有序介孔碳-石墨烯复合材料的制备及其对银纳米粒子吸附性能的研究
2013-04-06刘攀博黄英
刘攀博,黄英
(西北工业大学理学院应用化学系,空间应用物理与化学教育部重点实验室,陕西西安 710129)
有序介孔碳-石墨烯复合材料的制备及其对银纳米粒子吸附性能的研究
刘攀博,黄英
(西北工业大学理学院应用化学系,空间应用物理与化学教育部重点实验室,陕西西安 710129)
以溶剂挥发诱导有机-有机自组装法制备了有序介孔碳-石墨烯(OMC-RGO)复合材料。结果表明:当焙烧温度为800℃时,所得的OMC-RGO复合材料与OMC相比,其电导率从0.76S/m增加到32.5S/m,提高到42.8倍;但BET比表面积和孔容分别从670m2/g和0.40cm3/g下降到361m2/g和0.23cm3/g,降低的比率分别为46.1%和42.5%。随后,我们以OMC-RGO为载体,通过物理吸附制备了有序介孔碳-石墨烯-银纳米粒子(OMC-RGO-Ag)复合材料。结果表明:OMC-RGO表面上Ag纳米粒子的粒径在20~40nm之间;且OMC与RGO复合后,RGO表面上的含氧官能团有利于吸附较多的Ag纳米粒子。
有序介孔碳;石墨烯;银纳米粒子;复合材料
前言
有序介孔碳(OMC)具有较高的比表面积、较大的孔容和良好的热稳定性和机械稳定性,因此在吸附、分离、催化剂载体、电池电容和半导体等方面具有广泛的应用[1~4]。对其孔径或比表面积进行修饰还可以赋予其特殊的用途[5~7]。通常有序介孔碳材料的制备包括硬模板法[8~10]和软模板法[11~16]。然而,有序介孔碳有两个缺点:一是导电性较差,二是其孔壁上具有较少的官能团,很难实现功能化而负载金属粒子。通常是使用浓硫酸或浓硝酸作为氧化剂来增加其表面官能团的数量,但是其氧化过程容易使其结构的有序性遭到破坏[17,18]。石墨烯(RGO)是一种具有二维蜂窝纳米结构、由单一碳原子紧密排列组成的碳材料[19],因此可以用来提高OMC的导电性[20]。且其表面上具有较多的含氧官能团[21],与OMC复合后有利于吸附更多的金属粒子;同时,也不会破坏OMC的有序结构[22]。
本文以溶剂挥发诱导有机-有机自组装法制备了有序介孔碳-石墨烯(OMC-RGO)复合材料,并以此为载体,通过物理吸附制备了有序介孔碳-石墨烯-银纳米粒子(OMC-RGO-Ag)复合材料。通过XRD,N2吸附脱附,SEM和TEM等测试,研究了RGO对OMC结构、导电性和Ag离子吸附的影响。
1 实验部分
1.1 制备
氧化石墨(GO)的制备:以天然鳞片石墨作为前驱体,采用Hummers法合成GO[23]。
OMC-RGO的制备:1.0g F127溶于10.0g去离子水中搅拌10min,加入2.0g酚醛树脂前驱体(50wt%)和50mLGO溶液搅拌10min,将该溶液转移到培养皿中,室温挥发12h,再将培养皿转置于100℃烘箱中内放置24h,得到薄膜材料。将该薄膜材料从培养皿上刮下来,放入管式炉中,在Ar保护下,于800℃下焙烧3h。
OMC的制备:依照上述过程,不加GO溶液时制备OMC。
RGO的制备:将GO直接放入管式炉中,在Ar保护下,于800℃下焙烧3h。
OMC-RGO-Ag(RGO-Ag或OMC-Ag)的制备:将10mg OMC-RGO(RGO或OMC)加入5mL Ag纳米粒子溶液中超声2h,离心干燥。
