两个4-[(8-羟基-5-喹啉)偶氮]苯磺酸配合物的合成、结构及性质研究
2013-08-20罗亚楠许宪祝于晓洋曲小姝
罗亚楠 许宪祝 张 潇 于晓洋 曲小姝
(1 哈尔滨工业大学,城市水资源与水环境国家重点实验室,哈尔滨 150080)(2 吉林化工学院,化学与制药工程学院,吉林 132022)
Coordination chemistry has developed tremendously in the past 30 years, owing to the synthesis and characterization methods arising for compound structures[1-6]. In the constructions of coordination polymers, one important strategy is the extension of lowdimensional building blocks to high-dimensional networks through weak intermolecular interactions,including hydrogen-bonding, π…π stacking and Van der Waals interactions, etc.[7]. Large numbers of coordination polymers possessing diversity of topologies and properties have been obtained, which is conveyed in the wide choice of organic ligands[8-11]. Among them,the ligands containing oxygen and nitrogen atoms are the focus due to their various coordination modes[12-15],and many such ligands possessing strong coordination abilities, unique features or rigid frame structures have been chosen to construct specific function coordination polymers[16-18]. For instance, a rigid ligand 3,3′,5,5′-azobenzenetetracarboxylic acid possessing intense blue emission has been used to construct blue fluorescent materials with some d10metals[19]. As we know, azo-based systems represent one type of bridging aromatic carboxylate ligand employed in the generation of coordination networks. For example, the ligands, 3,3′-azodibenzoate (3,3′-ADB) and 4,4′-azodibenzoate (4,4′-ADB), have been employed in the synthesis of [Cd(3,3′-ADB)2·(H2NMe2)·(NH4)]nand{Tb2(4,4′-ADB)3[(CH3)2SO]4·16[(CH3)2SO]}n, respectively[20-22]. On the other hand, 8-hydroxyquinoline (8-HQ)derivatives are well-known ligands with high coordinated ability to metal ions and display excellent fluorescent performance. For example, the d10metals coordination polymers constructed with 7-iodo-8-hydroxyquinoline-5-sulfonic acid and mercury[23]or zinc[24], and the main group metals lithium[25]or aluminum[26]coordination polymers based on 8-hydroxyquinoline-5-sulfonato have been reported because of rich structures and optical properties. However, the ligands containing simultaneously 8-hydroxyquinoline and benzenesulfonic acid connected via an azo group have not been reported. Based on the above, we have designed and synthesized successfully a ligand, 4-[(8-hydroxy-5-quinolinyl)azo]-benzenesulfonic acid (H2L),which may be an excellent candidate for constructing the coordination polymers with diverse structures and outstanding fluorescent performance. To the best of our knowledge, no examples of crystal compounds containing H2L have been reported yet.
Here in, we report the syntheses of two compounds, namely [CuL(en)]·0.5H2O (1) and [CuL2][Cu(en)2]·2H2O (2). Interestingly, the crystalline structures of compounds 1 and 2 are both constructed from two discrete molecules (or ions), respectively,and the two discrete molecules (or ions) are connected by non-covalent interactions to form 3D supramolecular networks. Moreover, the coordination mode (Scheme 1) of H2L is identical to compounds 1 and 2, in which the quinoline nitrogen and deprotonated oxygen atoms of 8-HQ bind to Cu(Ⅱ)cation as the usual bidentate chelate and the sulfonate oxygen atoms does not participate in coordination. In addition, PXRD, IR, TGA experiments were carried out to check the phase purity and the stability of the two compounds. Fluorescent properties have also been investigated in solid state, and the results showed that the two compounds may have potential application in photoactive materials field.

