苯基多羧酸及含氮配体构筑的三维框架结构锌配位聚合物的合成、晶体结构和荧光性质
2013-08-20汪鹏飞吴国志
汪鹏飞 方 勤 吴国志 汪 新
(1 池州学院材料与化学工程系,化学材料与工程实验中心,池州247000)
(2 国家非金属矿深加工产品质量监督检验中心,池州247000)
0 Introduction
Coordination polymers (CPs) assembled through the coordination bonds between metal ions and multifunctional organic ligands are of current interest due to not only their intrinsic topological structures,but also their potential applications in catalysis[1-3], gas storage/separation[4-5], ion exchange[6-7], sensing[8],optics[9-10], magnetism[11-13], and so on. Most of these compounds, the structural diversities were exhibited by changing the synthetic strategy of CPs, solvent,ligand to metal salts ratio,the reaction temperature,the pH value of reaction system, the counter ions, ligand substituent[14-17].As a class of important organic ligands,the rigid benzenemulticarboxylic acids provide reliable coordination modes, forming many coordination polymers with new topologies and useful properties,such as benzene-dicarboxylic acids, benzene-tricarboxylic acids and benzene-tetracarboxylic acids[18-19]. However,the metal coordination polymers based the benzenemulticarboxylic acids with the halogen substitution groups have not been well documented[20-22].The 1,2,4-benzenetricarboxylic acid (H3btc) has proven to be a good candidate for construction of novel coordination polymers because of its rigidity in conformation as well as varieties in coordination modes and numbers[23-25]. On the other hand, the combination of benzenemulticarboxylate ligands with N-donor auxiliary ligands is a good choice for the construction of novel coordination polymers. To further understand the ability of the benzenemulticarboxylate containing the halogen substitution groups producing new metal coordination polymers. In this study, we have selected the 5-bromobenzene-1,2,4-tricarboxylic acid (BTCAH3)ligand and the ancillary ligand 4,4′-bipy to assemble new metal coordination polymers with interesting structure and physical properties. This paper presents the synthesis, crystal structure, thermal stability and luminescent properties of a new three-dimensional coordination polymer [Zn3(BTCA)2(4,4′-bipy)2] (1).
1 Experimental
1.1 Materials and methods
All commercially available chemicals are of reagent grade and used as received without further purification. IR (KBr pellets) spectra were recorded in the 400~4 000 cm-1range using a Bruker Spectrum One FTIR spectrometer. Elemental analyses were carried out on PE 240C Elemental Analyzer.Thermogravimetric analyses (TGA) were performed with a Perkin Elmer Pyris 1 TGA instrument in the range of 30~800 ℃under a nitrogen flow at a heating rate of 10 ℃·min-1. Powder X-ray diffraction patterns were performed on a Bruker D8 diffractometer using Cu Kα radiation (λ=0.154 056 nm). Luminescent spectra for polycrystalline samples were measured at room temperature on a Perkin Elmer LS 55 fluorescence spectrometer.
[Zn3(BTCA)2(4,4-bipy)2](1):A mixture of Zn(OAc)2·2H2O (33 mg, 0.15 mmol), BTCAH3(29 mg, 0.1 mmol),4,4′-bipy(16 mg,0.1 mmol)in H2O(10 mL)was placed in a perr Telflon-lined stainless steel vessel (25 mL),and then the vessel was sealed and heated at 160 ℃for 3 d. After being slowly cooled to room temperature,colorless needle-like crystals of 1 were collected(yield:45%, based on Zn). Anal. Calcd. for C38H20Br2N4O12Zn3(%):C 42.24,H 1.87, N 5.18; Found (%): C 42.04, H 1.98, N 5.03. IR spectrum (cm-1): 3 414 (w), 1 603 (s),1 482 (m), 1 377 (s), 1 217 (w), 1 061 (w), 1 007 (w),919 (w), 815 (m), 731 (w), 632 (w), 562 (w). The positions of experimental diffraction peaks accord with the simulated PXRD patterns well, which indicate the phase purity of the as-synthesized sample(Fig.S2)
1.2 Crystallographic studies
Suitable single crystal of 1 with dimensions 0.12 mm×0.08 mm×0.05 mm was selected and mounted in air onto thin glass fibres. 1 was measured at room temperature on a Bruker SMART APEX Ⅱ CCD diffractometer with graphic-monochromatic Mo Kα radiation (λ=0.071 073 nm). The data were collected in the θ range 1.85° to 26.00°, using a narrow-frame method with scan widths of 0.30° in ω and exposure of 10 s per frame. Numbers of collected and unique reflections of 1 are 1 0307 and 3 818 (Rint=0.060 1).The data were integrated using Siemens SAINT program[26], with the intensities corrected for Lorentz factor, polarization, air absorption, and absorption due to variation in the path through the detector faceplate.Absorption corrections were applied. The structure was solved by directed methods and refined on F2by full-matrix least squares using SHELXS-97[27]and SHELXL-97[28]. All the non-hydrogen atoms were refined anisotropically. All the hydrogen atoms were put in calculated positions. All hydrogen atoms were refined isotropically with isotropic parameters related to the non-hydrogen atoms to which they are bonded.Crystallographic and refinement details of 1 are listed in Table 1. Selected bond lengths and angles are given in Table 2.