1.2 性能测试
采用日本SHI-MADZU公司型号为XRD-7000X的X射线衍射仪测试样品结构。测试条件:采用Cu靶Kα辐射,入射波长λ=0.154060nm,电压40.0kV,电流40.0mA,扫描速度0.5°/min,扫描步长0.002°。采用美国micromeritics公司型号为ASPS2020的N2吸附/脱附物理吸附仪测试样品BET比表面积、孔容和孔径。样品在真空条件下于200℃预先脱气12h。BET比表面积采用Barrett-Emmet-Teller法计算得到;孔容和孔径由等温吸附分支采用Barrett-Joyner-Halanda(BJH)模型计算,其中孔容以相对压力P/P0=0.975处的吸附量计算。采用Gemini LEO1530型扫描电子显微镜(SEM)和Hitachi H800型透射电镜对表观形貌进行分析。电导率采用公式:R=ρ×L/A,其中R代表电导率,ρ代表电阻率,A代表样品与电极的接触面积,L代表电极之间的距离。
2 结果与讨论
2.1 XRD分析
由图1可知,OMC在2θ=25°和43°分别显示两个明显的衍射峰,对应于碳材料的(002)和(10)衍射[24]。与RGO复合后,OMC-RGO在25°衍射峰的强度低于OMC,说明其结构有一定程度的降低。对于OMC-Ag,在2θ=38.2°、44.5°、64.9°和77.9°处分别出现较不明显的衍射峰,对应于Ag纳米粒子(111)、(200)、(220)和(311)的衍射,说明OMC上负载少量的Ag纳米粒子。根据布拉格公式2dsinθ=nλ(n=1)(式中d为晶面间距离,θ为衍射角,n为衍射级数,λ为X射线波长),Ag纳米粒子(111)衍射峰对应的晶面间距离d=0.24nm。与RGO-Ag相比,OMC-Ag衍射峰的强度明显低于RGO-Ag,说明RGO上负载的Ag纳米粒子比OMC上负载的Ag纳米粒子多,这主要是因为RGO上具有含氧官能团,有利于吸附较多的金属粒子。而OMC-RGO-Ag衍射峰的强度与RGO-Ag相当,说明OMC与RGO复合后,可以提高其负载Ag纳米粒子的能力。

图1 OMC,OMC-RGO,OMC-Ag,RGO-Ag和OMC-RGO-Ag的XRD谱图Fig.1 XRDpatternsofOMC,OMC-RGO,OMC-Ag,RGO-Ag andOMC-RGO-Ag
2.2 N2吸附/脱附分析
从图2a可知,OMC呈现典型的LangmuirⅣ型吸附,在相对压力较低的区域,吸附层的厚度随着压力的增加而增加,在相对压力较高的区域出现明显的滞后环,表现为介孔材料的特征。OMC和OMC-RGO均在相对压力为0.5~0.6的范围内出现一个由毛细冷凝现象引起的明显的突跃,暗示其具有较窄的孔径分布,这与孔径分布的结果相同(图2b)。

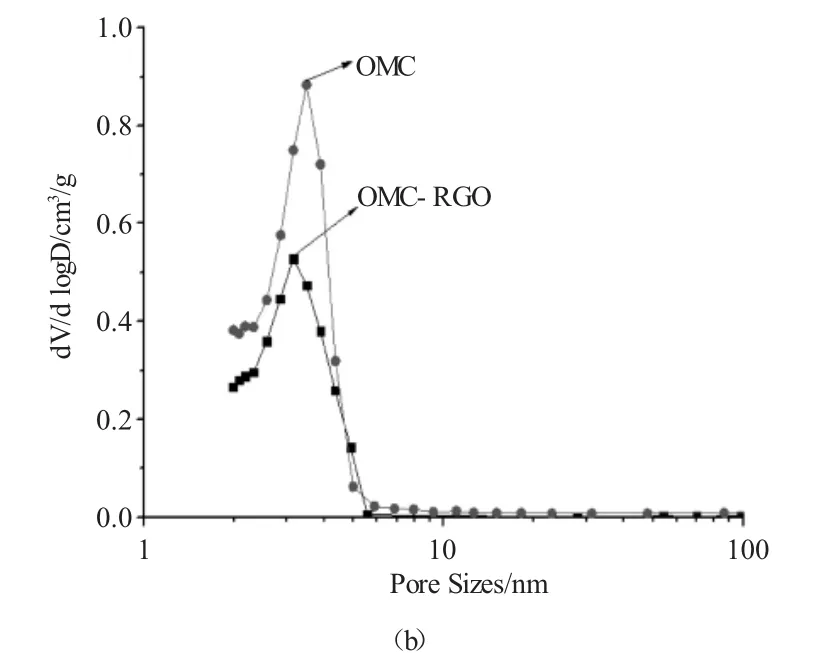
图2 OMC和OMC-RGO的N2吸附/脱附等温线(a)和孔径分布(b)。Fig.2 N2adsorption/desorption isotherms(a)and the pore size distributions(b)of OMC and OMC-RGO.
