三联吡啶羧基Zn(Ⅱ),Cu(Ⅱ)和Fe(Ⅱ)配合物研究
2013-08-20颜流水吴美凤邓春健
胡 斌 颜流水 吴美凤 邓春健 黄 伟
(1 南昌航空大学环境与化学工程学院,南昌 330063)
(2 南京大学化学化工学院,南京微结构国家实验室,配位化学国家重点实验室,南京 210093)
0 Introduction
Benefit to the strong binding affinity of 2,2′∶6′,2′-terpyridine (tpy) and their structural analogs containing three nitrogen atoms, they can coordinate with a variety of metal ions and they have attracted much attention in the fields of supramolecular and coordination chemistry[1-4]. Furthermore, they have been widely used as multifunctional materials for photovoltaic applications[5-7], optoelectronic device[8-9],magnetic properties[10], nanomaterials[11], biomedical activities[12-15], catalysts[16]and so on.

Scheme 1 Schematic illustration for the preparation of metal complexes 1, 2 and 3
Among them, the tpy derivatives having one or more carboxylate groups, such as [2,2′∶6′,2″-terpyridine]-4′-carboxylic acid, 4-([2,2′∶6′ ,2″-terpyridin]-4′-yl)benzoic acid and [2,2′∶6′,2″-terpyridine]-3,3″,4′-tricarboxylic acid, are excellent multidentate ligands to form novel metal complexes with high thermal and chemical stabilities and intriguing architectures and functions. In addition, their ruthenium (Ⅱ)complexes can be used in the investigations of dye sensitized solar cells by anchoring their carboxylate groups on TiO2[5,7,17-18]. With regard to ligand [2,2′∶6′,2″-terpyridine]-4′-carboxylic acid (HL) in this work[19-20], their structural reports are mainly involved in the Ru (Ⅱ)photosensitizers[17,21-22]but less related to other transition-metal ions[23]. Our purpose is to use this rigid multidentate chelating ligand as a building block to react with transition-metal ions forming self-assembled coordination complexes with interesting fluorescence properties. In our previous work, we have successfully constructed a family of Ru (Ⅱ), Cu(Ⅱ), Zn(Ⅱ), Cd(Ⅱ),Ni(Ⅱ), and Fe(Ⅱ)transition-metal complexes based on a tpyCl ligand with different counterions[24-26]. As related studies in this field, we report herein the syntheses,characterizations and luminescence properties of three mononuclear complexes (Scheme 1) formulated as[ML2]·4H2O (1, M=Zn(Ⅱ); 2, M=Cu(Ⅱ); 3, M=Fe(Ⅱ)).
1 Experimental
1.1 Materials and instruments
All reagents and solvents were of analytical grade and used without any further purification. The anhydrous solvents were drawn into syringes under a flow of dry N2gas and directly transferred into the reaction flask to avoid contamination. HL was prepared via a previously reported approach[19].
Elemental analyses (EA) for carbon, hydrogen,and nitrogen were performed on a Perkin-Elmer 1400C analyzer. Fourier transform infrared (FT-IR)spectra (4 000 ~400 cm-1) were recorded using a Nicolet FT-IR 170X spectrophotometer on KBr disks.Powder X-ray diffraction (PXRD) measurements were performed on a Philips X′ pert MPD Pro X-ray diffractometer using Cu Kα radiation(λ=0.154 18 nm),in which the X-ray tube was operated at 40 kV and 40 mA at room temperature. Luminescence spectra were recorded on a Hitachi 850 fluorescent spectrophotometer at room temperature (25 ℃).
1.2 Synthesis of the compounds
Preparation of complex 1: [ZnL2]·4H2O (1):