CCDC: 811925.

Table 1 Crystallographic data for compound 1
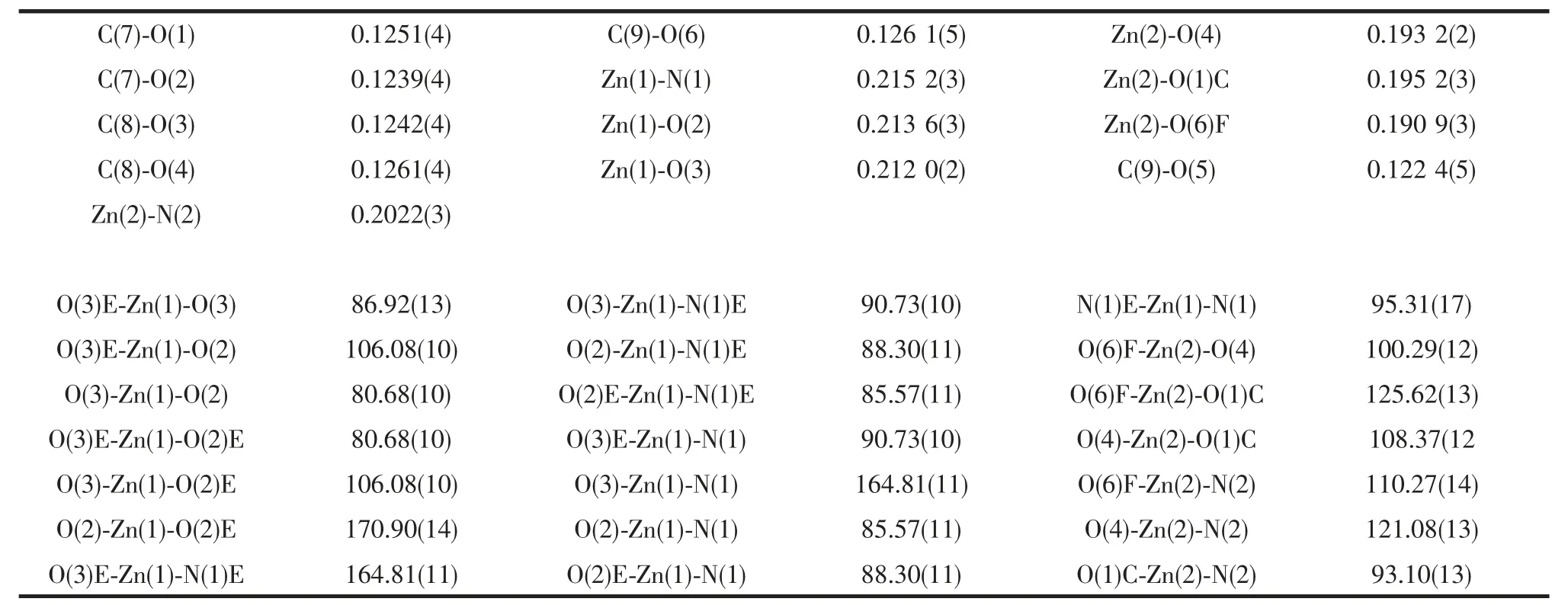
Table 2 Selected bond lengths (nm) and angle (°) for compound 1
2 Results and discussion
2.1 Description of crystal structure
Compound 1 crystallizes in a monoclinic space group C2/c. The asymmetric unit consists of one and a half Zn(Ⅱ)ions, one BTCA3-, one 4,4′-bipy molecule,as shown in Fig.1. Zn1 is six coordinated with octahedral coordination geometry defined by two nitrogen atoms from two different 4, 4′-bipy ligands,four carboxylate oxygen atoms from two different BTCA3-. Zn2 has a tetrahedral coordination environment surrounded by three carboxylate oxygen atoms from three different BTCA3-and one nitrogen atom from the 4,4′-bipy ligand. Zn-O bond distances are in the range of 0.190 9 (3)~0.213 6 (3) nm, and Zn-N bond distances are 0.202 2 (3) and 0.215 2 (3) nm,respectively. The completely deprotonated BTCA3-ligand displays a kind of coordination mode, as illustrated in Scheme 1, which adopts μ5bridging mode to link four different Zn atoms. The Zn1 and Zn2 atoms are linked by the oxygen atoms of the carboxylate group, forming to a three dimensional framework structure (Fig.2). The 4,4′ -bipy ligand coordinates the Zn atoms in the framework structure.

