植物血红蛋白基因功能研究进展
2018-06-19,,
,,
(1.中国科学院 东北地理与农业生态研究所 大豆分子设计育种重点实验室,吉林 长春 130102;2.中国科学院大学,北京 100049)
0 引 言
血红蛋白(Hemoglobin,Hb)广泛存在于动物、植物及微生物体内[1-2],起到O2输送与贮存以及运输CO2的作用。植物血红蛋白在植物根瘤固氮、抗逆胁迫、胚胎发育以及营养生长等方面具有重要作用[2-9]。自从1939年,Kubo从大豆根瘤中鉴定到植物血红蛋白以来,已经在绝大多数的物种中发现了植物血红蛋白的存在,并进行了深入的研究[10-11]。在2014年XVIII 氧结合和传感蛋白国际大会上,提出了植物血红蛋白(Phytoglobin)新的命名体系,现已被广泛采纳[12]。目前植物血红蛋白在决定细胞命运中新功能的发现等研究[13]已成为了一个新的研究热点[14],本文综述了国际上目前对于植物血红蛋白功能研究上的新进展。
1 植物血红蛋白类型和命名
植物血红蛋白最先在豆科植物的根瘤中发现。1938年,Pietz与Bakt发现蚕豆(Viciafaba)根瘤中的红色素是共生微生物增殖和生长的必要成分,红色素参与氧化还原反应[15]。1939年Kubo在大豆根瘤中分离出红色素,进一步的结晶表明红色素是一种类似于脊椎动物马肌红蛋白的血红蛋白(Haemoglobin)[16]。在最初的研究中,仅在豆科植物的根瘤中发现了血红蛋白的存在,Virtanen和Laine将存在于豆科植物根瘤中的血红蛋白命名为豆血红蛋白或根瘤血红蛋白(Leghemoglobin,Lb)[15]。随后的研究中发现,在与植物共生的根瘤中普遍存在与Lb相似的共生血红蛋白(Symbiotic hemoglobin,sHb)。Fukudome等根据植物系统进化起源和理化性质将sHb分为Class 1、Class 2和Class 3 3种类型[7]。
后续研究发现,在非豆科植物中存在非共生血红蛋白(Nonsymbiotic hemoglobin,nsHb)。Appleby等第一次在根瘤菌共生的非豆科双子叶植物糙叶山麻黄(Parasponiaandersonii)根瘤中发现并提纯了血红蛋白,打破了血红蛋白一直以来仅存在于豆科植物根瘤中的论断,并发现该血红蛋白不仅在共生的根瘤中表达,同时也在植物非共生部位表达[17]。随后在山黄麻(Trema)、大麦(Hordeumvulgare)、玉米(Zeamays)、小麦(Triticumaestivum)、黑麦(Secalecereale)、地钱(Marchantiapolymorpha)、水稻(Oryzasativa)、棉花(Gossypiumspp.)、拟南芥(Arabidopsisthaliana)及山黄麻属等多种单子叶和双子叶植物中发现nsHb[4,6],证明了在植物中广泛存在非共生血红蛋白[3]。根据与氧气亲和力的强弱可将nsHb分为nsHb-1和nsHb-2[9]。其中,nsHb-2能够结合氧气,这与sHb的功能类似,而nsHb-1的表达与低氧胁迫和营养物质过量供给密切相关,又被称为压力诱导的植物血红蛋白[18-19]。