根据N2吸附/脱附结果计算,OMC和OMC-RGO的孔结构参数如表1所示。
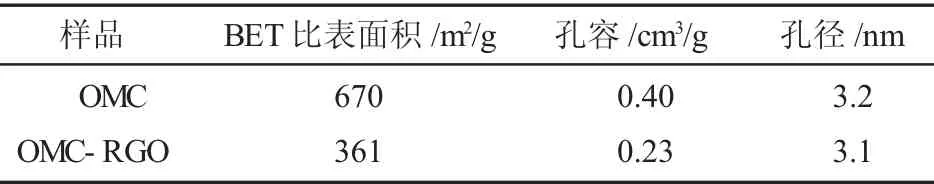
表1 OMC和OMC-RGO的孔结构参数。Table 1 Pore parameters of OMC和OMC-RGO
由表1可知,OMC的BET比表面积、孔容和孔径分别为670 m2/g,0.40 cm3/g和3.2nm。与RGO复合后,OMC-RGO的BET比表面积和孔容分别为361m2/g和0.23cm3/g,相对于OMC均有所降低,降低的比率分别为46.1%和42.5%。这可能是由于RGO覆盖在OMC表面,因而使其BET比表面积和孔容有所降低。但其孔径相对于OMC变化不大。
2.3形貌分析
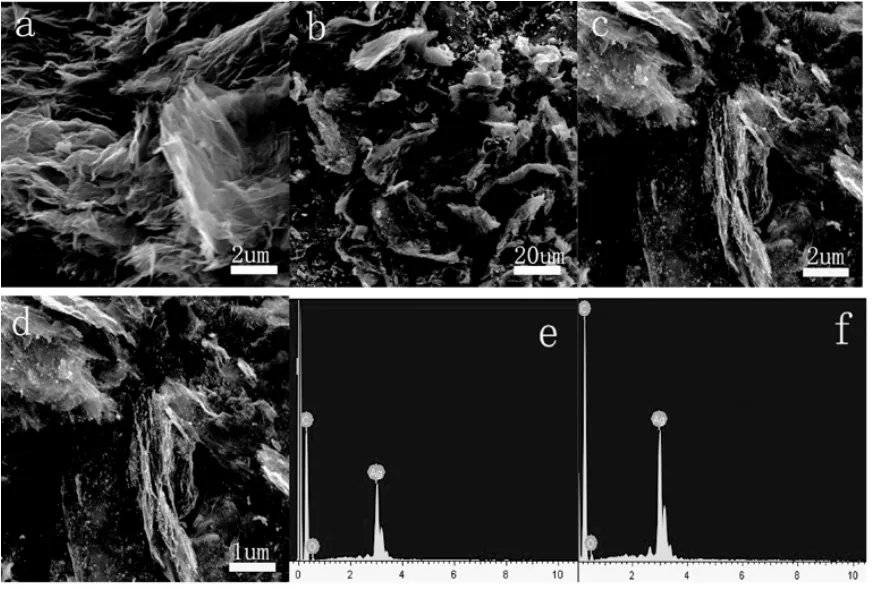
图3 RGO(a),OMC-RGO(b),RGO-Ag(c)和OMC-RGO-Ag(d)的SEM照片;RGO-Ag(e)和OMC-RGO-Ag(f)的EDS谱图。Fig.3 SEM images of RGO(a),OMC-RGO(b),RGO-Ag(c)and OMC-RGO-Ag(d);EDS spectra of RGO-Ag(e)and OMC-RGO-Ag(f).
由图3a可知,高温加热导致GO中的含氧官能团发生分解,同时产生剥离,生成极薄的RGO纳米片层。与OMC复合后,生成的RGO均匀地铺在OMC的表面(图3b)。图3c和图3d分别为RGO、OMC-RGO和Ag纳米粒子复合之后的SEM图。从图中可以看出,大量的Ag纳米粒子均匀地覆盖在RGO和OMC-RGO的表面,这主要是因为RGO中的含氧官能团能够吸附较多的Ag纳米粒子。RGO-Ag(图3e)和OMC-RGO-Ag(图3f)的EDS谱图显示,其表面上所负载的金属粒子为Ag,进一步说明RGO-Ag和OMC-RGO-Ag复合物的形成,这与XRD所以结果一致。

图4 RGO(a),OMC-RGO(b),RGO-Ag(c),OMC-Ag(d),OMCRGO-Ag的TEM照片;Ag的HRTEM照片(f)。Fig.4 TEM images of RGO(a),OMC-RGO(b),RGO-Ag(c),OMCAg(d),OMC-RGO-Ag(e)and HRTEM image of Ag(f).