A mixture of HL (0.028 g, 0.1 mmol), Zn(OAc)2·2H2O (0.022 g, 0.1 mmol), deionized water (1.0 mL),methanol (10.0 mL) and DMF (1.0 mL) was adjusted to pH=8.0 with triethylamine. The mixture was frozen and sealed under a vacuum in a thick-walled Pyrex tube, then placed inside an oven at 150 ℃for 96 h.The yellow block crystals were obtained in a yield of 0.019 g (55.1%) on the basis of HL. Main FT-IR absorptions (KBr pellets, ν / cm-1): 3 059 (m), 1 646(s), 1 623 (s), 1 599 (m), 1 576 (m), 1 554 (m), 1 463(w), 1 410 (m), 1 369 (m), 1 322 (s), 1 245 (w), 1 161(w), 1 013 (m), 805 (w), 781 (m), 745 (m), 731 (w),684 (w), 654 (w), 637 (w). Elemental Anal. Calcd. for C32H28ZnN6O8(%): C 55.70; N 12.18; H 4.09. Found(%): C 55.43; N 12.37; H 4.42.
[CuL2]·4H2O (2): The preparation of 2 is similar to that of 1 except that Cu(OAc)2·H2O (0.020 g, 0.1 mmol) was used instead of Zn(OAc)2·2H2O. Green block single crystals of 2 suitable for X-ray crystallographic analysis were obtained after one week. Yield:16.8 mg (48.8% based on HL). Main FT-IR absorptions(KBr pellets,ν/cm-1):3 392(b),3 050(w),1 604(m), 1 587(s), 1 560(s), 1 477(w), 1 413 (m), 1 394(s),1 367(m), 1 341(w), 1 325(m), 1 305(w), 1 253(w),1 161(w), 1 052(w), 1 022(w), 784(m), 745(w), 728(w),687 (m), and 654 (w). Elemental Anal. Calcd. for C32H28CuN6O8(%): C 55.85; N 12.21; H 4.10. Found(%): C 55.57; N 12.45; H 3.92.
[FeL2]·4H2O (3): complex 3 was prepared in the same method as that of 1 except that Fe(ClO4)2·6H2O(0.018 g, 0.05 mmol) was used instead of Zn (OAc)2·2H2O. Purple block single crystals of 3 suitable for Xray crystallographic analysis were obtained after one week. Yield: 12.8 mg (37.6% based on HL). Main FTIR absorptions (KBr pellets, ν / cm-1): 3 052(w), 1 660(w), 1 647(w), 1 602(w), 1 546(w), 1 536(m), 1 429(m), 1 374(w), 1 337(w), 1 113(w), 831(m), 558(m),529 (w), 477 (w). Elemental Anal. Calcd. for C32H28FeN6O8(%): C 56.48; N 12.35; H 4.15. Found(%): C 56.72; N 12.61; H 4.02.
Caution! Perchlorate salt of Fe(Ⅱ)used in this study is potentially explosive and should be prepared in small quantities. No such problems were encountered in any of the syntheses reported, but great care must always be exercised. In addition, the sealed tubes should be placed inside a protective case for protection against explosion when heating and the temperature should be less than 160 ℃.
1.3 X-ray data collection and solution
Single-crystal samples of 1~3 were glue-covered and mounted on glass fibers and then used for data collection at 291(2) K. The diffraction data were collected on a Bruker SMART 1K CCD diffractometer using graphite mono-chromated Mo Kα radiation (λ=0.071 073 nm). The crystal systems were determined by Laue symmetry and the space groups were assigned on the basis of systematic absences using XPREP.Absorption corrections were performed to all data and the structures were solved by direct methods and refined by full-matrix least-squares method on Fobs2by using the SHELXTL-PC software package[27-29]. All non-H atoms were anisotropically refined and all hydrogen atoms were inserted in the calculated positions assigned fixed isotropic thermal parameters and allowed to ride on their respective parent atoms.The summary of the crystal data, experimental details and refinement results for complexes 1~3 is listed in Table 1, while selected bond distances and bond angles related to the central Zn(Ⅱ), Cu(Ⅱ)and Fe(Ⅱ)ions are given in Table 2. Hydrogen bonding interactions in complexes 1~3 are shown in Table 3.
CCDC: 925947, 1; 925948, 2; 925949, 3.
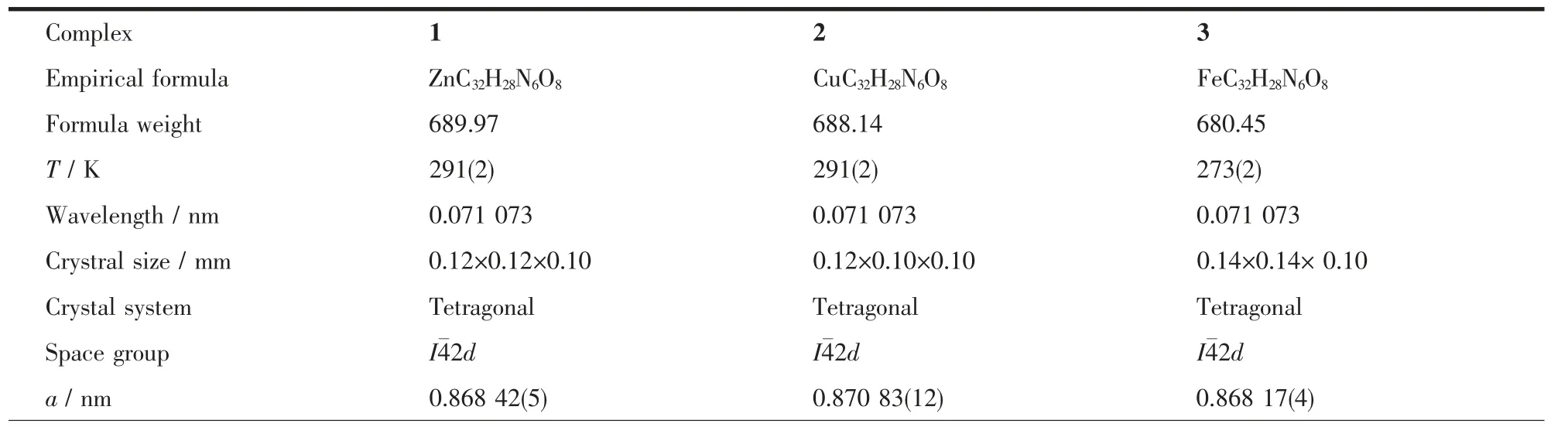
Table 1 Crystal data and structure refinement parameters for complexes 1~3

