Synthesis and Characterization of Tungsten Oxide Nanostructures
2013-09-29QINYuXiangBAOZhiYingHUMingSUNPengSchoolofElectronicsandInformationEngineeringTianjinUniversityTianjin300072
QIN Yu-Xiang BAO Zhi-Ying HU Ming SUN Peng(School of Electronics and Information Engineering,Tianjin University,Tianjin 300072)
Synthesis and Characterization of Tungsten Oxide Nanostructures
QIN Yu-Xiang*BAO Zhi-Ying HU Ming SUN Peng
(School of Electronics and Information Engineering,Tianjin University,Tianjin 300072)
One-and two-dimensional tungsten oxide nanostructures were synthesized by solvothermal method using tungsten hexachloride (WCl6)as the precursor.The effects of the solvent and the concentration of WCl6on the phase and the morphology of the as-synthesized tungsten oxide nanostructures were investigated and the NO2-sensing properties were evaluated.X-ray diffraction,field emission scanning electron microscope,transmission electron microscope,X-ray photoelectron spectroscopy were employed to characterize the as-synthesized products and the results indicate that the W18O49nanorod,W18O49nanowire and WO3nanosheet with monoclinic structure can be formed by adjusting the solvent and WCl6concentration.The NO2-sensing properties measurements show that the synthesized tungsten oxides have reversible response to NO2at different concentrations.In comparison with WO3nanosheet or W18O49nanorod,the W18O49nanowire exhibits much higher response to NO2gas.
tungsten oxide;nanostructure;solvothermal method;gas sensitive properties
Tungsten oxide is a versatile wide band gap semiconductor material,and it has wide applications in gas sensors[1-2],electrochromic devices[3]and photocatalysts[4].Especially,tungsten oxide has been found to be a promising material for detection of toxic gases such as NOx,H2S,Cl2,NH3[5-6].The gas sensing mechanism of oxide semiconductors lies in the fact that the electrical resistance of the materials will be changed when they are exposed to the gases.Consequently,the sensing response of oxides is highly dependent on their surface structure and morphology.A lot of sensing tests for tungsten oxide polycrystalline thin films indicate that the sensing response steeply increases when the grain size decreases.Recently,tungstennanostructuressuch asnanowires,nanobeltsand nanorods have been evaluated as ideal candidates for gas sensing applications due to their larger specific surface area and smaller dimensions compared to the Debye length[7-8].Typically,tungsten oxide nanostructures can be fabricated by various growth techniques[9-11].One interesting technique is solvothermal method featuring in simple operation and low cost.In this work,differenttungsten oxide nanostructures including nanorod,nanowire and nanosheet were synthesized by solvothermal method.The phase and morphology of the as-synthesized tungsten oxide nanostructures were characterized and the sensing properties of the material to NO2gas were also evaluated.
1 Experimental
1.1 Synthesis and characterization of tungsten oxide nanostructures
Tungsten oxide nanostructureswith different morphologies were synthesized by solvothermal method with tungsten hexachloride(WCl6)as the precursor and cyclohexanol or 1-propanol as the solvent.First,a pre-determined amount of WCl6was dissolved in 2~4 mL ethanol to form a solution.Then,cyclohexanol or 1-propanol was added to the solution which was subsequently transferred to and sealed in a 100 mL Teflon-lined stainless steel autoclave.The concentration of WCl6in the solvent of cyclohexanol or 1-propanol varied from 0.003 mol·L-1to 0.02 mol·L-1.The solvothermal reaction was conducted at 200℃for 6~9 h in an electric oven.After that,the autoclave was cooled naturally to room temperature.The final products were centrifuged and washed sequentially by deionized water and ethanol several times,and the obtained powder was dried at 70 ℃ for 6 h in air.