Fig.1 Building unit of 1 in ORTEP view

Scheme 1 Coordination mode of the BTCA3-anion in 1
In order to better understand the structure of 1, a topological analysis is performed using TOPOS 4.0 program[29-31]. Each BTCA3-anion is linked to four Zn(Ⅱ)ions (Fig.3a), and each Zn1 and Zn2 atoms are linked to two and three BTCA3-anion, respectively.Hence, the BTCA3-anion can be viewed as 4-connected node, both Zn1 and Zn2 atoms can be regarded as 3-connector by omitting the 4,4′-bipy ligands (Fig.3b). According to the simplification principle, the resulting structure of 1 is a 3-nodal and 4-connected net with Schläfli symbol of (4·7·84)2(4·74·8)2(74·82).
It is worthwhile to note that the a threedimensional coordination polymer [Zn6(btc)4(4,4′ -bipy)5]nthrough the reaction based on the Zn(NO3)2·6H2O, 1,2,4-benzenetricarboxylic acid (H3btc) and 4,4′-bipy has been reported by Long and co-workers[32],the structures of the two compounds are completely different, although they were synthesized under similar hydrothermal conditions expect that the 5-bromobenzene-1,2,4-tricarboxylic acid (BTCAH3)instead of H3btc. The halogen substitution group may play an important role in the formation of the target product. In order to understand the role of 4,4′-bipy in the formation of 1. The reaction mixture of the absent 4,4′ -bipy has been synthesized under the above condition, only the colorless solution is obtained. According to the experimental result, the 4,4′-bipy is found to be essential in the formation of 1.

Fig.2 Structure of 1 packing along the c axis

Fig.3 (a) Connection mode of each BTCA3-ligand to the Zn(Ⅱ)ions; (b) Schematic representation of the topology of 1
2.2 IR spectrum
In the IR spectra of 1, the features at 1 603 cm-1are attributed to the asymmetric stretching vibrations of the two carboxylate groups, and the strong vibration appearing around 1 482 and 1 377 cm-1corresponding to symmetric stretching vibrations of the carboxylate groups. The absence of the characteristic bands in the range of 1 760~1 680 cm-1attributed to protonated carboxylate groups in the compound indicates complete deprotonation upon reaction with Zn2+in the compound, in agreement with the single crystal X-ray diffraction analysis[33], comparable with those observed in the IR spectrum of the BTCAH3free ligand (1 720 cm-1).
2.3 Thermogravimetric analysis
To investigate the thermal stability of the compound, thermal analysis was carried out at the rate of 10 ℃·min-1under nitrogen atmosphere. As shown in Fig.S3, compound 1 is stable up to 400 ℃, which reveals the strong coordinated bonds as well as the rigid framework structure in 1. Above this temperature, the framework started to collapse accompanied by the decomposition of the organic components, leading to the formation of a stoichiometric amount of ZnO (Obsd. 41.50%, Calcd.38.80% ). Relatively high thermal stabilities of coordination polymers are also found in the other framework structures compounds containing the rigid multicarboxylate ligands with N-donor auxiliary ligands[34-36].
