).1H NMR spectra were recorded on a JEOLECX 500MHz spectrometer using DMSO solutionatroomtemperature.Thermogravimetric measurements were carried out in a nitrogen stream using a Netzsch STA449C apparatus with a heating rate of 10℃·min-1.X-Ray powder diffraction(XRPD) was carried out on a RIGAKU DMAX2500 apparatus.
1.2 Synthesis of the ligand L
1,3-bis(2-formylphenoxy)-2-propanol[17](0.3 g,1.0 mmol),excess of malononitrile(CH2(CN)2),and ammoniumacetate(NH4OAc)weremixedthoroughlyina mortar.The product so obtained was purified by column chromatography on silica gel using dichloromethane (CH2Cl2)as an eluent to afford yellow crystalline solid (0.26 g,65%).1H NMR(500MHz,DMSO-d6):8.623(s, 2H,C=CH),8.029~7.133(m,8H,C6H4),5.732(s,1H, CHOH),4.337(s,1H,OH),4.284~4.153(m,4H, OCH2CH2O).Anal.Calcd.(%)for C23H16N4O3:C,69.69; H,4.07;N,14.13;Found(%):C,69.62;H,4.00;N, 14.17.IR(KBr,cm1):3 524(m),3 046(w),2 924(w), 2 226(m),1 581(s),1 482(m),1 453(m),1 363(w),1 307 (w),1257(s),1163(w),1 116(w),1 045(w),1 006(w),947 (w),812(w),759(w),616(w),490(w).
1.3 Synthesis of[AgLSbF6]n·nCHCl3(1)
Hexafluoroantimonate(V)(AgSbF6,0.17 g,0.1 mmol)in chloroform(CHCl3,20 mL)was added dropwise with stirring to ligand L(0.2 g,0.5 mmol)in ethanol(20 mL)and stirring was continued at room temperature for several days.Slow evaporation of this solution yielded yellow block crystals suitable for X-ray analysis.The precipitate so formed was collected by filtration,and dried at room temperature to give 1(0.26 g,60%).Anal. Calcd.(%)for AgC24H17Cl3N4O3SbF6:C,33.54;H,1.99; N,6.52.Found(%):C,33.60;H,1.92;N,6.60.IR(KBr, cm-1):3 444(s),2 928(w),2 228(m),1 726(w),1 587(s), 1 483(m),1 454(m),1 362(w),1 310(w),1 253(s),1 166 (w),1115(w),1 005(w),809(w),757(s),615(s).
1.4 X-ray crystallographic study
X-ray diffraction data were collected at room temperature with graphite monochromated Mo Kα radiationonBrukerSmartApexsinglecrystal diffractometer by using a φ-ω scan method.Unit cell dimensionswereobtainedwithleast-squaresrefinements and multi-scan absorption corrections were applied by using SADABS program.The structures were solved by direct methods using the program SHELXL-97 in the winGX package[18].H atoms were placed in calculated positions,with C-H distances of 0.093~0.096 nm and NH=0.086 nm,and refined using a riding model with Uiso(H)=(1.2~1.5)Ueq(C).The ORTEP-3[19]drawing of the molecules is shown in Fig.1 and Fig.3.Crystal and experimental data are listed in Table 1,selected bond lengths and angles are given in Table 2.Hydrogen bonding geometry is given in Table 3 and Table 4.
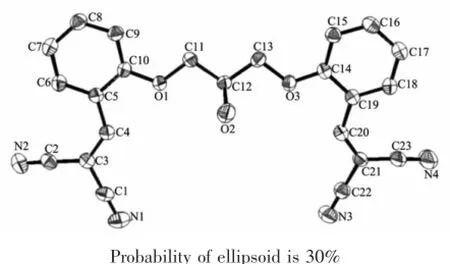
Fig.1Molecular structure of ligand L

Fig.2Dimers formed by the ligand through O2-H2…H2-O2 hydrogen bonds
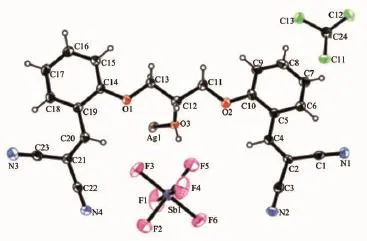
Fig.3Thermal ellipsoid plot at 25%level of the asymmetric units of the complex 1