进一步的研究表明,植物存在截短血红蛋白(Truncated hemoglobin,tHb)。Watts等在拟南芥中发现比正常血红蛋白少20~40氨基酸的植物血红蛋白,并将此命名为截短血红蛋白,它与nsHb在清除植物体内NO功能性质方面具有相似性,同时,tHb与其他两类植物血红蛋白相比具有更低的氧气亲和力[6]。另外,在棉花(Gossypiumspp.)、水稻(O.sativa)、栽培稻籼稻(O.sativavar.indica)和粳稻(O.sativavar.japonica)等植物中也发现了tHb[9],证明该种血红蛋白也广泛存在于植物体中。
2014年的新植物血红蛋白命名体系,为解决目前植物血红蛋白命名混乱的现状提供了一个很好的解决途径,开始被研究者接受和使用。在此系统中Phytogb0代表藻类、苔藓及裸子植物中的非共生血红蛋白(nsHb),Phytogb1代表被子植物中的非共生血红蛋白nsHb-1,Phytogb2代表被子植物中的非共生血红蛋白nsHb-2,SymPhytogb代表非豆科的共生血红蛋白(sHb),Lb代表豆科血红蛋白,Phytogb3代表藻类和陆生植物中的截短血红蛋白。各类蛋白质的性质详见表1。
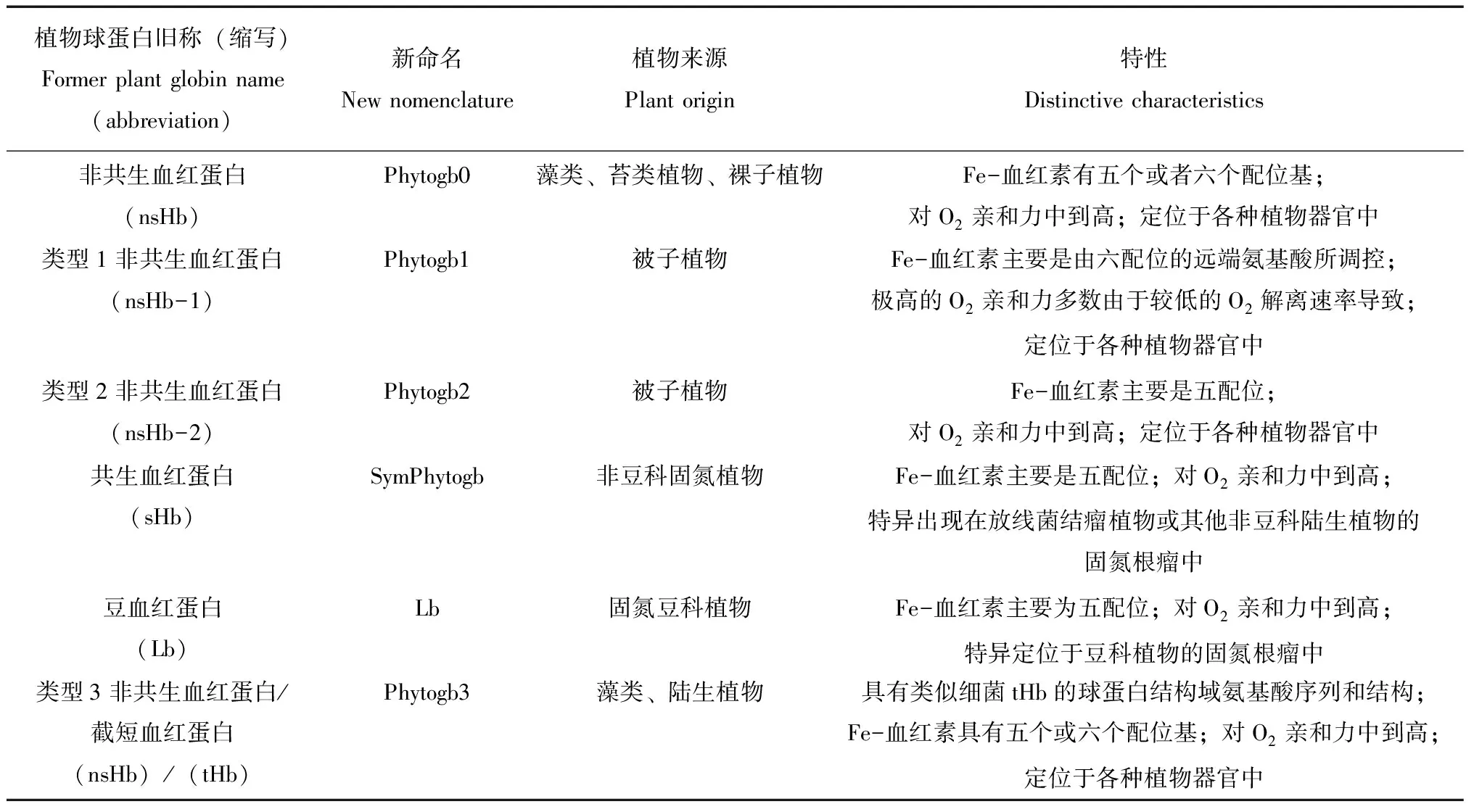
表1 植物(藻类、陆生植物)血红蛋白的系统和特征的命名法Table 1 System and characteristics of the accepted nomenclature for plant (algae+land plants) Phytoglobins
2 共生血红蛋白(sHb)功能
共生血红蛋白是由植物球蛋白和来自外源根瘤菌的亚铁血红素辅基(Ferroheme)组成的复合蛋白,它能在根瘤中特异表达,被认为是豆科植物根瘤固氮的重要指标[20]。植物根瘤的形成过程与植物血红蛋白具有密切关系:在形成初期即根瘤菌侵入及感染过程中,植物根瘤中不含有植物血红蛋白,不具有固氮功能;在根瘤发育过程中,随血红蛋白的形成,根瘤开始具有固氮功能;随根瘤及根瘤菌的退化,血红蛋白含量与固氮作用也随之降低,根瘤中植物血红蛋白含量与根瘤有效性呈正相关关系[21]。在豆科植物根瘤固氮过程中,植物共生血红蛋白存在于根瘤菌固氮的场所—细菌周膜,它对O2亲和力强,能够可逆地结合O2,促进O2扩散供给固氮微生物,降低菌体内部氧分压,将类菌体周围的O2环境维持在7~11 nmol·L-1,为固氮酶提供适宜氧环境,从而保护菌体内部的固氮酶活性[22],同时,促进游离氧的扩散[23]。
