Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
2013-09-29WANGChongChenSONGYanXiaWANGYuanLanWANGPengKeyLaboratoryofUrbanStormwaterSystemandWaterEnvironmentMOEBeijingUniversityofCivilEngineeringandArchitectureBeijing00044ChinaFacultyofScienceCenterSouthUniversityofForestryTech
WANG Chong-ChenSONG Yan-XiaWANG Yuan-Lan*,WANG Peng(Key Laboratory of Urban Stormwater System and Water Environment(MOE),Beijing University of Civil Engineering and Architecture,Beijing 00044,China)(Faculty of Science,Center South University of Forestry Technology,Changsha 40004,China)
Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
WANG Chong-Chen*,1SONG Yan-Xia2WANG Yuan-Lan*,2WANG Peng1
(1Key Laboratory of Urban Stormwater System and Water Environment(MOE),Beijing University of Civil Engineering and Architecture,Beijing 100044,China)(2Faculty of Science,Center South University of Forestry Technology,Changsha 410004,China)
The reaction of AgNO3,4,4′-bipyridine(bpy)/flexible 1,2-di(4-pyridyl)ethylene(dpe),diphenic acid(H2da)in acetic acid/alcohol aqueous solution produces,respectively,block-like crystals of[Ag2(bpy)2(Hda)2](HAc)2·2H2O(1),and[Ag2(dpe)2(da)]·4H2O(2)at room temperature.Both the above-stated compounds consist of parallel 1D infinite cationic chains,[AgL]∞(L=bpy and dpe),interspersed with organic da2-anions which play the role of charge compensation in the crystal structure.The lattice water molecules are situated among these chains,and stabilized by rich hydrogen-bonding interactions,playing a role in the orientation of the da2-in the crystal packing.Optical absorption properties and band gaps of title compounds were determined with UV/Vis/NIR diffuse reflectance spectra,and the results show that the Egcan be assessed at 3.3 eV for both compound 1 and 2.CCDC:768737,1;768736,2.
coordination polymer;diphenic acid;bipyridine-like ligand;crystal structure;optical properties
0 Introduction
The design and assembly of metal-organic frameworks(MOFs)with novel structures has received more and more attentions due to their versatile architecture as well as their special properties like electrical conductivity,magnetism,host-guest chemistry and catalysis[1-9].The construction of coordination polymers is highly influenced by factors like coordination nature of metal ions,the structural characteristic of the poly-dentate organic ligands,the metal-ligand ratio,and the possible counter-ions[10].The key to construction of a desired framework is the selection of organic ligands,and in some cases,a subtle alteration of organic ligands could lead to different architecture,such as[Ag(bpp)][Ag2(bpp)2(ox)]NO3and[Ag2(ppa)2(ox)]·9H2O[10](bpp=1,3-bis(pyridyl)propane; ppa=N-(4-pyridinylmethyl)-4-pyridinecarboamide;ox=oxalate),[Ag(bpp)]2(tdc)·8H2O[11]and[Ag2(bpy)2(H2O)](tdc)·6H2O[12](bpy=4,4′-bipyridine;tdc=thiophene-2,5-dicarboxylate).4,4′-Bipyridine and other pyridyl-donor ligands like 1,2-di(4-pyridyl)ethylene(dpe)can construct a large range of infinite frameworks,including honeycomb,diamond,square grids,ladder,brick,octahedraland T-shaped[3-14].While diphenic acid(H2da)is one of the candidates used as a ligand to build multi-dimensional architectures,in which its two carboxylate groups can coordinate with metal ions via multi-coordinate ways to form a series of metal-organic frameworks bearing different structures and interesting properties[15-18].The combination of center metal ions with neutral N-donor ligands and anionic O-donor ligands can generate more interesting structures which can not be obtained only via one type of ligands[17-21].Furthermore,the prospect of introducing the second or more organic ligands into a reaction system provides further impetus for research on metal-organic supramolecular frameworks.