The crystalline phases of the tungsten oxides were analyzed using a RIGAKU D/MAX 2500V/PC X-ray diffractometer(XRD)fitted with a curved graphite diffracted-beam monochromator and 0.15 mm receiving slit and scintillation counter as the detector.The 2θ investigation region was in the range of 10°~80°with a step of 0.02°and a scanning speed of 4°·min-1.Cu Kα1(λ=0.154 06 nm)radiation was obtained after Kα1stripping from a Cu X-ray target operated at 40 kV and 200 mA.The morphology and crystalline structure were observed by a FEI Nanosem 430 field emission scanning electron microscope(FESEM)at an accelerating voltage of 10 or 15 kV and a TECNAI G2F-20 field emission transmission electron microscope (FETEM)operated at 300 kV of accelerated voltage.The chemical state of the tungsten oxide nanostructures was studied by X-ray photoelectron spectroscopy (XPS)using PERKIN ELEMER PHI-1600 ESCA with Mg Kα source and a charge neutralizer.X-ray irradiation was generated under 250 W.All the binding energies were corrected for charging effect by calibration on the graphite C1s peak at 284.6 eV.
1.2 NO2-sensing properties test
Gas sensors were fabricated by pouring a few drops of tungsten oxide powder-suspended ethanol onto the cleaned alumina substrates attached with a pair of interdigitated Pt electrodes with a thickness of 100 nm.The electrodes were deposited by using RF magnetron sputtering method,and the dropping suspension was prepared by ultrasonically dispersing tungsten oxide powders in ethanol for 30 min.The coated film was dried in air for 10 min.The above drop-coating process was repeated for 3 times to achieve the desired film thickness.Finally,the resulted films were heated in air using an infra-red dryer for 1 h,to evaporate the solvents residues in the coating layers.
The NO2gas sensing measurements were carried out in a computer-controlled gas sensing characterization system.The sensors were placed on the heating plate fixed in test chamber,and the operating temperature of the sensors was achieved by adjusting the temperature controller of the heat plate.The pure NO2gas was injected into the chamber directly to get the desired concentration.A professional digital multimeter with the function of measuring range automatic adjustment was used for continuously monitoring the resistance change of the sensors during the whole measurement process and the sampling interval was set to 1s.The gas response was defined as(Rg-R0)/R0,where Rgand R0are the resistance of a sensitive filmin a measuring gas and that in clean air,respectively.
2 Results and discussion
2.1 Effect of WCl6concentration on morphologies of tungsten oxide
The morphologies of tungsten oxide nanostructures synthesized at different WCl6concentrations in 1-propanol are shown in Fig.1(a)~(d).The solvothermal reaction temperature and reaction time were maintained constant at 200 ℃ and 9 h,respectively.It can be seen from Fig.1(a),the product synthesized at a concentration of 0.003 mol·L-1exhibits ultrathin nanowire structure with diameters of several ten nanometers and lengths up to several micrometers.Further TEM investigation can identify the bundled feature,giving evidence that several nanowires with diameters of 5~10 nm assembled along their main growth direction and formed a bundled structure,as shown in the inset in Fig.1(a).This bundle structure is often formed in 1-D nanostructured materials to minimize the surface energy of the system and the lateral capillary force may act as the driving force for the nanorod assembly along the axis direction[12-13].Increasing WCl6concentration to 0.01 mol·L-1,it is obvious from Fig.2 (b)that the nanowire bundles become thicker,indicating that a possible agglomeration between the adjacent nanowires or nanowire bundles has occurred.The estimated bundle diameter is 40~60 nm.The inset TEM image in Fig.1(a)shows that each nanowire in the bundles is with a diameter of about 10 nm.

Fig.1 SEM images of tungsten oxide nanostructures synthesize in 1-propanol at different WCl6concentration
When the concentration increases to 0.015 mol·L-1,apparent evolution in the morphology can be observed.As shown in Fig.1(c),the product synthesized at 0.015 mol·L-1is a mixture structure of nanowires and nanosheets.Up to a much higher WCl6concentration of 0.02 mol·L-1,a pure nanosheets structure with thicknesses of 10~30 nm is obtained (Fig.1(d)).From these results,it can be speculated that the WCl6concentration has a great effect on the specific morphologies of tungsten oxide nanostructures synthesized by solvothermal method.This is in agreement with the previous report[14].Low solution concentration contributestothelowersupersaturation oftungsten source,promoting the growth oftungsten oxide nanowires[15].At higher concentration,the highly saturated WCl6could prohibit the growth of tungsten oxide nanowires along the main growth direction.