在百脉根中通过RNAi沉默血红蛋白基因,发现当根瘤中的游离氧含量增加时,类菌体内的固氮酶受损,甚至丧失固氮能力,证实了sHb在植物根瘤共生固氮过程中具有关键性作用[24]。sHb通过结合O2,促进游离氧扩散,从而为类菌体提供低氧环境和ATP,并保护对O2敏感的固氮酶活性,这对特定环境下植物细胞呼吸代谢和耐涝具有重要意义。sHb的作用在植物-微生物共生中尤为重要,植物通过sHb调节NO并结合植物激素调控自身防御反应,NO通过与生长素(Auxin)相互作用参与植物根系的发育,并为极端条件提供ATP和低氧环境,维持固氮酶活性[25-27]。
3 非共生血红蛋白(nsHb)功能

植物非共生血红蛋白不仅能够运输和存储氧气,促进氧气扩散,控制微生物菌群体内的NO水平,并利用NO控制O2的水平,从而控制氧化还原反应信号通路[9,29-31];还可通过控制类菌体与自由基之间的活性和反应结合并运输CO、硫化物和油脂等,使植物免受其害[8-9,32-33];在结瘤植物中通过NO参与发生在土壤、微生物和植物中的不同生物过程,触发不同的植物-微生物互作的必要途径[27],作为干扰激素合成、影响其活性的重要信号分子[34],此外,nsHb还具有清除含氯化物等功能[8,35]。

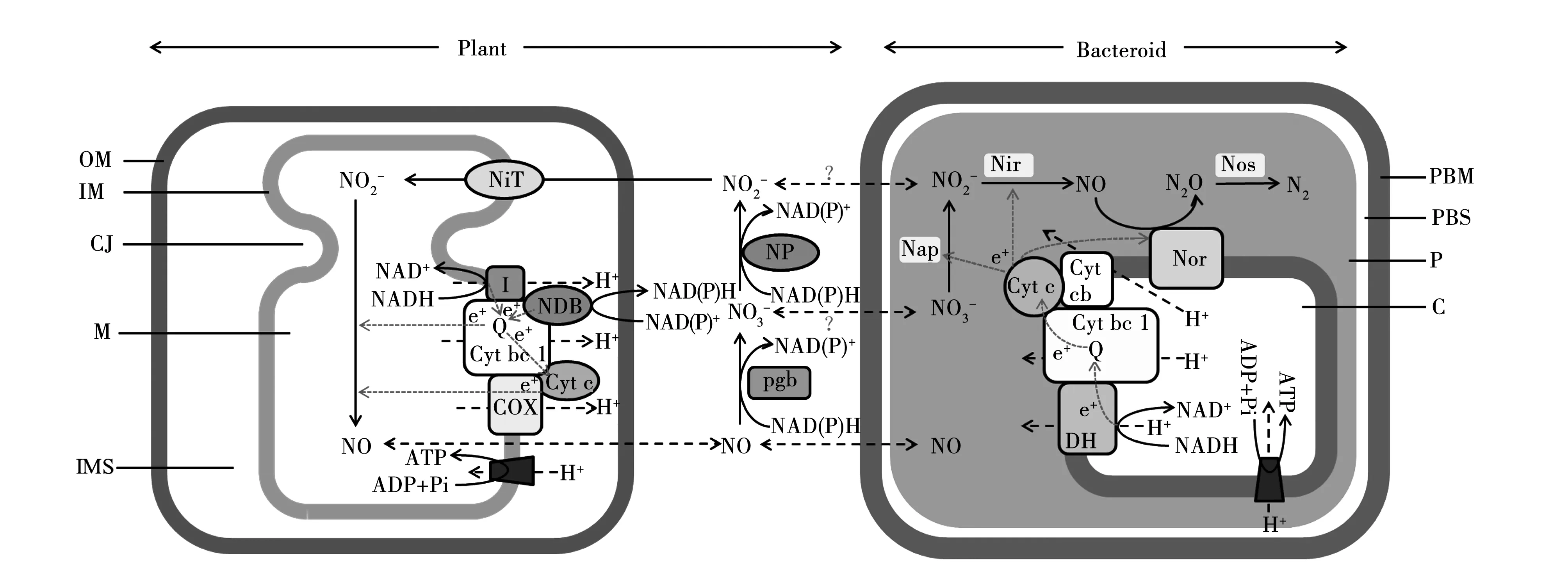
注:参考[37]和[38]文献并做修改。虚线代表潜在的或者假设的物质转运机制。外膜:Outer Membrane(OM);内膜:Inner Membrane(IM);嵴:Cristae Junction(CJ);基质:Matrix(M);线粒体膜间隙:Mitochondrial Intermembrane Space(IMS);类菌体细胞膜:Peribacteroid Membrane(PBM);类菌体间隙:Peribacteroid Space(PBS);周质:Periplasm(P);细胞基质:Cytosol(C);亚硝酸盐转运蛋白:Nitrite Transporter(NiT);线粒体复合体Ⅰ:Mitochondrial Complex I (Ⅰ);辅酶Q:Ubiquinone(Q);细胞色素bc1(复合体Ⅲ):Cytochrome bc1(Complex III)(Cyt bc1);细胞色素氧化酶: Cytochrome Oxidase(COX);细胞色素C:Cytochrome c(Cyt c);硝酸盐还原酶:Nitrate Reductase(NR) ;植物球蛋白:Phytoglobin(Pgb);硝酸盐还原酶:Nitrate Reductase(Nap);亚硝酸盐还原酶:Nitrite Reductase(Nir);NO还原酶:NO Reductase(Nor);一氧化二氮还原酶:Nitrous Oxide Reductase(Nos);细胞色素cb:Cytochrome cb(Cyt cb);NADH-对苯二酚氧化还原酶:NADH-quinol oxidoreductase(DH)。