Scheme 1 Coordination mode of H2L ligand in compounds 1 and 2
1 Experimental
1.1 Materials and general methods
All the starting materials were reagent grade and used as purchased without further purification.Distilled water was used throughout. The radical ligand H2L, 4-[(8-hydroxy-5-quinolinyl)azo]-benzenesulfonic acid, was prepared according to the literature method[27-28]. The elemental analyses were carried on a Perkin-Elmer 240C elemental analyzer. The infrared(IR) spectra were recorded (400~4 000 cm-1region)on a Nicolet Impact 410 FT-IR spectrometer using KBr pellets. Thermogravimetric analyses (TGA) were performed under oxygen atmosphere with a heating rate of 10 ℃·min-1using a Perkin-Elmer TGA 7 thermogravimetric analyzer.Fluorescence spectrum was performed on a HITACHIF-7000 Fluorescence Spectrophotometer at room temperature. PXRD were collected on a Siemens D5005 diffractometer by using Cu Kα(λ=0.154 18 nm)with a graphite monochromator.
1.2 Syntheses of complexes
[CuL(en)]·0.5H2O (1): A mixture of H2L (5 mg,0.015 mmol), CuCl2·2H2O (30 mg, 0.18 mmol), N,Ndimethylacetamide (DMA, 15 mL), H2O (2 mL) and en(0.012 5 mL) was stirred at room temperature for 1 h until a clear purple solution formed, finally sealed in a 25 mL glass bottle and heated at 100 ℃for 3 d.Afterwards, it was cooled to 25 ℃at a rate of 4 ℃·h-1.Brown sheet crystals were obtained after washing with DMA and drying in air. Yield: 55% based on copper.Anal. Calcd. for C34H36Cu2N10O9S2(%): C 44.39; H 3.94; N 15.23; Found(%): C 44.31; H 3.88; N 15.18.
[CuL2][Cu(en)2]·2H2O (2): This compound was prepared in a similar manner as compound 1 except for H2L (10 mg, 0.030 mmol), DMA (12 mL) and H2O(6 mL). Brown sheet crystals were obtained after washing with DMA and drying in air. Yield: 32%based on copper. Anal. Calcd. for C34H38Cu2N10O10S2(%): C 43.54; H 4.08; N 14.93; Found(%): C 43.04; H 3.99; N 14.88.
1.3 Crystal structure determination
Compounds 1 and 2 were stable under ambient conditions and brown single crystals were glued on thin glass fibers. Diffraction intensities for two compounds were collected at 296 K on Bruker Smart Apex ⅡCCD diffractometer (Mo Kα, 0.071 073 nm).An empirical absorption correction was applied to the date using the SADABS program. The structures were solved by the direct method and refined by full matrix least squares (SHELX-97)[29]. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were located geometrically by the program OLEX2[30].The final formula was derived from crystallographic data combined with elemental and thermogravimetric analyses data. A summary of the crystallographic data and structural determination for compounds 1 and 2 is listed in Table 1.
CCDC: 881778, 1; 889463, 2.
2 Results and discussion
2.1 Description of crystal structures

Table 1 Crystal data and structure refinements for compounds 1 and 2
Single-Crystal X-ray diffraction analysis reveals that the asymmetric unit of compound 1 contains two[CuL(en)] molecules A and B together with one disordered water molecule (Fig.1). In molecules A and B, Cu1 and Cu2 are both coordinated by one nitrogen atom, one oxygen atom from the quinolinol group of one L2-ligand and two nitrogen atoms from an en molecule, forming distorted square geometries,selected bond lengths and bond angles are provided in Table 2. The bond lengths of Cu1-N1, Cu1-N2, Cu1-O1 and Cu1-N9 are 0.197 7(4), 0.199 9(4), 0.195 3(4)and 0.200 2(4) nm, and the bond lengths of Cu2-N3, Cu2-N4, Cu2-O2 and Cu2-N10 are 0.196 4(5),0.199 0(5),0.193 1(4)and 0.199 7(4)nm,respectively.The angles of N1-Cu1-N2, N2-Cu1-O1, O1-Cu1-N9 and N9-Cu1-N1 are 84.57(17)°,93.07(16)°,83.17(15)°and 99.84 (17)°, and the angles of N3-Cu2-N4, N4-Cu2-O2,O2-Cu2-N10 and N10-Cu2-N3 are 84.70(20)°,91.43(19)°, 83.85(18)° and 100.01(19)°, respectively.From above data can see, molecules A and B of compound 1 are similar in the structure but the bond lengths and angles are different.