Table 1Crystallographic data for ligand L and 1

Table 2Selected bond lengths(nm)and bond angles(°)for ligand and complex 1

Table 3Hydrogen Bond Lengths(nm)and Bond Angles(°)for ligand L

Table 4C-H…π interactions in complex 1
CCDC:1008802,L;1008804,1.
2 Results and discussion
2.1 Crystal structures of ligand L and complex1
The bond lengths and bond angles of ligand L are listed in Table 2,and its crystal structure is shown in Fig.1.The hydroxyl groups present in the flexible ligand are connected by the ether chain bridge.Fig.1 and values listed in Table 2 show that the two pairs of C(CN)2are located toward the end of the base in the same direction.However,the two benzene rings are not in a plane,and the dihedral angle between them is 168.37°.The ligand forms dimers through O2-H2…H2-O2 hydrogen bonds(Fig.2 and Table 3).
The crystal structure of the complex 1 is shown in Fig.3.Single-crystal XRD analysis reveals that compound 1 crystallizes in the monoclinic space group P21/n.The asymmetric unit is composed of a Ag(Ⅰ)center,a unique ligand L,an SbF6anion,and CHCl3solventmolecules(Fig.3).EachAg(Ⅰ)ionis tetracoordinated in a distorted tetrahedron coordination environment to three N atoms and one O atom from four different ligands.The Ag1-N1,Ag1-N2,and Ag1-N4 bond lengths are 0.240 5(4),0.217 0(4),and 0.223 1(5)nm,respectively.The Ag1-O3 bond length is 0.246 7(3)nm,N-Ag-N bond angles are in the range of 87.14(15)°~138.57(15)°,and N-Ag-O bond angles are in the range of 91.32(12)°~109.46(12)°. The ligands act as a μ4-bridge connecting four Ag(Ⅰ)ions,with three nitrogen atoms of the three cyano groups,respectively and one oxygen atom of hydroxyl group.The two cyano nitrogen atoms at one end of ligand L coordinate with two Ag(Ⅰ)ions independently. In contrast,only one of the two cyano nitrogen atoms at the other end coordinates with a Ag(Ⅰ)ion;the uncoordinatednitrogenatomundergoesweak interaction with the molecules of solvent CHCl3.The two adjacent cyano nitrogen atoms at the two ends of ligand L and the two cyano nitrogen atoms at one end of another ligand L coordinate with two Ag(Ⅰ)ions simultaneouslyandthus,forma24-member macrocycle.Such 24-member macrocycles alternately extend to either side and form a one-dimensional(1D) ribbon structure(Fig.4),in which the hydroxyl oxygen atoms of ligand L coordinate individually to Ag(Ⅰ)ion, bridgingthe1DribbonstructuresintotheCPs consisting of two-dimensional(2D)mesh structure.In this 2D mesh structure(Fig.5),C-H…π interactionsexist between the hydrogen on C4(H4)and ring Cg1, with an C4-H4…Cg(1)angle of 93.33°,a H4…Cg(1) distance of 0.315 45 nm,where Cg(1)is the centroid of the phenyl ring formed by atoms C5,C6,C7,C8,C9, and C10(Table 4).The ligands act as four-connecting nodes;the Ag atoms act as four-connecting links.
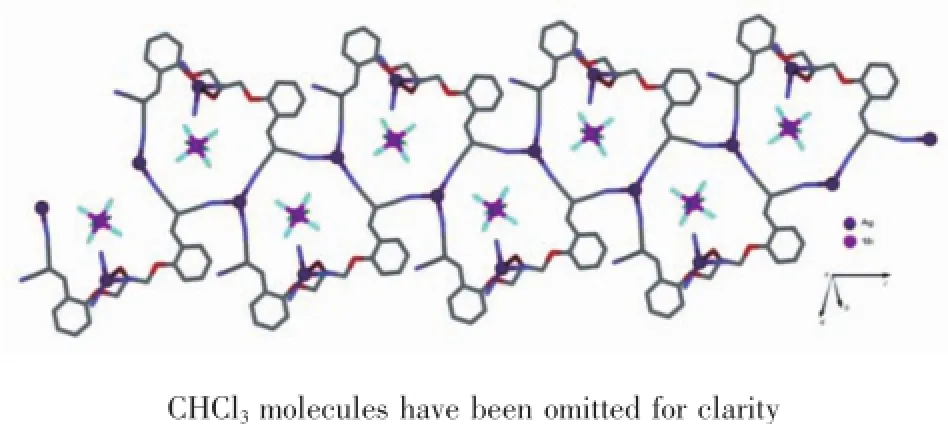
Fig.41D ribbon structure in the complex 1
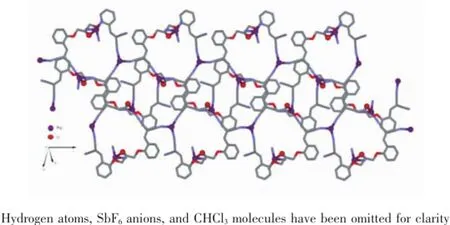
Fig.5View of 2D coordination layer structure in the complex 1
2.2 PXRD analysis
To examine the phase purity of the complexes, the PXRD patterns of title complex was obtained at room temperature.As shown in Fig.6,the peak positions of the simulated and experimental PXRD patterns were in agreement,demonstrating good phase purityforthecomplex.Thedissimilaritiesin intensities may be due to the preferred orientation of the crystalline powder samples.