Note:Revised[37] and [38] references.Dashed lines represent potential or bypothetical material transport mechanism.图1 低氧条件下根瘤中线粒体Pgb-NO循环Fig.1 Schematic of mitochondrial Pgb-NO cycle pathway operation in hypoxic nodules
4 截短血红蛋白(tHb)功能
截短血红蛋白与非共生植物血红蛋白在清除NO、CO和H2S等方面的功能相似,而不同的特性及分子结构又体现出不同于共生、非共生植物血红蛋白的功能[6]。截短血红蛋白具有保守的家族特征和序列的多样性,由于配体结构是由截短血红蛋白氮调控[39],因而异常灵活,使其在植物进化中对O2有从高到低的亲和力,对环境变化具有快速的功能适应性[40-41]。截短血红蛋白和共生血红蛋白进化起源不同,其最早在原核生物及真核藻类中被发现[6],在百脉根和弗兰克氏菌属中被诱导产生,通过调控自身的表达参与植物体内NO的内稳态,后来在原核生物、蓝细菌、细菌、古生菌、真核藻类及植物中均被发现[36,42-43]。
5 植物血红蛋白调控胚胎发育及抗逆胁迫的功能
细胞程序性死亡(Programmed cell death,PCD)是影响植物体细胞胚胎发生的重要因素之一[50]。植物血红蛋白能够调节NO含量,而NO作为植物调控PCD的重要信号分子进一步调控植物体细胞胚胎发育。植物特定血红蛋白的表达可减少NO含量,改变植物对逆境胁迫的反应,终止PCD[51]。同时,体细胞胚胎发生能够调控特定细胞血红蛋白表达,改变胚胎发育进程的模式系统,影响植物激素生物合成、细胞代谢过程的PCD和胚性潜能的表达[50]。细胞内NO含量增加会导致与PCD相关的Zn2+和ROS含量增加,同时降低负反馈调控IAA-的MYC2表达,影响体细胞胚胎发生[50]。在NO含量升高时ZmPgb能诱导玉米(Z.mays)在体细胞胚胎发生过程中发生PCD[52];在拟南芥中,NO能激活茉莉酸生物合成通路中的关键酶丙二烯氧化合酶和脂氧化酶2基因的表达,促进拟南芥胚胎组织内JA含量增加[53],与NO共同调节体细胞内的抑制剂MYC2和茉莉酸酯蛋白基因(JASMONATE-ZIM-PROTEIN,JAZ1),同时,JA通过植物血红蛋白(Class 2 Pgb)调控体细胞胚胎发生[35,47]。