Continued Table 1

Table 2 Selected bond distances (nm) and angles (°) for complexes 1~3

Continued Table 2

Table 3 Hydrogen bonding interactions in complexes 1~3
2 Results and discussion
2.1 Synthesis and spectral characterizations

Fig.1 Solid-state fluorescence emission spectra of HL and complex 1 at room temperature
Three complexes 1~3 were prepared by a simple solvothermal method in a thick-walled Pyrex tube with stoichiometric zinc (Ⅱ)acetate (copper (Ⅱ)acetate or ferrous(Ⅱ)perchlorate) and HL for 4 d, as shown in scheme 1. The formation of stable, neutral and divalent metal complexes was verified by their elemental and FT-IR spectral analyses. To better understand the nature of the luminescence properties,fluorescence emission of complex 1 in the solid state is illustrated in Fig.1. The ligand shows one intense emission peak at 400 nm (λex=347 nm), which is assigned to the π-π* electronic transitions. Compared with the photoluminescence spectrum of free ligand,the intraligand π-π* transition between the heterocyclic aromatic rings in complex 1 shows an obvious bathochromic shift and enhancement from 400 to 494 nm (λex=397 nm), because the ligand coordinated with the Zn2+ion increases electron delocalization on the complex backbone. In contrast, no emission peaks can be detected in the photoluminescence spectra of complexes of 2 and 3 (data are not shown here). The fluorescence deactivation can be attributed to an increase of energy gap required for electron transfer between the central Cu2+and Fe2+ion and the fluorophore ligand (HL) after coordination. In addition, the pure phase of mononuclear complexes 1 ~3 are also confirmed by PXRD patterns as shown in Fig.SI1-3.
2.2 Single-crystal structures of mononuclear Zn(Ⅱ), Cu(Ⅱ)and Fe(Ⅱ)complexes 1~3
As shown in Fig.2~4, X-ray single-crystal structural determination of complexes 1~3 reveals that they are all crystallize in the tetragonal I42d space group in which the crystallographically imposed center of symmetry is observed. The asymmetric unit of 1~3 is composed of one central metal ion (Zn(Ⅱ)for 1, Cu(Ⅱ)for 2 and Fe (Ⅱ)for 3), two ligands, and four lattice water molecules. The central metal ions are sixcoordinated by six nitrogen atoms from two tridentate L ligands to form a compressed octahedral coordination environment leaving the two protonated carboxylate groups free of coordinative bond. In the molecular structures of 1~3, the ZnN bond lengths are 0.206 7(3) and 0.217 4(3) nm and the Cu-N distances are 0.197 1(4) and 0. 216 9(4) nm, while the Fe-N distances are shorter at 0.188 5(2) and 0.198 9(2) nm,respectively (Table 2). These measured MN bond lengths are in good agreement with those reported in literature[30-31]. The carboxylic groups are not completely coplanar with the central terpyridine ring with dihedral angles of 25.6(3)°, 23.1(4)° and 18.6(2)° in 1~3. The related two CO bond lengths are the same as 0.122 4(4), 0.122 2(6) and 0.122 2(3) nm in 1~3,indicative of the delocalized and deprotonated forms of carboxylic groups.

Fig.2 ORTEP diagram of the molecular structure of complex 1

Fig.3 ORTEP diagram of the molecular structure of complex 2

Fig.4 ORTEP diagram of the molecular structure of complex 3

Fig.5 Perspective view of the π-π stacking interactions in the crystal packing of complex 1

Fig.6 Perspective view of the π-π stacking interactions in the crystal packing of complex 2
In the crystal packing of 1~3, hydrogen bonding interactions are found between the carboxylic oxygen atoms and the protons of one of the terpyridine ring(Table 3). It is also noteworthy that π-π stacking interactions are observed between adjacent aromatic rings and the centroid-to-centroid separations between neighboring pyridine rings are 0.366 4(6) nm for complex 1,0.367 3(6)nm for complex 2 and 0.371 7(4)nm for complex 3. As can be seen in Fig.5~7, complex 1 ~3 are extended into three-dimensional supramolecular networks by virtue of these π-π stacking and hydrogen bonding interactions, which will significantly stabilize the overall solid-state structures.