2.2 Effect of solvent on morphologies of tungsten oxide
Fig.2(a)and(c)respectively show the morphologies of tungsten oxide nanostructures synthesized in the solvents of 1-propanol and cyclohexanol with a constant WCl6concentration of 0.005 mol·L-1.The solvothermal reaction in cyclohexanol and 1-propanol were conducted at 200℃for 6 h and 9 h,respectively,because there was no any precipitates obtained in 1-propanol when reacting for 6 h.The SEM image shown in Fig.2(a)exhibits that the product obtained in cyclohexanol is mainly composed of short nanorods with diameter of 30~40 nm and length of 300~400 nm.Several thinner nanorods assemble together along the axis direction and form nanorod bundles.Fig.2(b)shows the TEM image of one nanorod bundle.The stacked bundles shown in Fig.2(b)can be as-cribed to physical agglomeration,which can be easily dispersed by ultrasonic vibration.When substituting 1-propanol with cyclohexanol,the nanowire bundles exhibiting thin and long features are obtained,as shown in Fig.2(c).

Fig.2 (a)SEM and(b)TEM images of the tungsten oxide synthesized in cyclohexanol;(c)SEM image of the tungsten oxide synthesized in 1-propanol
2.3 Structure characterization of tungsten oxide nanostructures
XRD results are show in Fig.3.As shown in Fig.3(a)and(b),the XRD patterns for the bundled tungsten oxide nanorods and nanowires synthesized by solvothermal method are very similar as evidence of the comparability of diffraction peaks in 2θ position and intensity.The main two diffraction peaks of both products can be well indexed to the monoclinic phase of W18O49with lattice parameters of a=1.832 nm,b=0.379 nm,c=1.404 nm and β =115.04°(PDF No.65-1291).It is also observed that there are no other impurity phase peaks.The strongest peak intensity of(010)plane indicates that the growth is preferentially along the b-axis,i.e.the [010]direction.In the present experiments,the monoclinic W18O49structures obtained in the solvent of cyclohexanol and 1-propanol are identical,which can be seen from the same diffraction peaks of monoclinic W18O49in Fig.3.The formation oftheseone-dimensionalnanostructures arises from the anisotropic properties of monoclinic W18O49crystals[16].The XRD pattern of the tungsten oxide nanosheet shown in Fig.3(c)corresponds to the monoclinic structure of WO3with lattice of a=0.729 7 nm,b=0.753 9 nm,c=0.768 8 nm and β=90.91°(PDF No.43-1035).From thisXRD pattern,thetwo strongest diffraction peaks appear at 2θ=23.58°and 2θ=24.34°corresponding to(020)and(200)facets and the peak intensity of the (002)reflection is much weaker,which implies that the nanosheets grow along the[010]and[100]crystallographic direction and are enclosed by ±(001)facets.

Fig.3 XRD patterns of the as-synthesized tungsten oxide nanostructures
Fig.4(a)~(c)show the high resolution(HR)TEM images of the nanorod,nanowire and nanosheet.Here,the lattice spacings for nanorod and nanowire are 0.378 nm and 0.380 nm,respectively,corresponding to (010)plane of monoclinic W18O49according to PDF No.65-1291.This result indicates that the as-synthesized one-dimensional nanostructures all consist of monoclinic W18O49and the dominant growth direction is along the b-axis direction.The HRTEM of tungsten oxide nanosheet indicates that the crystal plane distances are about 0.363 nm and 0.378 nm,almost equal to the crystal plane distance of monoclinic WO3(200)and (020)planes,respectively.The results shown in Fig.4 are in agreement with the ones from XRD characterization(Fig.3).
