一锅法合成3,4-二氢嘧啶-2(1H)-酮
2012-09-13魏振中王绍辉洪美玲
魏振中,王绍辉,洪美玲
(淮北师范大学 化学与材料科学学院,安徽 淮北 235000)
一锅法合成3,4-二氢嘧啶-2(1H)-酮
魏振中,王绍辉,洪美玲
(淮北师范大学 化学与材料科学学院,安徽 淮北 235000)
苦味酸催化下,芳香醛、β-酮酸酯、脲或硫脲(物质的量比1∶1∶1.15)在水中环化缩合,合成3,4-二氢嘧啶-2-酮及其衍生物.反应时间2~3 h,产率75% ~98%.
Biginelli反应;3,4-二氢嘧啶-2-酮;苦味酸
1893年Biginelli首次报道乙酰乙酸乙酯、芳香醛和脲在浓盐酸催化下于乙醇中加热回流18 h,得到3,4-二氢嘧啶-2-酮类化合物,这一合成法称为Biginelli反应.3,4-二氢嘧啶-2-酮具有广泛的生物活性和药理活性[1],目前合成该类化合物有很多改进的方法.常见的催化剂有:酒石酸[2]、超分子[3-4]、室温离子液体[5-7]、硝酸铋[8]、硝酸铈铵[9]、三氯化铁[10]、磷酸二氢钾[11]、三聚磺酸[12]、硼酸[13]、三溴化铟[14]、苯硼酸[15]等,并且微波辐射[16-18]、超声波技术[19]等都已应用于该反应.这些方法大大改进原始Biginelli反应中存在的不足,缩短反应时间,提高反应效率,大大增加反应产率.但是这些方法还存在着它的不足之处:如操作麻烦、催化剂价格昂贵、污染环境、微波辐射耗能大,且不能用于大量的工业化生产等.因此研究新的方法或寻找新的催化剂来改进Biginelli反应十分的有必要.
研究发现在水为溶剂的条件下,用少量苦味酸为催化剂可以高效、快速地催化Biginelli反应,大大缩短反应时间,提高反应效率、操作简便,适合工业化.反应合成路线如下:
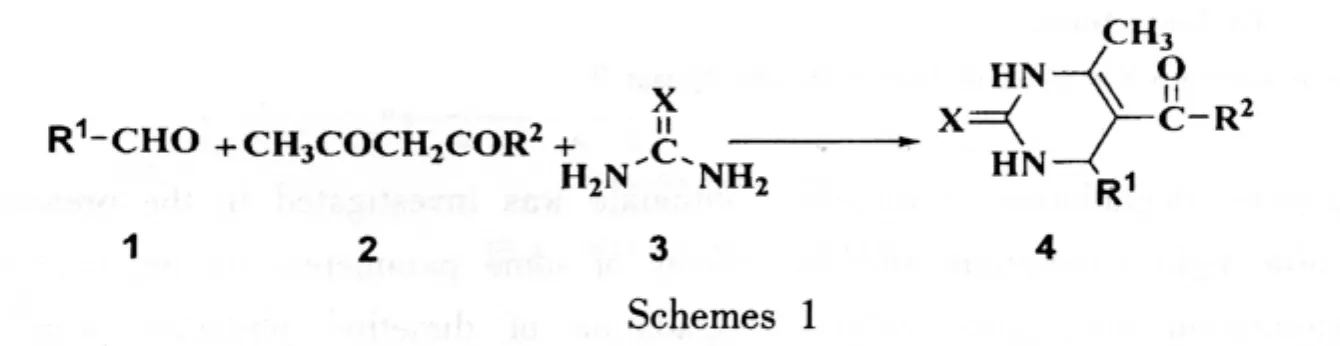
1 实验部分
1.1 仪器和试剂
WPS-1型数字熔点仪(温度计未校正);Bruker核磁共振仪(TMS为内标,400 Hz);PE-2400型元素自动分析仪;TLC进行跟踪反应,薄层层析硅胶(青岛海洋化工有限公司GF254);所用试剂均为AR级,水为二次蒸馏水.
1.2 3,4-二氢嘧啶-2(1H)酮合成步骤
1.2.1 化合物4b的合成
在25 mL的圆底烧瓶中,加入尿素0.480 g,(8.0 mmol)、乙酰乙酸乙酯0.975 g,(7.5 mmol)、苯甲醛0.795 g(7.5 mmol)、苦味酸0.1 g和水15 mL,加热回流,1 h后有沉淀产生,2 h后反应完全.冷却至0℃,静置过夜后抽滤,滤饼干燥得4b.