Fig.7 Perspective view of the π-π stacking interactions in the crystal packing of complex 3
3 Conclusions
In conclusion, a [2,2′∶6′,2″-terpyridine]-4′-carboxylic acid ligand is used to prepare three new mononuclear zinc(Ⅱ), copper(Ⅱ)and ferrous(Ⅱ)complexes 1~3 under solvothermal synthetic conditions. Ligand L shows a tridentate chelating fashion where the deprotonated carboxylate unit is free of coordinative bond. The solid-state luminescence spectrum of complex 1 indicates the blue photoluminescence.Further work is being undertaken in our lab on the preparation and properties of heterometal coordination polymers based upon these mononuclear metal complexes where the uncoordinated and deprotonated carboxylate groups in 1~3 are used to coordinate with other metal ions with or without the presence of certain auxiliary ligands.
[1] Wild A, Winter A, Schlutter F, et al. Chem. Soc. Rev., 2011,40(3):1459-1511
[2] Yuan S C, Chen H B, Wang H C. Prog. Chem., 2009,21(10):2132-2152
[3] Pan Y X, Tong B, Zhi J G, et al. Prog. Chem., 2009,21(9):1763-1771
[4] Constable E C. Chem. Soc. Rev., 2007,36(2):246-253
[5] Nazeeruddin M K, Pechy P, Renouard T, et al. J. Am. Chem.Soc., 2001,123(8):1613-1624
[6] Cooke M W, Hanan G S, Loiseau F, et al. J. Am. Chem.Soc., 2007,129(34):10479-10488
[7] Chou C C, Wu K L, Chi Y, et al. Angew. Chem.-Int. Ed.,2011,50(9):2054-2058
[8] Winter A, Friebe C, Chiper M, et al. J. Polym. Sci. Pol.Chem., 2009,47(16):4083-4098
[9] Seo K, Konchenko A V, Lee J, et al. J. Am. Chem. Soc.,2008,130(8):2553-2559
[10]Hayami S, Komatsu Y, Shimizu T, et al. Coord. Chem. Rev.,2011,255(17-18):1981-1990
[11]Winter A, Hager M D, Newkome G R, et al. Adv. Mater.,2011,23(48):5728-5748
[12]Wu P, Wong E L M, Ma D L, et al. Chem.-Eur. J., 2009,15(15):3652-3656
[13]Manner V W, DiPasquale A G, Mayer J M. J. Am. Chem.Soc., 2008,130(23):7210-7211
[14]Eryazici I, Moorefield C N, Newkome G R. Chem. Rev.,2008,108(6):1834-1895
[15]Cummings S D. Coord. Chem. Rev., 2009,253(9-10):1495-1516
[16]Sinha P, Raghuvanshi D S, Singh K N, et al. Polyhedron,2012,31(1):227-234
[17]Wolpher H, Sinha S, Pan J X, et al. Inorg. Chem., 2007,46(3):638-651
[18]Schulze B, Escudero D, Friebe C, et al. Chem.-Eur. J., 2012,18(13):4010-4025
[19]Constable E C, Dunphy E L, Housecroft C E, et al. Dalton Trans., 2007,(38):4323-4332
[20]Husson J, Beley M, Kirsch G. Tetrahedron Lett., 2003,44(9):1767-1770
[21]Cooke M W, Santoni M P, Hanan G S, et al. Inorg. Chem.,2008,47(14):6112-6114
[22]Park H J, Kim K H, Choi S Y, et al. Inorg. Chem., 2010,49(16):7340-7352
[23]Jarosz P, Du P W, Schneider J, et al. Inorg. Chem., 2009,48(20):9653-9663
[24]You W, Huang W, Fan Y, et al. J. Coord. Chem., 2009,62(13):2125-2137
[25]Huang W, You W, Wang L, et al. Inorg. Chim. Acta, 2009,362(7):2127-2135
[26]Huang W, Qian H F. J. Mol. Struct., 2008,874(1-3):64-76
[27]Siemens, SAINT v4 Software Reference Manual, Siemens Analytical X-Ray Systems, Inc., Madison, Wisconsin, USA,2000.
[28]Sheldrick G M. SADABS, Program for Empirical Absorption Correction of Area Dectector Data, Univ. of Gottingen,Germany, 2000.
[29]Sheldrick G M. SHELXTL, Version 6.10 Software Reference Manual, Siemens Analytical X-Ray Systems, Inc., Madison,Wisconsin, USA, 2000.
[30]Wang P, Li Z, Lü G C, et al. Inorg. Chem. Commun., 2012,18:87-91
[31]Beves J E, Bray D J, Clegg J K, et al. Inorg. Chim. Acta,2008,361(9-10):2582-2590