XPS spectra are shown in Fig.5,which highlights the survey and the high-resolution spectra of W4fpeaks.Tungsten is identified in the survey spectrum(Fig.5(a))by the presence of the W4f,W4d,and W4p transitions.Also labeled are the OKLL and O1s features and the C1s line probably coming from the surface contamination during the annealing treatment.Fig.5(b)shows the high-resolution spectra of the W4f region,which contains the W4f7/2and W4f5/2peaks with binding energies of 35.2/35.2/35.7 eV and 37.2/37.2/37.8 eV for nanorod/nanowire/nanosheet,respectively.It is well established that the fully oxidized tungsten oxide (WO3)has the doublet due to W4f7/2at 35.8 eV and W4f5/2at 38 eV,which represents the W6+[17].In Fig.5(b),the position shift of the peaks corresponding to W4f7/2and W4f5/2indicates that there are some oxygen vacancies existing in three tungsten oxide nanostructures.The larger peaks shift of tungsten oxide nanorod and nanowire implies much more oxygen vacancies.Because the electron density in semiconductors depends on the density of oxygen vacancies,the vacancies play a significant role in the gas detection mechanism for the oxide semiconductors[18],and the existence of oxygen vacancies is much beneficial to the gas-sensing application of this kind of materials.From Fig.5(b),it also can be seen that,for the nanorods and nanowires,the binding energies for the peaks of W4f7/2and W4f5/2are the same,which suggests the same W valence state.This result is consistent with the one of XRD measurements that the nanorod and nanowire has the same crystalline structure of monoclinic W18O49.

Fig.4 HR-TEM images of as-synthesized(a)nanowire,(b)nanorod and(c)nanosheet

Fig.5 XPS spectra of tungsten oxide nanorod,nanowire and nanosheet
2.4 Gas sensing properties
The gas-sensing properties of the as-synthesized tungsten oxide nanostructures were evaluated upon exposure to NO2gas.Fig.6 shows the dynamic responses of tungsten oxide nanorod,nanowire and nanosheet to NO2gas at an operating temperature of 200℃.As shown in the figure,the measured resistances increase upon exposure to NO2gas.This result is expected because the oxidizing analyte NO2withdraws electrons from the n-type tungsten oxide surface and induces the formation of electron-depleted space-charge layers[19].Notably,the resistances could almost recover to its initial value after NO2removal,indicating a good reversibility of these nanostructure materials.Fig.7 shows the effect of NO2concentration in the range of 1~20 μL·L-1on the responses of tungsten oxide nanostructures at 200℃.From the figure,the W18O49nanowire shows the highest response at different NO2concentrations,while the lowest response is obtained from the W18O49nanorod.The response values of the W18O49nanowires upon exposure to 1,5,10 and 20 μL·L-1NO2are 13.4,123.6,203.4 and 332.3,while those of the WO3nanorod are 6.9,49.7,89.1 and 152.8,respectively.

Fig.6 Dynamic response of tungsten oxide nanostructure to varying NO2concentration at an operating temperature of 200℃

Fig.7 NO2gas response of tungsten oxide nanostructures as a function of NO2concentration at an operating temperature of 200℃