用上述合成方法换用不同的R1和脲(X=O或X=S)分别进行加热反应,可得到一系列产物.将不同R1和脲(X=O或X=S)的详细结构附于表1中,部分化合物的表征数据如下.
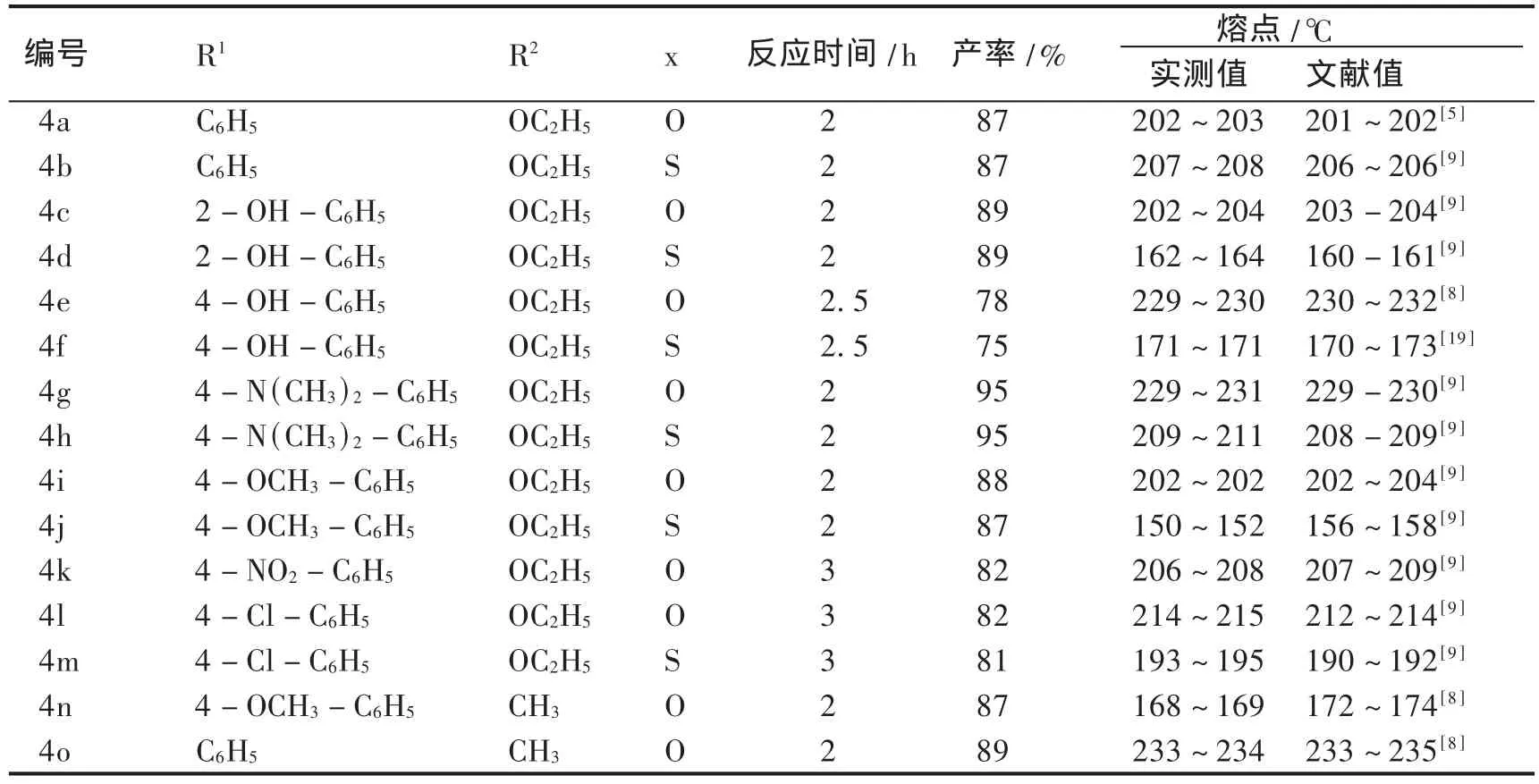
表1 3,4-二氢嘧啶-2-酮及其衍生物的产率和熔点Table 1 Yield and melting point of 3,4-Dihydropyrim idine-2-Ones and their derivatives
4a:1H NMR(DMSO-d6,400 MHz)δ:9.33(s,1H),7.91(s,1H),7.31~7.57(m,5H),5.38(d,J= 6.4 Hz,1H),4.07(q,J=6.0 Hz,2H),2.37(s,3H),1.16(t,J=6.0 Hz,3H).Anal.calcd for C14H16N2O3: C 64.60,H 6.20,N 10.76,O 18.44;found C 64.37,H 6.42,N 10.71,O 18.52.