Fig.6PXRD patterns of complex 1
2.3 Photoluminescent properties of Ligand L and Complex 1
The photoluminescence spectra of the ligand L and complex 1 are shown in Fig.7.The ligand L exhibited photoluminescence with emission maxima at 474 nm(λex=337 nm).Presumably,the peak originated from the π*→n or π*→π transitions[20].Intense emissions occurred at 525 nm(λex=338 nm)for complex 1,which was attributed to the intraligand π→π* transitions modified by metal coordination[21].The enhancementandslightred-shiftofcomplex1 compared to that of the ligand L probably resulted from the fact that the coordination of Ag(Ⅰ)ions increased the conformational rigidity of the ligand and;thus,reduced the loss of energy by thermal vibrational decay[22-23].

Fig.7Fluorescence spectra of the ligand and complex
[1]CHENG Xiao-Ning(程小宁),ZHANG Wei-Xiong(张伟雄), XUE Wei(薛玮),et al.Chinese.J.Inorg.Chem.(无机化学学报),2014,30(1):142-148
[2]Chen X Y,Huang R B,Zheng L S,et al.Inorg.Chem., 2014,53:5246-5252
[3]Manus L M,Holbrook R J,Atesin T A,et al.Inorg.Chem., 2013,52:1069-1076
[4]ZHANG Chun-Li(张春丽),QIN Ling(覃玲),ZHENG He-Gen (郑和根).Chinese J.Inorg.Chem.(无机化学学报),2014,30 (4):800-804
[5]Halper S R,Do L,Stork J R,et al.J.Am.Chem.Soc., 2006,128:15255-15268
[6]Fan L M,Zhang X T,Sun Z,et al.Cryst.Growth Des., 2013,13,2462-2475
[7]Zhou X,Lu Y,Zhai L L,et al.RSC Adv.,2013,3:1732-1734
[8]ZHANG Qi-Long(张奇龙),ZHANG Yun-Qian(张云黔),ZHU Bi-Xue(朱必学).Chinese J.Inorg.Chem.(无机化学学报), 2011,27(11):2191-2194
[9]Omary M A,Elbjeirami O,Gamage C S,et al.Inorg.Chem., 2009,48:1784-1786
[10]Hu X H,Liang Y,Li C,et al.Dalton Trans.,2014,43:2458 -2464
[11]Zhang Q L,Zhu B X,Zhang Y Q,et al.Cryst.Growth Des., 2011,11:5688-5695
[12]Liu S J,Huang Y B,Lin Z J,et al.RSC Adv.,2013,3:9279 -9287
[13]Ma L F,Wang L Y,Du M,et al.Inorg.Chem.,2010,49: 365-367
[14]Yang Y,Yang J,Du P,et al.CrystEngComm,2014,16: 6380-6390
[15]Wang X L,Sui F F,Lin H Y,et al.CrystEngComm, 2013,15:7274-7284
[16]Dong Y B,Jiang Y Y,Li J,et al.J.Am.Chem.Soc., 2007,129:4520-4521
[17]ZHAO Bin(赵斌),XIA Min(夏闽),WANG Xue-Zhi(王学之), et al.Chinese J.Chemical Reagents(化学试剂),2000,22: 224-227
[18]Sheldrick G M.Acta Crystallogr.,1990,A46,467
[19]Sheldrick G M.SHELX-97,University of Göttingen,Germany, 1997.
[20]Zhang X T,Fan L M,Sun Z,et al.CrystEngComm, 2013,15:4910-4916