同时,植物血红蛋白在抗逆胁迫中具有重要作用。在受到低氧胁迫时,抑制玉米(Z.mays)血红蛋白基因(ZmPgb1.1或ZmPgb1.2)表达会导致根部顶端分生组织异常分化,抑制根部生长;此外,Pgbs通过调节NO,间接调控乙烯含量,控制活性氧含量(Reactive Oxygen Spices,ROS),实现低氧胁迫下保护根尖分生组织的功能[53]。Pgb保护细胞分生功能的作用可能是植物应对诸如盐分和干旱等胁迫的反应,如紫花针茅(Stipapurpurea)中的StipurPhytogb1具有提高转基因拟南芥在干旱和渍涝条件下的耐受能力[27],进一步研究发现,这是由StipurPhytogb1通过抑制NO的积累,调节抗氧化酶活性,进而诱导清除活性氧的相关通路来提升上述耐受能力。谷类作物在水淹等极端条件下,过量表达Pgb基因,可增强光合进程中有关的抗氧化酶的活性,抑制抗坏血酸含量及ROS活性,减轻水淹胁迫压力,确保植物在极端条件胁迫下具有更高的光合速率;反之,植物抗氧化酶活力降低,光合速率下降[54]。
在拟南芥中,重度PEG胁迫产生水分亏缺时,Class 1 Phytoglobin(AtPgb1)的表达有利于延迟其顶端分生组织的死亡和退化[55]。当根部受到极端胁迫时,抑制AtPgb1表达导致细胞内部发生PCD,这个过程可通过ROS和乙烯的调节得到缓解[56],达到部分恢复植物根部生长的作用[57-58]。PEG胁迫致使植物根部组织细胞生长受阻是先于根尖分生组织细胞结构变化之前发生的,包括细胞和特化组织的缺失,这可能会导致调节植物激素的PIN1和PIN4的改变,进而增加根部细胞极性[59-61]。抑制AtPgb1表达能延缓WOX5的相应表达[62],调控静态细胞中心(Quiescent center,QCs)的功能[63],导致早期营养生长中茎组织细胞功能差异和分生组织大小的快速减少[64-65]。同时发现,拟南芥根部的其它组织细胞主要基因如SCARECROW(SCR)的表达调控内皮层和QCs的功能,通过非自主细胞信号保持根部原始细胞的分化能力[66-67];WEREWOLF(WER)的表达会严重影响植物侧根的生长和功能,这同样会显著影响AtPgb1抑制时PEG胁迫处理对根部生长及功能的影响[55,68]。AtPgb1在PEG胁迫时起到保护拟南芥根部正常功能的作用,同时确保根部组织细胞在极端胁迫下通过保留特定的细胞行使分生组织分化生长的功能[69]。
6 展 望
关于植物血红蛋白的起源、进化及功能等方面,前人已进行了总结[5,7-8,17,20,22,46,70],而植物中血红蛋白基因在体细胞胚胎发生中所起的作用才刚刚起步;未来植物血红球蛋白在植物发育中所发挥的作用将会得到进一步的揭示;植物血红蛋白与NO信号决定细胞命运以及其在植物抗逆境胁迫上的深入研究将为人们重新认识其在植物生长发育的作用开辟一个新的视角。
参考文献(References):
[1] SUZUKI T,IMAI K.Evolution of myoglobin[J].Cellular and Molecular Life Sciences,1998,54(9): 979-1004.