4b:1H NMR(DMSO-d6,400 MHz)δ:10.53(s,1H),9.42(s,1H),7.31~7.82(m,5H),5.44(d,J= 4.0 Hz,1H),4.28(q,J=6.8 Hz,2H),2.48(s,3H),1.19(t,J=6.8 Hz,3H)Anal.calcd for C14H16N2O2S: C 60.85,H 5.84,N 10.14,O 11.58,S 11.60;found C 60.60,H 5.80,N 10.17,O 18.81,S 11.60.
4c:1H NMR(DMSO-d6,400 MHz)δ:9.40(s,1H),8.94(s,1H),7.62(s,1H),7.01-6.62(m,4H),5.24 (d,J=3.2 Hz,1H),4.03(q,J=8.0 Hz,2H),2.26(s,3H),1.10(t,J=8.0 Hz,3H).Anal.calcd for C14H16N2O4:C 60.86,H 5.84,N 10.14,O 23.16;found C 60.53,H 5.80,N 10.17,O 23.50.
4d:1H NMR(DMSO-d6,400 MHz)δ:10.13(s,1H),9.33(s,1H),7.65(s,1H),7.21-6.70(m,4H),5.33(d,J=8.0 Hz,1H),4.05(q,J=7.6 Hz,2H),2.27(s,3H),1.14(t,J=7.6 Hz,3H).Anal.calcd for C14H16N2O3S:C 57.52,H 5.52,N 9.58,O 16.42,S 10.97;found C 57.40,H 5.40,N 9.54,O 16.68,S 10.99.
4e:1H NMR(DMSO-d6,400 MHz)δ:9.67(s,1H),9.21(s,1H),8.76(s,1H),7.05-6.55(m,4H),5.16 (d,J=6.0 Hz,1H),4.05(q,J=7.2 Hz,2H),2.12(s,3H),1.07(t,J=7.2 Hz,3H).Anal.calcd for C14H16N2O4:C 60.86,H 5.84,N 10.14,O 23.16;found C 60.82,H 6.00,N 10.05,O 23.53.
4f:1H NMR(DMSO-d6,400 MHz)δ:10.27(s,1H),9.53(s,1H),9.44(s,1H),6.80-7.00(m,4H),5.03(d,J=6.4 Hz,1H),4.02(q,J=8.0 Hz,2H),2.26(s,3H),1.13(t,J=8.0 Hz,3H).Anal.calcd for C14H16N2O3S:C 57.52,H 5.52,N 9.58,O 16.42,S 10.97;found C 57.50,H 5.50,N 9.47,O 16.59,S 10.85.
4g:1H NMR(DMSO-d6,400 MHz)δ:9.54(s,1H),7.83(s,1H),6.76~7.15(m,4H),5.31(d,J= 8.0 Hz,1H),4.04(q,J=6.0 Hz,2H),2.98(s,6H),2.36(s,3H),1.15(t,J=6.0 Hz,3H).Anal.calcd for C16H21N3O3:C 63.35,H 6.98,N 13.85,O 15.82;found C 64.00,H 6.90,N 13.67,O 15.45.
4h:1H NMR(DMSO-d6,400 MHz)δ:10.14(s,1H),8.24(s,1H),6.70~7.20(m,4H),5.15(d,J= 6.0 Hz,1H),3.97(q,J=7.2 Hz,2H),2.98(s,6H),2.29(s,3H),1.13(t,J=7.2 Hz,3H).Anal.calcd for C16H21N3O2S:C 60.16,H 6.63,N 13.16,O 10.02,S 10.04;found C 60.12,H 6.53,N 13.17,O 10.00,S 10.09.