Tungsten oxide is a typical n-type semiconductor,and its gas-sensing mechanism belongs to the surfacecontrolled type,i.e.the change in resistance of tungsten oxide is primarily caused by the adsorption and desorption of the gas molecules on the surface of the sensing film[20-21].Atmospheric oxygen absorbed on the oxide surface captures electrons from the conduction band of tungsten oxide and forms chemisorbed O-,O2-and O2-,creating a thin electron-depleted space-charge layer at the surface of the oxide.When the tungsten oxide is exposed to the oxidizing NO2,the gas molecules can be directly adsorbed onto the surface by extracting electrons from the conduction band(Eq.(1)and(2))or they can interact with the chemisorbed oxygen on the surface(Eq.(3)and(4))[22-23]:
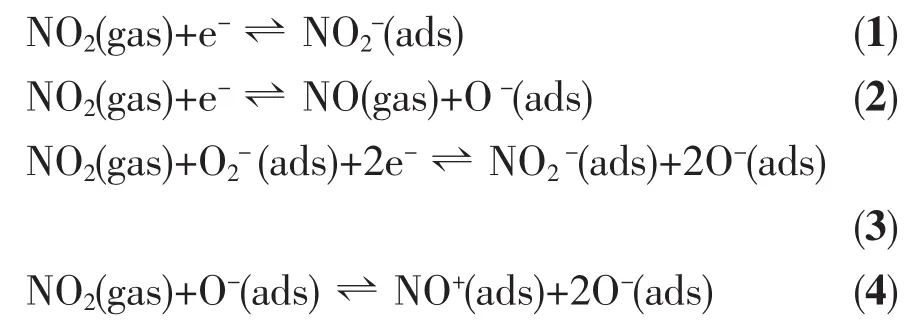
These reactions consume further electrons in the conduction band of tungsten oxide,leading to an increase of the electron-depleted layer and the height of the Schottky barrier,which then leads to the increase in the resistance.Therefore,large specific surface area is beneficial to achieving a high gas response.In our experiments,Brunauer-Emmett-Teller(BET)gas-sorption measurements were employed to evaluate the specific surface area of the as-synthesized tungsten oxide nanostructures by using Quantachrome NOVA automated gas sorption system.The measurements results show that the W18O49nanowires have much larger specific surface area (90 m2·g-1)than the WO3nanosheets(47 m2·g-1)or W18O49nanorods(69 m2·g-1).The larger surface area can provide more adsorptiondesorption sites and a larger amount of surface adsorbed oxygen species interacting with detected gas molecules.Thus,W18O49nanowires with higher specific surface area can show much larger change in resistance upon exposure to NO2than the WO3nanosheets or W18O49nanorod with lower specific surface area.Another important factor for high response of W18O49nanowire is its non-stoichiometric crystal structure[24].There exist much more oxygen vacancies in the crystal structure of non-stoichiometric W18O49than fully oxidized WO3[17],as indicated from the XPS results in Fig.5.The large amounts of oxygen vacanciescan serve as adsorption sites of gas molecular and effect on the electron density in oxide,which is beneficial to achieving much higher gas response[18,25].Above analysis can explain why the W18O49nanowires exhibit higher response than the WO3nanosheets or W18O49nanorod.However,as shown in Fig.7,the non-stoichiometric W18O49nanorod with higher specific surface area shows lower NO2response than the stoichiometric WO3nanosheet with lower specific surface area.This result can be explained from their different microstructure.Comparing the SEM images of WO3nanosheet and W18O49nanorod(Fig.1(d)and Fig.2(a)),it is clear that,differing from the compact structure of nanorod,the nanosheets support each other and form a loose and porous structure which is convenient for the diffusion of NO2gas in the bulk of nanosheets film.It is possible that the loose and porous structure dominate the high response of WO3nanosheet.
3 Conclusion
Tungsten oxide nanostructures including nanowire,nanorod and nanosheet were synthesized by solvothermal method with tungsten hexachloride (WCl6)as the precursor.One-dimensional W18O49nanowire bundles are obtained in 1-propanol at WCl6concentration below 0.01 mol·L-1,while the structure of pure two-dimensional WO3nanosheet was formed at concentration of 0.02 mol·L-1.In the solvent of cyclohexanol,W18O49nanorod is formed at WCl6concentration of 0.005 mol·L-1.The as-synthesized tungsten oxide nanostructure exhibits reversible response to NO2at different concentrations.In comparison with WO3nanosheet or W18O49nanorod,the W18O49nanowire exhibits much higher response to NO2gas due to its much larger specific surface area and non-stoichiometric crystal structure.