Fig.1 Coordination environment of Cu1 and Cu2 in compound 1 with thermal ellipsoids at 30% probability

Table 2 Selected bond lengths (nm) and angles (°) for compounds 1 and 2
It is noteworthy that the cohesion of these molecules is strengthened by hydrogen bonding interactions, and hydrogen bond distances (nm) and angles (°) for compound 1 are listed in Table 3. In compound 1, N2 acting as H-donor bonds to thesulfonate oxygen atom O6iiiof the neighboring molecule A (N2…O6iii0.301 4(5) nm, N2-H2C…O6iii160°), thus the molecules A are linked into onedimensional (1D) chains through the N -H … O hydrogen bonding interactions in a head-to-tail manner. Similarly, the molecules B are connected into 1D chains through the hydrogen bonding interaction between N4 and sulfonate oxygen atom O3vof the adjacent molecule (N4…O3v0.302 0(7) nm, N4-H4A…O3v167°) (Fig.2a). Further, N1 and N3 acting as hydrogen bonding donors link, respectively, to the sulfonate oxygen atoms O5iiand O7iiiof the two neighboring molecules A and B (N1…O5ii0.304 6(6)nm, N1-H1C…O5ii163°; N3…O7iii0.288 3 (5) nm,N3-H3A…O7iii131°), which link the 1D chains A and B into two-dimensional (2D) layer structures in AB-A-B chains manner. Interestingly, through the hydrogen bonding linkages, the oxygen atoms and the nitrogen atoms show wavy structures along a axis and the neighboring chains A and B form a special “fish scales” structure (Fig.2a). Finally, four hydrogen bonds between the layers and layers to pillar the neighboring 2D layers into 3D supramolecular network(Fig.2b) (N1…O7iii0.293 9 (6) nm, N1-H1D…O7iii158°; N2…O1iv0.305 6 (6) nm, N2-H2D…O1iv160°;N3…O5i0.296 7 (7) nm, N3-H3A…O5i160°; N4…O2vi0.318 1 (8) nm, N4-H4B…O2vi171°). In particular, the π … π stacking interactions between molecules play important roles in stabilization of the supramolecular structure of compound 1, selected π…π interactions geometry data for compound 1 are listed in Table 4. There are π…π stacking interactions between L2-ligands in neighboring layers. As shown in Fig.2c, those planar L2-ligand molecules are parallel to each other. The distances of center to center, Cg3…Cg5iii, Cg4…Cg5iii, Cg8…Cg10i, Cg9…Cg10i, between adjacent quinoline and benzene rings are 0.357 8(3), 0.378 2(3), 0.365 3(4), 0.446 2(5) nm and the dihedral angle are 3.5(3)°, 4.4(3)°, 4.2(3),5.7(4)°, respectively, estimated by using PLATON program[31-32](Cg3 is the centroid of N9/C3/C4/C5/C6/C11, Cg4 is the centroid of C6/C7/C8/C9/C10/C11,Cg5iiiis the centroid of C12iii/C13iii/C14iii/C15iii/C16iii/C17iii, Cg8 is the centroid of N10/C25/C24/C28/C27/C26, Cg9 is the centroid of C20/C21/C22/C23/C24/C25 and Cg10iis the centroid of C29i/C30i/C31i/C32i/
C33i/C34iof L2-ligand).

Table 3 Hydrogen bond distances and angles in compounds 1 and 2

Fig.2 (a) Hydrogen bonding interactions between nitrogen and oxygen atoms connect N and O atoms into 2D layer structures;(b) 2D layers are linked into a 3D supramolecular network through interlayer hydrogen bonding interactions; (c) π…π stacking interactions in compound 1

Fig.3 Coordination environment of Cu1 and Cu2 in compound 2 with thermal ellipsoids at 30% probability