4i:1H NMR(DMSO-d6,400 MHz)δ:9.45(s,1H),8.15(s,1H),6.69~7.10(m,4H),5.30(d,J= 5.2 Hz,1H),4.04(q,J=8.0 Hz,2H),3.98(s,3H),2.30(s,3H),1.17(t,J=8.0 Hz,3H).Anal.calcd forC15H18N2O4:C 62.06,H 6.25,N 9.65,O 22.04;found C 61.92,H 6.27,N 9.56,O 22.15.
4j:1H NMR(DMSO-d6,400 MHz)δ:10.44(s,1H),9.69(s,1H),6.78~7.43(m,4H),5.44(d,J= 4.4 Hz,1H),4.08(q,J=6.0 Hz,2H),3.91(s,3H),2.42(s,3H),1.17(t,J=6.0 Hz,3H).Anal.calcd for C15H18N2O3S:C 58.80,H 5.92,N 9.14,O 15.67,S 10.47;found C 58.81,H 5.98,N 9.07,O 15.65,S 10.49.
4k:1H NMR(DMSO-d6,400 MHz)δ:9.75(s,1H),7.87~8.40(m,4H),8.05(s,1H),5.51(d,J= 4.0 Hz,1H),4.13(q,J=5.6 Hz,2H),2.43(s,3H),1.23(t,J=5.6 Hz,3H).Anal.calcd for C14H15N3O5: C 55.08,H 4.95,N 13.76,O 26.20;found C 55.10,H 5.00,N 13.79,O 26.26.
4l:1H NMR(DMSO-d6,400 MHz)δ:9.53(s,1H),8.55(s,1H),7.27~7.40(m,4H),5.41(d,J= 6.0 Hz,1H),4.12(q,J=7.6 Hz,2H),2.30(s,3H),1.53(t,J=7.6 Hz,3H).Anal.calcd for C14H15ClN2O3: C 57.05,H 5.13,Cl 12.03,N 9.50,O 16.29;found C 57.03,H 5.15,Cl 12.05,N 9.51,O 16.26.
4m:1H NMR(DMSO-d6,400 MHz)δ:10.35(s,1H),9.65(s,1H),7.79~8.13(m,4H),5.35(d,J= 4.0 Hz,1H),4.07(q,J=7.2 Hz,2H),2.29(s,3H),1.09(t,J=7.2 Hz,3H).Anal.calcd for C14H15ClN2O2S: C 54.10,H 4.86,Cl 11.41,N 9.01,O 10.30,S 10.32;found C 54.08,H 4.85,Cl 11.40,N 9.02,O 10.31,S 10.34.
4n:1H NMR(DMSO-d6,400 MHz)δ:8.53(s,1H),8.26(s,1H),6.97~7.37(m,4H),5.97(d,J= 4.0 Hz,1H),3.95(s,3H),3.37(s,3H),2.40(s,3H).Anal.calcd for C14H16N2O3:C 64.60,H 6.20,N 10.76,O 18.44;found C 64.64,H 6.18,N 10.77,O 18.41.
4o:1H NMR(DMSO-d6,400 MHz)δ:8.33(s,1H),8.05(s,1H),7.03~7.67(m,5H),5.83(d,J= 3.6 Hz,1H),3.49(s,3H),2.37(s,3H).Anal.calcd for C13H14N2O2:C 67.81,H 6.13,N 12.17,O 13.90;found C 67.80,H 6.14,N 12.19,O 13.88.
2 结论
对原始的Biginelli合成法进行简单的改进,寻找得到一种简单易行的合成3,4-二氢嘧啶-2-酮及其衍生物的方法,具有良好的应用前景.
[1]KAPPE CO.100 years of the biginelli dihydropyrimidine synthesis[J].Tetrahedron,1993,49(32):6 937-6 963.
[2]SANGRAM G,SUNDARABABU B,BURKHARD K.Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid-ureamixtures[J].Green Chem,2011(13):1 009-1 013.
[3]DANIEL L,DA S,SERGIO A F,et al.P-sulfonic acid calixarenes as efficient and reusable organocatalysts for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones[J].Tetrahedron Letters,2011,52:6 328-6 330.
[4]SEKINEH A,MAHMOOD T,BEHNAM JK.Supramolecular synthesis of 3,4-dihydropyrimidin-2(1H)-one/thiones under neat conditions[J].Chinese Chemical Letters,2011,22(2):127-130.