We are trying to utilize rigid 4,4′-bipyridine(-bpy)/flexible 1,2-di(4-pyridyl)ethylene(dpe)and diphenic acid(H2da)to generate some metal-organic frameworks(MOFs)with novel crystal structures.In this paper,we report the synthesis,crystal structures,and optical absorptance properties of two new and novel metalorganic frameworks assembled by AgNO3,da and 4,4′-bpy/dpe,namely[Ag2(bpy)2(Hda)2](HAc)2·2H2O(1)and[Ag2(dpe)2(da)]·4H2O(2).The single-crystal diffraction results revealed that both complexes contain fascinating sandwich-like framework,in which the anionic sheets built up from da2-anions and lattice water molecules via rich hydrogen-bonding interactions were inserted between the cationic silver complex layers.And the Optical absorption properties of complexes 1 and 2 were determined with UV/Vis/NIR diffuse reflectance spectra.
1 Experimental
1.1 Materials and general methods
All commercially available chemicals were of reagent grade,and used as received without further purification.Elemental analysis for the title complexes was performed by Elementar Vario EL-Ⅲ.Infrared(IR)spectra,in the region of 400~4000 cm-1,were recorded on PerkinElmer Spectrum 100 Fourier Transform infrared spectrophotometer.UV-Vis-NIR diffuse reflectance spectra of the solid samples were measured by Shimadzu UV-3100 spectrophotometer,in which barium sulfate (BaSO4)was used as the standard with 100%reflectance[22].
1.2 Synthesis of complex 1
An ammonia solution(25 mL)containing 0.0085 g AgNO3(0.05 mmol)and 0.012 g diphenic acid~ (0.05 mmol,H2da)was added dropwise to an acetic acid solution (25 mL)of 0.007 8 g 4,4′-bipyridine(0.05 mmol,bpy).The clear mixture was stirred for a few minutes and then allowed to evaporate slowly at room temperature.Block-like brown crystals of[Ag2(bpy)2(Hda)2](HAc)2·2H2O (1)appeared after several weeks.Anal.calcd.for C52H46Ag2N4O14(%):C,53.49;H,3.94;N,4.80;O,19.20.Found(%):C,53.51;H,4.00;N,4.61;O,19.50.IR (KBr,cm-1):3 432,3 050,2 456,1 940,1 702,1 598,1 486,1 438,1 389,1 276,1 214,1 143,1 099,1 066,1 005,852,805,756,651,626,617,576,530,488.
1.3 Synthesis of complex 2
An ammonia solution(25 mL)containing 0.008 5 g AgNO3(0.05 mmol)and 0.012 g diphenic Acid(0.05 mmol,H2da)was added drop-wise to an alcohol solution(25 mL)of 0.009 1 g 1,2-di(4-pyridyl)ethylene(0.05 mmol,dpe).The clear mixture was stirred for a few minutes and then allowed to evaporate slowly at room temperature.Block-like yellow crystals of[Ag2(dpe)2(da)]·4H2O (2)appeared after several weeks.Anal.Calcd.for C38H36Ag2N4O8(%):C,51.09;H,3.94;N,6.27;O,14.34.Found(%):C,52.22;H,4.01;N,6.16;O,14.55.IR(KBr,cm-1):3434,3039,1950,1857,1598,1 498,1 435,1 424,1 416,1 390,1 302,1 257,1 205,1 152,1 108,1 089,1 074,1 010,998,973,955,847,838,827,781,759,716,672,659,554,492.
1.4 X-ray crystallography
Diffraction intensities for complexes 1 and 2,were recorded with a Bruker Smart Apex CCD area detector diffractometer with graphite-monochromatized Mo Kα radiation (λ=0.071073 nm)using φ-ω mode at 298(2)K.Semi-empirical absorption correction were applied using the SADABS program[23].The structure were solved by direct methods[24]and refined by full-matrix least-squares on F2using SHELXS 97 and SHELXL 97 programs respectively[24-25].All non-hydrogen atoms were refined anisotropically and hydrogen atoms were placed in geometrically calculated positions.Crystallographic data and structural refinements for compound 1 and 2 are summarized in Table 1.Selected bond lengths and angles for both compounds are listed in Table 2.
CCDC:768737,1;768736,2.