2.4 Luminescent property
The metal-organic hybrid coordination polymers with d10electronic configuration of metal centers are promising candidates for photoactive materials with potential applications[37-39]. The luminescent properties of compound 1 and the free BTCAH3ligand are studied in the solid state at room temperature. As illustrated in Fig.4, the intense broad emission bands at 484 nm (λex=370 nm) for 1. The free organic BTCAH3ligand shows intense emission bands at 550 nm (λex=380 nm). The ligand emission peak could be probably attributed to the π*-n or π*-π transition[40-42].Obviously, the emission arising from the free ligand is not observed. The absence of ligand-based emission suggested energy transfer from the ligand to the central metal ions during luminescence. Thus, the luminescence could be assigned to the emission of ligand-to-metal charge-transfer (LMCT)[9-10].
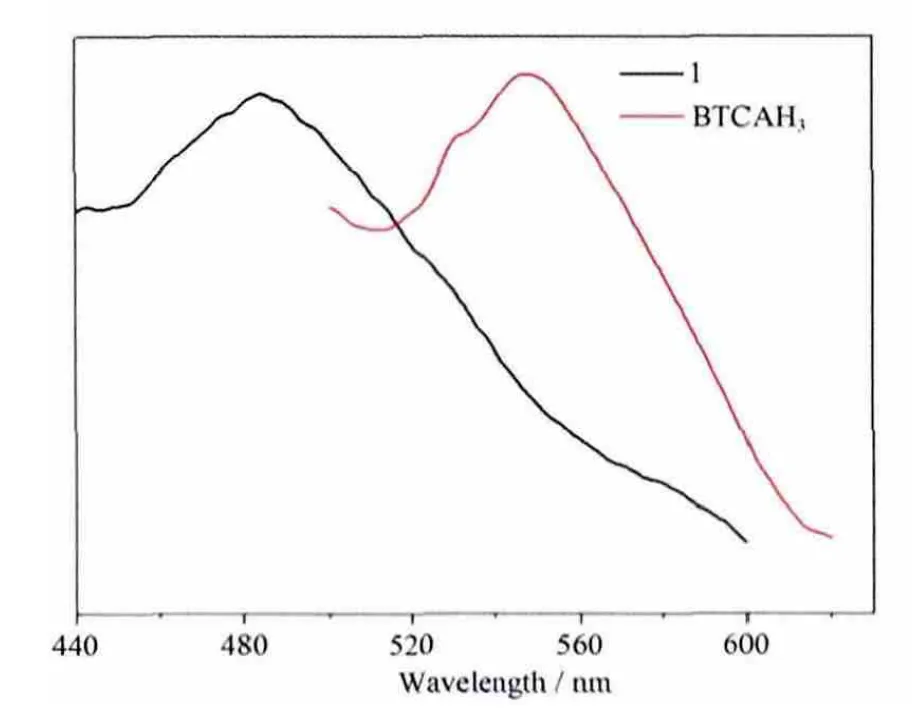
Fig.4 Solid-state emission spectrum of 1
3 Conclusions
In summary, this paper reports a new Zn(Ⅱ)coordination polymer, [Zn3(BTCA)2(4,4′-bipy)2] (1)under hydrothermal reaction. The compound was characterized by single crystal X-ray diffraction, IR spectroscopy, elemental analysis, thermogravimetric analysis and luminescent property. Compound 1 possesses a three-dimensional framework structure,which is a novel example 3-nodal and 4-connected net with (4·7·84)2(4·74·8)2(74·82) topological network. In addition, the thermal stability and luminescent properties of 1 were investigated. Further work is in progress to explore new inorganic-organic hybrid materials based on the 5-bromobenzene-1,2,4-tricarboxylic acid ligand with interesting structures and functional properties.