[5]GARIMA,VISHNU P S,LAL D S Y.Biginelli reaction starting directly from alcohols[J].Tetrahedron Letters,2010,51:6 436-6 438.
[6]彭家建,邓友全.室温离子液体催化“一锅法”合成3,4-二氢嘧啶-2-酮[J].有机化学,2002,22(1):71-73.
[7]Fang Dong,Luo Jun,Zhou Xinli,et al.One-pot green procedure for Biginelli reaction catalyzed by novel task-specific roomtemperature ionic liquids[J].Journal of Molecular Catalysis A:Chemical,2007,274:208-211.
[8]杨兆平,盛寿日,林淑英,等.硝酸铋催化“一锅法”合成3,4-二氢嘧啶-2-酮[J].江西师范大学学报,2007,31(3):262-267.
[9]MOJGAN K,RAHIM H,ABDOLJALIL M,et al.Efficient and green synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones using imidazol-1-yl-acetic acid as a novel,reusable and water-soluble organocatalyst[J].Catalysis Communications,2011,15:123-126.
[10]路军,马怀让,李万华.三氯化铁催化的一锅法合成3,4-二氢嘧啶-2-酮[J].有机化学,2000,20(5):815-819.
[11]付岩,张爱黎,杜红梅,等.磷酸二氢钾催化合成5-乙氧羰基-4-苯基-6-甲基-3,4-二氢嘧啶-2-酮[J].化学与生物工程,2007,24(11):28-30.
[12]FARHAD S,MOHAMMAD A Z,JALAL A.Melamine trisulfonic acid:A new,efficient and recyclable catalyst for the synthesis of3,4-dihydropyrimidin-2(1H)-ones-thiones in the absence of solvent[J].Chinese Chemical Letters,2011,22(3):318-321.
[13]TU Shujiang,FANG Fang,MIAO Chunbao,et al.One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using boric acid as catalyst[J].Tetrahedron Letters,2003,44:6 153-6 155.
[14]傅南雁,袁耀锋,庞美丽,等.利用三溴化铟催化的Biginelli反应原位合成3,4-二氢嘧啶-2-酮[J].高等学校化学学报,2003,24(1):79-81.
[15]DEBACHE A,BOUMOUD B,AMIMOUR M,et al.Phenylboronic acid as a mild and efficient catalyst for Biginelli reaction[J].Tetrahedron Letters,2006,47:5 697-5 699.
[16]屠树江,房芳,高原,等.微波辐射下4-(2-氯苯基)-6-甲基-5-乙氧羰基-3,4-二氢嘧啶-2-酮的合成和晶体结构[J].结构化学,2003,22(5):617-619.
[17]王东超,袁腾飞,杨西宁,等.微波辐射下FeCl3·6H2O催化绿色合成3,4-二氢嘧啶-2-酮[J].有机化学,2007,27(8): 1 034-1 037.
[18]丁欣宇,景晓辉,施磊.超声波辐射合成4-(4-氯苯基)-6-甲基-5-乙氧羰基-3,4-二氢嘧啶-2(1H)-酮[J].化学试剂,2006,28(4):249-251.
[19]RANU B C,HAJRA A,JANA U.Indium(III)chloride catalyzed one-pot synthesis of dihydropyrimidinones by a threecomponent coupling of 1,3-dicarbonyl compounds,aldehydes and urea:an improved procedure for the Biginelli reaction[J].J Org Chem,2000,65:6 270-6 272.
Abstract:3,4-dihydropyrimidine-2(1H)-Ones and their derivatives were synthesized in high yields by onepot three component beginelli reactions of aldehydes,β-dicarbonyl compounds,urea or thiourea catalytic by picric acid.The reaction time need 2-3 h,The final yield was 75% -98%.
Key words:Biginelli reaction;3,4-dihydropyrimidine-2(1H)-Ones;picric acid
One-Pot Synthesis of 3,4-Dihydropyrim idine-2(1H)-Ones
WEIZhen-zhong,WANG Shao-hui,HONG Mei-ling
(School of Chemistry and Materials Science,Huaibei Normal University,235000,Huaibei,Anhui,China)
O 626.4
A
2095-0691(2012)03-0046-04
2012-04-05
中科院重点实验室开放基金(PCOM201105);安徽省含能材料重点实验室基金项目(KLEM2009009)
魏振中(1979- ),男,安徽巢湖人,硕士,讲师,主要从事药物合成研究.