[21]Liu F J,Sun D,Hao H J,et al.Cryst.Growth Des., 2012,12:354-361
[22]Zheng S L,Zhang J P,Chen X M,et al.J.Solid State Chem.,2003,172:45-52
[23]Yi L,Zhu L N,Ding B,et al.Inorg.Chem.Commun., 2003,6:1209-1212
Synthesis,Crystal Structures,and Fluorescent Properties of Ag(I)Complex Containing 1,3-Bis(2-(2,2-dicyanovinyl)phenoxy)-2-propanol Ligand
ZHANG Qi-Long*,1WANG Huan-Yu1HU Peng1ZHU Bi-Xue2
(1Department of Chemistry,Guiyang Medical College,Guiyang 550004,China)
(2Key laboratory of Macrocycle and Supramolecular Chemistry,Guiyang 550025,China)
1,3-bis(2-formylphenoxy)-2-propanol underwent an ammonium acetate-catalyzed condensation reaction with dicyanomethane to afford the ligand 1,3-bis(2-(2,2-dicyanovinyl)phenoxy)-2-propanol(L).Further,the ligand L was made to react with hexafluoroantimonate(AgSbF6)which resulted in the formation of coordination polymer [AgLSbF6]n·nCHCl3(1).Complex 1 so-obtained was characterized by Fourier transform infrared spectroscopy,elemental analysis,and single crystal X-ray diffraction.The results of X-ray crystallographic analysis indicated that the ligand L crystallized in the monoclinic system,space group P21/n,with the following crystal cell parameters: a=0.990 2(11)nm,b=2.181(2)nm,c=1.012 2(11)nm,β=109.374(10)°,V=2.062(4)nm3,Z=4,Dc=1.277 g·cm-3, Mr=396.40,μ=0.087 mm-1,F(000)=824,R1=0.064 2,and wR2=0.1174(observed reflections withI>2σ(I)). Complex 1 crystallized in the monoclinic system,space group P21/n,with the following crystallographic data:a= 1.270 57(11)nm,b=1.456 44(13)nm,c=1.669 85(14)nm,β=105.643(3)°,V=2.975 6(4)nm3,Z=4,Dc=1.918 g· cm-3,Mr=859.39,μ=1.907 mm-1,F(000)=1664,R1=0.041 7,and wR2=0.103 2(observed reflections with I>2σ(I)). In the crystal structure,each ligand L acted as a tetradentate ligand coordinating four Ag(Ⅰ)centers,and each Ag(Ⅱ)was connected to four ligands L,forming two-dimensional layered structure.Moreover,the fluorescent properties of ligand L and complex 1 were investigated in the solid state at room temperature.CCDC:1008802,L;1008804,1.
3-bis(2-(2,2-dicyanovinyl)phenoxy)-2-propanol;crystal structure;Silver(Ⅱ)Complex;Fluorescent property
O614.81+2;O614.24+1
A
1001-4861(2015)02-0353-08
10.11862/CJIC.2015.030
2014-08-01。收修改稿日期:2014-09-17。
国家自然科学基金(No.21061003)资助项目。
*通讯联系人。E-mail:gzuqlzhang@126.com