Table 1 Details of X-ray data collection and refinement for complexes 1 and 2

Table 2 Selected bond lengths(nm)and angles(°)for complex 1 and 2
2 Results and discussion
2.1 Crystallographic analysis of complex 1
The analysis of the crystal structure reveals that[Ag2(bpy)2(Hda)2](HAc)2·2H2O(1)is made up of neutral chains of[Ag2(bpy)2(Hda)2]∞,acetic acid and water molecules.In the neutral chains of[Ag2(bpy)2(Hda)2]∞,each Ag atom is coordinated,in a slightly distorted tetrahedral geometry,by two nitrogen atoms from two different bpy ligands(Ag-N 0.222 2(3)nm;N-Ag-N 169.80(10)°),and two oxygen atoms from two different bpda ligands(Ag-O 0.258 9(3)nm and 0.259 3(3)nm;N-Ag-O 88.44(10)°~92.08(9)°),as illustrated in Fig.1a and Table 2.And bpy acts as typical bidentate ligand,linking two Ag atoms via nitrogen atoms from two pyridyl rings.Da act as both counterions,balancing the cationic charge of Ag,and bidentate ligands with coordination mode as illustrated in Scheme 1a,joining two Ag atoms via two oxygen atoms from the same COO-group,while the two oxygen atoms on the other COOH group are terminal.
Inthe[Ag2(bpy)2(Hda)2](HAc)2·2H2O,theda anions coordinate and weakly bridge adjacent Ag centres,thus reducing the effective positive charge of each Ag,which allows the cations to approach more closely.Therefore,the apparent Ag…Ag interactions are formed with distances of 0.308 34(7)nm,which is comparable to those(0.2970(2)~0.342(3)nm)found in other Ag coordination polymers with pyridyl-donor ligands[10-11,27].The adjacent chains are interconnected by da ligands via oxygen atoms with chelating mode and Ag…Ag interactions in a “head to head”fashion,binding two adjacent chains together to afford infinite “ladders”(Fig.1b),which are further strengthened by aromatic ππ interactions(0.311 43(4)nm).The adjacent chains are joined into 3D sandwich-like network by rich hydrogen-bonding interactions (Table 3)formed by lattice water molecules and acetic acid.

Scheme 1 Two coordination modes of diphenic ion in complex 1 and 2

Fig.1 (a)Asymmetric unit of[Ag2(bpy)2(Hda)2](HAc)2·2H2O(1)and coordination environments around Ag cations(H atoms are omitted for clarity);(b)double chains viewed from a axis in 1 built by da ligands and Ag…Ag interactions
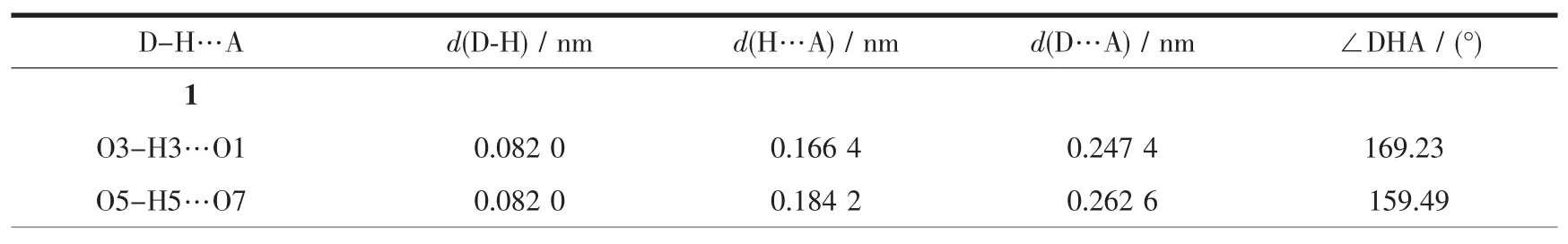
Table 3 Hydrogen bonds for complex 1 and 2

Continued Table 3
2.2 Crystallographic analysis of complex 2