由图4a可知,RGO具有典型的透明褶皱形态,这主要是由于其具有较大的宽高比。由图4b可以看出,RGO覆盖在具有二维六方结构的OMC的表面,说明所制备的复合物为OMC-RGO。图4b中黄色方框为RGO的HRTEM照片,从RGO的边缘可以看出其具有多层结构。RGO的存在对提高OMC电导率有很大的影响。未复合RGO前,OMC的电导率为0.76 S/m;与RGO复合后,形成的OMC-RGO复合物的电导率增加到32.5 S/m,相比OMC提高到42.8倍。焙烧后,由于RGO上仍然存在一定数量的含氧官能团,有利于吸附较多的Ag金属粒子,这与图4c所示的结果一致。且Ag金属粒子的粒径处于20~40nm之间,这比以原位化学还原法制备的Ag纳米粒子具有较大的粒径[25]。而二维六方结构OMC上具有较少的官能团,不利于Ag金属粒子的吸附,因此形成的OMC-Ag上具有较少的Ag金属粒子(图4d)。当OMC与RGO复合后,RGO的存在有利于吸附更多的Ag金属粒子(图4e),这与XRD所得的结果一致。由图4f可以看出,形成的Ag纳米粒子的晶格间距为0.24nm,对应于Ag(111)晶面间距,这与XRD所计算的结果一致。
3 结论
以溶剂挥发诱导有机-有机自组装法制备了有序介孔碳-石墨烯(OMC-RGO)复合材料,其电导率相对于OMC提高到42.8倍,但BET比表面积和孔容相对于OMC分别降低了46.1%和42.5%。以所制备的OMC-RGO为载体,通过物理吸附制备了OMC-RGO-Ag复合材料,其Ag金属粒子的粒径在20~40nm之间,且RGO的存在有利于吸附较多的Ag金属粒子。
[1]LEE J,KIM J,HYEON T.Recent Progress in the Synthesis of Porous Carbon Materials[J].Adv.Mater,2006,18(16):2073~2094.
[2]WAN Y,WANG H Y,ZHAO Q F,et al.Ordered Mesoporous Pd/ Silica Carbon as a Highly Active Heterogeneous Catalyst for Coupling Reaction of Chlorobenzene in Aqueous Media[J].J.Am.Chem.Soc,2009,131(12):4541~4550.
[3]ZHUANG X,WAN Y,FENG C M,et al.Highly Efficient Adsorption of Bulky Dye Molecules in Wastewater on Ordered Mesoporous Carbons[J].Chem.Mater,2009,21(4):706~716.
[4]LIANG C,LI Z,DAI S.Mesoporous Carbon Materials:Synthesis and Modification[J].Angew.Chem.Int.Ed,2008,47(20):3696~3717.
[5]SHIN Y S,FRYXELL G E,UM W,et al.Sulfur-Functionalized Mesoporous Carbon[J].Ad.Funct.Mater,2007,17(15):2897~2901.
[6]WANG X Q,JIANG D E,DAI S.Surface Modification of Ordered Mesoporous Carbons via 1,3-Dipolar Cycloaddition of A-zomethine Ylides[J].Chem.Mater,2008,20(15):4800~4802.
[7]STEIN A,WANG Z Y,FIERKE M A.Functionalization of Porous Carbon Materials with Designed Pore Architecture[J].Adv.Mater, 2009,21(3):265~293.
[8]LIU N N,YIN L W,WANG C X,et al.Adjusting the texture and nitrogen content of ordered mesoporous nitrogen-doped carbon materials prepared using SBA-15 silica as a template[J].Carbon,2010,48(12):3579~3591.
[9]KRUK M,DUFOUR B,CELER E B,et al.Adsorption and Structural Properties of Ordered Mesoporous Carbons Synthesized by Using Various Carbon Precursors and Ordered Siliceous P6mm and Ia3d Mesostructures as Templates[J].J.Phys.Chem.B, 2005,109(49):23263~23268.
[10]FAN J,YU C Z,LEI J,et al.Low-Temperature Strategy to Synthesize Highly Ordered Mesoporous Silicas with Very Large Pores[J].J.Am.Chem.Soc,2005,127(31):10794~10795.
[11]JIN J,NISHIYAMA N,EGASHIRA Y,et al.Pore structure and pore size controls of ordered mesoporous carbons prepared from resorcinol/formaldehyde/triblock polymers[J].Microporous and Mesoporous Materials,2009,118(1~3):218~223.