[1] Ma L Q, Abney C, Lin W B. Chem. Soc. Rev., 2009,38:1248-1256
[2] Corma A,García H,Llabrés Xamena F X.Chem.Rev.,2010,110:4606-4655
[3] Hasegawa S, Horike S, Matsuda R, et al. J. Am. Chem. Soc.,2007,129:2607-2614
[4] Murray L J, Dinc M, Long J R. Chem. Soc. Rev., 2009,38:1294-1314
[5] Li J R, Kuppler R J, Zhou H C. Chem. Soc. Rev., 2009,38:1477-1504
[6] Dinc M, Long J R. J. Am. Chem. Soc., 2007,129:11172-11176
[7] Zhao J A, Mi L W, Hu J Y, et al. J. Am. Chem. Soc., 2008,130:15222-15223
[8] Maspoch D, Ruiz-Molina D, Veciana J. Chem. Soc. Rev.,2007,36:770-818
[9] Allendorf M D, Bauer C A, Bhakta R K, et al. Chem. Soc.Rev., 2009,38:1330-1352
[10]Cui Y J, Yue Y F, Chen B L, et al. Chem. Rev., 2012,112:1126-1162
[11]Kahn O. Molecular Magnetism. New York: VCH Publishers,Inc., 1993.
[12]Carling C L. Magnetochemistry. Berlin: Springer, 1986.
[13]Gatteschi D, Sessoli R, Villain J. Molecular Nanomagnets.New York: Oxford University Press, 2006.
[14]Zang S Q, Dong M M, Hou H W, et al. Cryst. Growth. Des.,2012,12:1239-1246
[15]Li C P, Du M. Chem. Commun., 2011,47:5958-5972
[16]Yin P X, Zhang J, Yao Y G, et al. CrystEngComm, 2011,13:3536 -3544
[17]Chen M, Chen S S, Sun W Y, et al. Cryst. Growth Des.,2011,11:1901-1912
[18]Eddaoudi M, O′Keeffe M, Yaghi O M, et al. Science,2002,295:469-472
[19]Song X Z, Song S Y, Zhang H J, et al. Cryst. Growth Des.,2012,12:253-263
[20]Eddaoudi M, O′Keeffe M, Yaghi O M, et al. J. Am. Chem.Soc., 2002,124:376-377
[21]Zang S Q, Fan Y J, Hou H W, et al. Cryst. Growth Des.,2011,11:3395-3405
[22]WANG Peng-Fei(汪鹏飞), WU Guo-Zhi(吴国志), WANG Xin(汪新). Chinese J. Inorg. Chem. (Wuji Huaxue Xuebao),2012,28(3):579-583
[23]Wang L, Shi Z, Feng S H, et al. Inorg. Chem., 2006,45:2474-2478
[24]Yao J, Meng Q J, Lu C S, et al. CrystEngComm, 2008,10:1379-1383
[25]Ren C, Hou L, Wang Y Y, et al. Dalton Trans., 2011,40:793-804
[26]SAINT, Program for Data Extraction and Reduction,Siemens Analytical X-ray Instruments, Madison, WI, 1994-1996.
[27]Sheldrick G M. SHELXS-97, Program for Crystal Structure Solution,University of Göttingen,Göhttingen,Germany,1997.
[28]Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany,1997.
[29]O′Keeffe M, Peskov M A, Yaghi O M, et al. Acc. Chem.Res., 2008,41:1782-1789
[30]The Reticuler Chemistry Structure Resource Database can be accessed at http://rcsr.anu.edu.au
[31]Blatov V A, Shevchenko A P. TOPOS 4.0; Samara State University, Russia, 2008.
[32]Zheng P Q, Long L S, Zheng L S, et al. Appl. Organometal.Chem., 2003,17:739-740
[33]Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordinated Compounds. 5th Ed. New York: Wiley & Sons,1997.
[34]Xia H Y, Liu G X, Nishihara S, et al. J. Inorg. Organomet.Polym., 2010,20:110-117
[35]Li H H, Shi W, Cheng P, et al. Cryst. Growth Des.,2012,12:2602-2612
[36]Liao S Y, Gu W, Yang L Y, et al. Cryst. Growth Des.,2012,12:3927-3936
[37]Wu Q G, Esteghamatian M, Xu N X, et al. Chem. Mater.,2000,12:79-83
[38]Lee E Y, Jang S Y, Suh M P. J. Am. Chem. Soc., 2005,127:6374-6381
[39]He Y H, Feng Y L, Lan Y Z, et al. Cryst. Growth Des.,2008,8:3586-3594
[40]Chen W, Wang J Y, Chen J S, et al. Inorg. Chem., 2003,42:944-946
[41]Wang X L, Chao Q, Wang E B, et al. Inorg. Chem.,2004,43:1850-1856
[42]Ji C C, Li Y Z, Zheng H G, et al. Cryst. Growth Des.,2011,11:480-487