The crystal structure reveals that[Ag2(dpe)2(da)]·4H2O is made up of 1D infinite neutral double chains[Ag2(dpe)2(da)]∞and lattice water molecules.The Ag(1)atom is coordinated in a slightly distorted tetrahedral geometry with two nitrogen atoms from two different dpe ligands(Ag1-N 0.219 3(3)nm;N-Ag1-N 153.26°),and two oxygen atoms from two COO-group attached on different benzene rings of the same da ligand(Ag1-O 0.251 1(4)and 0.2539(4)nm;N-Ag1-O 109.50(14)°;OAg1-O 104.40(12)°).While Ag(2)atom,in distorted trigonal geometry,is coordinated by two nitrogen atoms from two different dpe ligands(Ag-N 0.217 9(4)and 0.2201(4)nm)and an oxygen atom from COO-group(Ag-O 0.2431(4)nm).As illustrated in Fig.2a and Table 2.The dpe ligand acts as typical 4,4′-bipyridinelike bidentate ligand,linking two Ag atoms via nitrogen atoms from two pyridyl rings to form infinite 1D chain.While da acts as tridentate ligand with coordination mode as shown in Scheme 1b,in which one Ag atom was coordinated by two oxygen atoms,with chelating mode,from two different COO-group attached on two different benzene rings of the same ligand molecule;the other Ag atom was coordinated by an oxygen atom with mono mode.
It is noteworthy that in 2 the ligand-unsupported Ag…Ag(0.3539 nm)and Ag…N (0.3450 nm)weak interactions also play important role on connecting the adjacent[Ag(dpe)]+chains,as shown in Fig.2b.The 1D double chains are connected into 2D sheet by rich hydrogen-bonding interactions formed by lattice water moleculessituatingamongthe[Ag2(dpe)2(da)]molecules.

Fig.2 (a)Asymmetric unit of[Ag2(dpe)2(da)]·4H2O;(2)coordination environments around Ag cations(H atoms are omitted for clarity);(b)Ag…N and Ag…Ag interactions between the[Ag(dpe)]∞chains
2.3 Optical energy gap
In order to explore the conductivity of the title compound,the measurement of diffuse reflectivity for a powder sample was used to obtain its band gapEg;which agrees well with that obtained by absorption measurement from a single crystal[27].The band gapEgwas determined as the intersection point between the energy axis and the line extrapolated from the linear portion of the absorption edge in a plot of KubelkaMunk functionFagainst energyE.KubelkaMunk function,F=(1-R)2/(2R),was converted from the recorded diffuse reflectance data,whereRis the reflectance of an infinitely thick layer at a given wavelength.TheFversusEplots for the compound 1 and 2 are shown in Fig.3,where steep absorption edges are displayed and theEgof both compounds can be assessed at 3.3 eV.

Fig.3 F-Eplots for compound 1 and 2
3 Conclusions
In conclusion,we present two novel and interesting metal-organic frameworksbuiltfrom rigid 4,4′-bipyridine(4,4′-bpy)/flexible 1,2-di(4-pyridyl)ethylene(dpe),diphenic acid (H2da)and Ag(Ⅰ)ions.The isolations of 1 and 2 imply that the utilization of different ligands can lead to new types metal coordination compounds with fascinating architectures.And the reflectance spectrum measurements revealed the presence of optical band gaps with 3.3 eV compound 1 and 2,respectively.