Fig.4 (a) Hydrogen bonding interactions between nitrogen and oxygen atoms connect unit Ⅰand unit Ⅱinto 2D layer structures; (b) 2D layers are linked into a 3D supramolecular network through interlayer hydrogen bonding interactions; (c) π…π stacking interactions between adjacent [CuL2]2-anions in compound 2
Single-Crystal X-ray diffraction analysis reveals that compound 2 is constructed from two ions. As shown in Fig.3, the structure consists of discrete centrosymmetric [Cu(en)2]2+cations and [CuL2]2-anions together with two crystallization water molecules. Cu1 is coordinated by two nitrogen atoms (N3 and N3i) and two oxygen atoms (O4 and O4i) from the quinolinol groups of two L2-ligands, and Cu2 is chelated by four nitrogen atoms (N1, N1ii, N2 and N2ii) from two en molecules. The coordination environments of the two Cu (II) are both distorted square geometries, selected bond lengths and bond angles are provided in Table 2. The bond lengths for Cu-N are in the range (0.195 6 (2)~0.201 7 (2) nm), and the bond lengths for Cu-O are 0.1925 (2) nm. The angles of N3-Cu1-O4 and N2-Cu2-N1 are 85.14(8)° and 84.96(9)°, respectively.
In compound 2, the en molecules, sulfonate groups and two crystallization water molecules play an important role in the formation of hydrogen bonds,and hydrogen bond distances (nm) and angles (°) for compound 2 are listed in Table 3. N1 and N2 acting as hydrogen bonding donors link, respectively, to the sulfonate oxygen atoms O1iii, O2iiiand O2vof neighboring two L2-ligands (N1…O1iii0.319 4(4) nm,N1-H1B…O1iii150°; N1…O2iii0.335 9 (4) nm, N1-H1B…O2iii147°; N2…O2v0.294 6(4) nm, N2-H2B…O2v165°), which connect [Cu (en)2]2+cations and[CuL2]2-anions into 1D chain structures (Fig.4a). N1 of the en molecules acting as hydrogen bonding donor links to the sulfonate oxygen atoms O1 (N1 …O1 0.312 3(3) nm, N1-H1A…O1 148°) and the 1D chain structures are linked into 2D layer structures (Fig.4a).In addition, The en molecules N2 and the crystallization water O5 acting as hydrogen bonding donors link, respectively, to the sulfonate oxygen atoms O3iv, O1viand O3v(N2…O3iv0.299 1(3) nm,N2-H2A…O3iv146°; O5…O1vi0.298 8 (4) nm, O5-H5A…O1vi173°; O5…O3v0.318 3(3) nm, O5-H5B…O3v165°) to form a N-H …O hydrogen bonding linkages which pillar the neighboring 2D layers into a 3D supramolecular network (Fig.4b). Moreover, it is obvious that compound 2 involves abundant π…π stacking interactions, selected π … π interactions geometry data for compound 2 are listed in Table 4.The neighboring two L2-ligands are parallel to each other in intermolecules, and π…π interactions exist between quinoline and benzene rings (Fig.4c). The distances of center to center, Cg3…Cg5v, Cg4…Cg5v,Cg4i…Cg3v, Cg4i…Cg4v, between adjacent quinoline and benzene rings are 0.365 2(2),0.394 6(2),0.371 3(2),0.356 6(2) nm and the dihedral angle are 11.8(2)°,13.5(2)°, 1.9(2)°, 0°, respectively, estimated by using PLATON program[31-32](Cg3 is the centroid of N3/C1/C2/C3/C4/C5, Cg4 is the centroid of C4/C5/C6/C7/C8/C9, Cg5vis the centroid of C10v/C11v/C12v/C13v/C14v/C15v, Cg4iis the centroid of C4i/C5i/C6i/C7i/C8i/C9i,Cg3vis the centroid of N3v/C1v/C2v/C3v/C4v/C5vand Cg4vis the centroid of C4v/C5v/C6v/C7v/C8v/C9vof L2-ligand). Hydrogen bonding and π … π stacking interactions are weaker than the coordination bonds,but they play important roles in stabilizing the molecular packing in crystal engineering.
2.2 IR spectra

Table 4 Selected π…π interactions geometries for compounds 1 and 2
The IR spectrums of compounds 1 and 2 were measured and listed in Fig.5 and Fig.6.Bands at 1 650 and 1 257 cm-1for compound 1 (1 663 and 1 259 cm-1for compound 2) correspond to stretching vibrations of the benzene skeleton, band at 1 583 cm-1(1 584 cm-1for compound 2) corresponds to C =N stretching vibration of the pyridine rings, band at 1 510 cm-1(1 504 cm-1for compound 2) corresponds to N=N,bands at 1 470 and 1 397 cm-1(1 465 and 1 405 cm-1for compound 2) correspond to C-H bending vibrations, bands at 1 330 and 1 190 cm-1(1 326 and 1 187 cm-1for compound 2) correspond to S=O of the sulfonate groups, and bands at 1 110, 1 057 and 1 021 cm-1(1 114, 1 054 and 1 022 cm-1for compound 2)correspond to sulfonate groups[33]. The O-H stretching band of the water of crystallization is observed at 3 447 cm-1(3 436 cm-1for compound 2).IR spectrums of compounds 1 and 2 are similar because there are identical functional groups in compounds 1 and 2,however, the slightly differences of their IR spectrums are owing to the different coordination modes of the ligands.