[12]SIMANJUNTAK F H,JIN J,NISHIYAMA N,et al.Ordered mesoporous carbon films prepared from 1,5-dihydroxynaphthalene/ triblock copolymer composites[J].Carbon,2009,47(10):2531~2533.
[13]TANAKA S,KATAYAMA Y,TATE M P,et al.Fabrication of continuous mesoporous carbon films with face-centered orthorhombic symmetry through a soft templating pathway[J].J.Mater.Chem,2007,17(34):3639~3645.
[14]ZHAO D Y,MENG Y,GU D,et al.A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic-Organic Self-Assembly[J].Chem.Mater,2006,18(18): 4447~4464.
[15]ZHAO D Y,ZHANG F Q,MENG Y,et al.An Aqueous Cooperative Assembly Route To Synthesize Ordered Mesoporous Carbons with Controlled Structures and Morphology[J].Chem.Mater,2006,18(22):5279~5288.
[16]WANG X Q,LIANG C D,DAI S.Facile Synthesis of Ordered Mesoporous Carbons with High Thermal Stability by Self-Assembly of Resorcinol Formaldehyde and Block Copolymers under Highly Acidic Conditions[J].Langmuir,2008,24(14):7500~7505.
[17]RYOO R,JOO S H,JUN S,et al.Ordered mesoporous carbon molecular sieves by templating synthesis:the structural varieties[J].Stud.Surf.Sci.Catal,2001,135:1121~1128.
[18]LU A H,LI W C,MURATOVA N,et al.Evidence for C-C bond cleavage by H2O2in a mesoporous CMK-5 type carbon at room temperature[J].Chem.Commun,2005,47:5184~5186.
[19]NOVOSELOV K S,GEIM A K,MOROZOV S V,et al.Electric Field Effect in Atomically Thin Carbon Films[J].Science, 2004,306(22):666~669.
[20]WANG L,SUN L,TIAN C G,et al.A novel soft template strategy to fabricate mesoporous carbon/graphene composites as highperformance supercapacitor electrodes[J].RSC Advances,2012, 2:8359~8367.
[21]KUILA T,BOSE S,HONG C E,et al.Preparation of water-dispersible graphene by facile surface modification of graphite oxide[J].Nanotechnology,2011,22:1033~1037.
[22]SUN X,HE J P,TANG J,et al.Structural and electrochemical characterizationoforderedmesoporouscarbon-reduced graphene oxide nanocomposites[J].J.Mater.Chem,2012,22: 10900~10910.
[23]HUMMERS W S AND OFFEMAN R E.Preparation of Graphitic Oxide[J].J.Am.Chem.Soc,1958,80(6):1339~1339.
[24]Yang H F,Yan Y,Liu Y et al.A Simple Melt Impregnation Method to Synthesize Ordered Mesoporous Carbon and Carbon Nanofiber Bundles with Graphitized Structure from Pitches[J].J.Phys.Chem.B,2004,108(45):17320~17328.
[25]Liu S,Tian J Q,Wang L,et al.A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizingagentanditssubsequentdecorationwithAg nanoparticles for enzymeless hydrogen peroxide detection[J].Carbon,2011,49(10):3158~3164.
Preparation of Ordered Mesoporous Carbon-Reduced Graphene Oxide Composites and the Research on Adsorption of Ag Nanoparticles
LIU Pan-bo and HUANG Ying
(Key Laboratory of Space Applied Physics and Chemistry,Ministry of Education,Northwestern Polytechnical University,Xi'an 710129,China)
The ordered mesoporous carbon-reduced graphene oxide(OMC-RGO)composites were prepared through organic-organic selfassembly method.The results showed that the conductivity of OMC-RGO increased from 0.76S/m to 32.5S/m,which was about 23-fold in comparison with OMC,but the BET surface area and pore volume of OMC-RGO(361m2/g,0.23cm3/g)were lower than OMC(670m2/g,0.40cm3/g)by calcination at 800℃.Then the OMC-RGO-Ag nanocomposites were prepared by directly adsorption of Ag nanoparticles with using OMC-RGO as matrix,the results showed that the average diameter of Ag on the surface of RGO-OMC was between 20 and 40 nm,the presence of oxygen-containing groups on the surface of RGO was favorable to adsorb more Ag nanoparticles.
Ordered mesoporous carbon;reduced graphene oxide;Ag nanoparticles;composites
TQ 322.99
A
1001-0017(2013)02-0018-04
2012-12-20
刘攀博(1986-),男,陕西西安人,博士,主要从事石墨烯的研究。