[1]Zhang L J,Wei Y G,Wang C,et al.J.Solid State Chem.,2004,177:3433-3428
[2]Zhang G,Guo H,Wu N,et al.Chin.J.Chem.,2003,21:40-43
[3]Zhang J,Liu C,Zhang D,et al.Inorg.Chim.Acta,2007,360:3553-3559
[4]Liu C,Gao S,Kou H,et al.Cryst.Growth Des.,2006,6:94-98
[5]Sun C,Gao S,Jin L.Eur.J.Inorg.Chem.,2006:2411-2421
[6]Batten S R,Robson R.Angew.Chem.Int.Ed.,1998,37:1460-1494
[7]Blake A J,Champness N R,Hubberstey P,et al.Coord.Chem.Rev.,1999,183:117-138
[8]Moulton B,Zaworotko M J.Chem.Rev.,2001,101:1629-1658
[9]Hagrman P J,Hagrman D,Zubieta J.Angew.Chem.Int.Ed.,1999,38:2638-2684
[10]Tong M L,Wu Y M,Ru J,et al.Inorg.Chem.,2002,41:4846-4848
[11]YU Jing-Hua(于景华),DING Chang-Jiang(丁长江),HAN Ke-Fei(韩克飞),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22:607-611
[12]YU Jing-Hua(于景华),DU Hong-Guang(杜洪光),HAN Ke-Fei(韩克飞),et al.J.Beijing University Chem.Tech.:Natural Science Edition(Beijing Huagong Daxue Xuebao),2006,33:61-64
[13]Wang C C.Asian J.Chem.,2009,21:4755-4762
[14]Wang C C,Yin C.Z.Kristallogr.NCS.,2008,223:13-15
[15]Malaestean I L,Speldrich M,Baca S G,et al.Eur.J.Inorg.Chem.,2009:1011-1018
[16]Gou L,Zhang B,Hu H M,et al.J.Mol.Struct.,2008,889:244-250
[17]Wang R,Jiang F,Han L,et al.J.Mol.Struct.,2004,699:79-84
[18]Wang R,Zhou Y,Sun Y.Cryst.Growth Design.,2005,5:251-256
[19]Lo S M L,Chui S S Y,Shek L Y,et al.J.Am.Chem.Soc.,2000,122:6293-6294
[20]Shi Z,Feng S H,Sun,Y,et al.Inorg.Chem.,2001,40:5312-5313
[21]Tao J,Tong M L,Chen X M.J.Chem.Soc.,Dalton Trans.,2000:3669-3674
[22]Li J,Chen Z,Wang X,et al.J.Alloys Compds.,1997,28:262-263
[23]Sheldrick G M.SADABS,Program for Empirical Absorption Correction of Area Detector Data,University of Göttingen,Germany,1997.
[24]Sheldrick G M.SHELXS97,Program for Crystal Structure Solution,University of Göttingen,Germany,1997.
[25]Sheldrick G M.SHELXL97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[26]Wang C C,Ma H W.Z.Kristallogr.NCS.,2007,222:101-103
[27]McCarthy T J,Ngeyi S P,Liao J H,et al.Chem.Mater.,1993,5:331-336
两个含双配体的银(Ⅰ)配合物的晶体结构和光学性能
王崇臣*,1宋燕霞2王元兰*,2王 鹏1
(1北京建筑工程学院,城市雨水系统与水环境省部共建教育部重点实验室,北京 100044)(2中南林业科技大学理学院,长沙 410004)
室温下,AgNO3、4,4′-联吡啶(bpy)/1,2-二(4-吡啶基)乙烯(dpe)与联苯二甲酸(H2da)在乙酸/乙醇水溶液中反应数周后分别得到块状晶体[Ag2(bpy)2(Hda)2](HAc)2·2H2O(1)和[Ag2(dpe)2(da)]·4H2O(2)。上述两种晶体均包含无限扩展的一维阳离子链[AgL]∞(L=bpy和dpe),而穿插其中的有机阴离子da2-起到了平衡电荷的作用。结晶水分布于离子链之间并形成氢键,对晶体堆积起到了定位作用。UV/Vis/NIR漫反射光谱测定结果表明化合物1和2的光学能隙Eg均约为3.3 eV。
配位聚合物;联苯二甲酸;类联吡啶;晶体结构;光学性能
O614.122
:A
:1001-4861(2011)02-0361-06
2010-07-25。收修改稿日期:2010-10-10。
北京市属高等学校人才强教计划项目(No.PHR201008372和PHR201106124)和北京建筑工程学院科研基金(No.100902602)资助。
*通讯联系人。 E-mail:chongchenwang@126.com,csfuyl@163.com;会员登记号:S060000959M。
猜你喜欢
杂志排行
无机化学学报的其它文章
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis,Crystal Structure,Luminescent and Electrochemical Properties of a New Binuclear Cd(Ⅱ)Complex with 2,4-DAA as Ligand
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