Fig.5 IR spectra of compound 1
Fig.6 IR spectra of compound 2
2.3 Powder X-ray diffractions
We examined the structural homogeneity of bulk powder samples of compound 1 through comparison of experimental and simulated PXRD patterns. The peak positions of the experimental patterns are nearly in agreement with those of the simulated ones generated from single-crystal X-ray diffraction data (Fig.7),suggesting that the product of compound 1 is pure single phase.
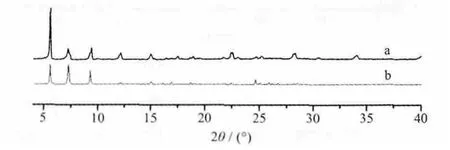
Fig.7 Experimental (a) and simulative (b) powder X-ray diffraction patterns for compound 1
2.4 TGA
TGA curve (Fig.8) shows weight losses of compound 1: The weight loss of 2.12% (Calcd. 1.96%)from 85 ℃to 115 ℃is attributed to the release of the crystallization H2O. The total weight loss of 77.92%from 328 to 980 ℃can be attributed to the release of en and L2-(Calcd. 78.63%).
TGA curve (Fig.9) shows weight losses of compound 2: Compound 2 losses its crystallization H2O from 130 to 270 ℃(Expt. 4.20%, Calcd. 3.83%).The total weight loss of 66.86% from 270 to 937 ℃corresponds to the removal of the en and L2-(Calcd.66.32%).

Fig.8 TGA curve of compound 1
2.5 Fluorescent properties
The fluorescent properties of compounds 1, 2 and the free ligand H2L have been carried out in the solid state at room temperature under the same condition.As shown in Fig.10, compounds 1 and 2 both display the emissions maxima at 367 nm when excited at 234 nm. The free ligand exhibits emission maximum at 354 nm upon excitation at 236 nm. Compounds 1 and 2 are red-shifted with respect to the free ligand H2L,which can likely be attributed to ligand based π→π*or n→π* electronic transitions including the -N=Nbased π→π* transition[34-35]. The coordination of H2L to the metal ion does not affect these ligand-specific transitions in its products 1 and 2 substantially except for some minor changes. The fluorescence makes them potentially useful photoactive materials.