[1]Li X L,Lou T J,Sun X M,et al.Inorg.Chem.,2004,43:5442-5449
[2]Ponzoni A,Comini E,Sberveglieri G,et al.Appl.Phys.Lett.,2006,88:203101
[3]Santato C,Odziemkowski M,Ulmann M.J.Am.Chem.Soc.,2001,123:10639-10649
[4]DU Jun-Ping(杜俊平),CHEN Qi-Yuan(陈启元),ZHAO Juan(赵 娟),etal.ChineseJ.Inorg.Chem.(WujiHuaxueXuebao),2007,23:1005-1010
[5]LI Ling(李 玲),PAN Qing-Yi(潘庆谊),CHENG Zhi-Xuan(程知萱),et al.J.Inorg.Mater.,2006,21:151-156
[6]Choi Y G,Sakai G,Shimanoe K,et al.Sens.Actuators B,2004,101:107-111
[7]Pan Z W,Dai Z R,Wang Z L.Science,2001,291:1947-1949
[8]Cui Y,Lieber C M.Science,2001,291:851-853
[9]SunSB,ZouZD,MinGH.Mater.Charact.,2009,60:437-440
[10]Ha J H,Muralidharan P,Kim D K.J.Alloy.Compd.,2009,475:446-451
[11]Huang K,Pan Q T,Yang F,et al.Appl.Surf.Sci.,2007,253:8923-8927
[12]Pfeifer J,Badaljan E,Tekulabuxbaum P,et al.J.Cryst.Growth,1996,169:727-733
[13]Kwan S,Kim F,Akana J,et al.Chem.Commun.,2001,5:447-448
[14]Moon J,Carasso M L,Krarup H G,et al.J.Mater.Res.,1999,14:866-875
[15]Choi H G,Jung Y H,Kim D K.J.Am.Ceram.Soc.,2005,88:1684-1686
[16]Xia Y N,Yang P D,Sun Y G,et al.Adv.Mater.,2003,15:353-389
[17]Liao C C,Chen F R,Kai J J.Sol.Energy Mater.Sol.Cells,2007,91:1258-1266
[18]Gopel W,Schierbaum K D.Sens.Actuators B,1995,26-27:1-12
[19]Park C O,Akbar S A.J.Mater.Sci.,2003,38:4611-4637
[20]Mizsei J.Sens.Actuators B,1993,23:173-176
[21]Rothschild A,Komem Y.J.Appl.Phys.,2004,9:6374-6380
[22]Safonova O V,Delabouglise G,Chenevier B,et al.Mater.Sci.Eng.C,2002,21:105-111
[23]Sayago I,Gutirrez J,Ars L,et al.Sens.Actuators B,1995,25:512-515
[24]Zhao Y M,Zhu Y Q.Sens.Actuators B,2009,137:27-31
[25]Jiménez I,Centeno M A,Scotti R,et al.J.Electrochem.Soc.,2003,150:72-80
钨氧化物纳米结构的合成与表征
秦玉香*包智颖 胡 明 孙 鹏
(天津大学电子信息工程学院,天津 300072)
采用溶剂热法以WCl6作为前体合成出了一维和二维的钨氧化物纳米结构,研究了反应溶剂和前体浓度对钨氧化物物相和形貌的影响并评价了各种钨氧化物纳米结构对NO2气体的敏感性能。XRD、SEM、TEM和XPS的表征结果表明,通过改变溶剂和调整WCl6浓度,可分别获得单斜的W18O49纳米棒、W18O49纳米线和WO3纳米片结构。气敏性能测试结果表明,钨氧化物纳米结构对NO2气体表现出良好的可逆性,与W18O49纳米棒和WO3纳米片相比,W18O49纳米线对NO2具有更高的灵敏度。
氧化钨;纳米结构;溶剂热法;气敏性能
O649
:A
:1001-4861(2010)12-2259-07
2010-05-31。收修改稿日期:2010-08-24。
秦玉香,女,35岁,博士,副教授;研究方向:敏感材料与微传感器。
国家自然科学基金(No.60801018),天津市自然科学基金(No.09JCYBJC01100),教育部博士点新教师基金(No.200800561109)资助项目。
*通讯联系人。 E-mail:qinyuxiang@tju.edu.cn
猜你喜欢
杂志排行
无机化学学报的其它文章
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis,Crystal Structure,Luminescent and Electrochemical Properties of a New Binuclear Cd(Ⅱ)Complex with 2,4-DAA as Ligand
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