Fig.9 TGA curve of compound 2

Fig.10 Solid-state fluorescent spectrum of the H2L(a, a′), compound 1 (b, b′) and compound 2(c, c′) at room temperature
3 Conclusions
The self-assembly reaction of the rigid ligand H2L and Cu (Ⅱ)salts yields two Cu (Ⅱ)coordination polymers [CuL(en)]·0.5H2O (1) and [CuL2][Cu(en)2]·2H2O (2). The solvent ratio of DMA and H2O (7.5∶1 in 1 and 2∶1 in 2) and the metal/ligand ratio (6∶1 in 1 and 3∶1 in 2) have a remarkable influence on the selfassembly of the rigid ligand with metal atoms and result the different motifs of compound 1 and 2.Compound 1 contains two [CuL(en)] molecules A and B, and the neighboring molecules A and B form a special “fish scales” structure. Compound 2 is constructed from[Cu(en)2]2+cations and[CuL2]2-anions.The crystalline structures of compounds 1 and 2 are both connected by non-covalent interactions to form 3D supramolecular networks.The fluorescent properties of compounds 1 and 2 were also determined in solid state at room temperature. This work demonstrates that the azo ligands could be used in producing new frameworks and topologies of coordination compounds with potential fluorescent properties. On the basis of this work, further syntheses and structural studies of new coordination polymers with azo ligands are also under way in our laboratory.
[1] Daniel M C, Astruc D. Chem. Rev., 2004,104:293-346
[2] Kitagawa S, Kitaura R, Noro S. Angew. Chem. Int. Ed.,2004,43:2334-2375
[3] Eddaoudi M, Moler D B, Li H L, et al. Acc. Chem. Res.,2001,34(4):319-330
[4] YANG Ying-Qun (杨颖群), CHEN Man-Sheng (陈满生),CHEN Zhi-Min (陈 志 敏), et al. Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2011,27(9):1847-1851
[5] Yaghi O M, O′Keeffe M, Ockwig N W, et al. Nature, 2003,423:705-714
[6] Tranchemontagne D J, Mendoza-Cortés J L, O′Keeffe M,et al. Chem. Soc. Rev., 2009,38:1257-1283
[7] Kolotuchin S V, Fenlon E E, Wilson S R, et al. Angew.Chem. Int. Ed., 1995,34:2654-2657
[8] Wang X L, Qin C, Wu S X, et al. Angew. Chem. Int. Ed.,2009,48(29):5291-5295
[9] Dikarev E V, Li B, Chernyshev V V, et al. Chem. Commun.,2005:3274-3276
[10]ZHU Dun-Ru(朱敦如), ZHOU Jun(周俊), YANG Jie(杨捷), et al. J. Nanjing Univ. Tech.(Nanjing Gongye Daxue Xuebao), 2007,29:103-110
[11]XIA Jun(夏军), ZHANG Ming(张明), ZHAO Bin(赵斌),et al. Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2006,22(8):1406-1410
[12]Larsen R W. J. Am. Chem. Soc., 2008,130:11246-11247
[13]Xu G C, Ding Y J, Okamura T A, et al. CrystEngComm,2008,10:1052-1062
[14]Luo F, Che Y X, Zheng J M. Cryst. Growth Des., 2008,8:176-178
[15]Zeng M H, Wang Q X, Tan Y X, et al. J. Am. Chem. Soc.,2010,132:2561-2563
[16]Long J R, Bloch E D, Britt D, et al. J. Am. Chem. Soc.,2010,132:14382-14384
[17]Katsoulis D E. Chem. Rev., 1998,98:359-388
[18]Müller A. Serain C. Acc. Chem. Res., 2000,33:2-10
[19]Liu W L, Ye L H, Liu X F, et al. CrystEngComm, 2008,10:1395-1403
[20]Chen Z F, Xiong R G, Abrahams B F, et al. J. Chem. Soc.Dalton Trans., 2001,17:2453-2455
[21]Reineke T M, Eddaoudi M, Moler D B, et al. J. Am. Chem.Soc., 2000,122:4843-4844
[22]Chen Z F, Zhang Z L, Tan Y H, et al. CrystEngComm,2008,10:217-231
[23]Balasubramani K, Thomas P, Bocelli G, et al. J. Coord.Chem., 2005,58:1689-1694
[24]Lu Y G, Cheng W, Meng X R, et al. J. Mol. Struct., 2008,875:183-188
[25]Thomas M P, Murugesan S. J. Coord. Chem., 2006,59:1167-1172
[26]Camerel F, Barberá J, Otsuki J, et al. Adv. Mater., 2008,20:3462-3467
[27]Park J S, Jeong S, Dho S K, et al. Dyes Pigm., 2010,87:49-54
[28]Aytül S, Zeynel S, Nermin E. Dyes Pigm., 2008,76(2):470-476
[29]Sheldrick G M. SHELXTL V. 5.10. Structure Determination Software Suite, Bruker AXS, Madison, 1998.
[30]Dolomanov O V, Bourhis L J, Gildea R J, et al. J. Appl.Cryst., 2009,42:339-341
[31]Spek A L, Appl J. J. Appl. Crystallogr., 2003,36:7-13
[32]Spek A L. PLATON, A Multipurpose Crystallographic Tool,Utrecht University, Netherlands, 2006.
[33]Yuan L, Qin C, Wang X L, et al. Solid State Sci., 2008,10:967-975
[34]Yu X Y, Ye L, Zhang X, et al. Dalton Trans., 2010,39(44):10617-10625
[35]Lan A J, Padmanabhan M, Li K H, et al. Inorg. Chim. Acta,2011,366:68-75