[3] TAYLOR E R,NIE X Z,MACGREGOR A W,et al.A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions[J].Plant Molecular Biology,1994,24(6): 853-862.
[4] HEBELSTRUP K H,IGAMBERDIEV A U,HILL R D.Metabolic effects of hemoglobin gene expression in plants[J].Gene,2007,398(1-2): 86-93.
[5] HUNT P W,WATTS R A,TREVASKIS B,et al.Expression and evolution of functionally distinct haemoglobin genes in plants[J].Plant Molecular Biology,2001,47(5): 677-692.
[6] WATTS R A,HUNT P W,HVITVED A N,et al.A hemoglobin from plants homologous to truncated hemoglobins of microorganisms[J].Proceedings of the National Academy of Sciences of the United States of America,2001,98(18): 10119-10124.
[7] SHANKAR A,FERNANDES J L,KAUR K,et al.Rice phytoglobin regulate responses under low mineral nutrients and abiotic stresses inArabidopsisthaliana[J].Plant Cell and Environment,2017,41(1):215-230.
[8] DAMIANI I,PAULY N,PUPPO A,et al.Reactive oxygen species and nitric oxide control early steps of theLegume-Rhizobiumsymbiotic interaction[J].Frontiers in Plant Science,2016,7(384): 454-461.
[9] ARREDONDOPETER R,MORAN J F,SARATH G.Rice(Oryza) hemoglobins[J].Fl000 Research,2014,3: 253-270.
[10] 吴玉厚,吴冰洁,周国立,等.植物血红蛋白研究进展[J].生物学教学,2008,33(1): 10-12,14.
WU Y H,WU B J,ZHOU G L,et al.Research progress in plant hemoglobin[J].Biology Teaching,2008,33(1): 10-12,14.
[11] 徐慧妮,赵秀玲,何小钊,等.植物非共生血红蛋白的研究进展[J].植物生理学报,2012,3: 217-222.
XU H N,ZHAO X L,HE X Z,et al.Research progress in plant non-symbiotic hemoglobin[J].Plant Physiology Journal,2012,48 (3): 217-222.
[12] HILL R,HARGROVE M,ARREDONDO-PETER R.Phytoglobin:A novel nomenclature for plant globins accepted by the globin community at the 2014 XVIII conference on oxygen-binding and sensing proteins[J].Fl000 Research,2016,5: 212.
[13] STASOLLA C,HILL R D.Determining cellular responses:Phytoglobins may direct the traffic[J].Trends in Plant Science,2017,22(10): 820-822.
[14] MIRA M M,HUANG S,HILL R D,et al.Protection of root apex meristem during stress responses[J].Plant Signaling and Behavior,2018,13(2): e1428517.
[15] KEILIN D,WANG Y L.Haemoglobin in the root nodules of leguminous plants[J].Nature,1945,155: 227-229.
[16] KUBO H.Hemoprotein from the root nodules of legumes[J].Acta Phytochim,1939,11: 195-200.
[17] APPLEBY C A,TJEPKEMA J D,TRINICK M J.Hemoglobin in a nonleguminous plant,Parasponia:Possible genetic origin and function in nitrogen fixation[J].Science,1983,220(4600): 951-953.
[18] IVARSON E,LEIVAERIKSSON N,AHLMAN A,et al.Effects of overexpression ofWRI1 and hemoglobin genes on the seed oil content ofLepidiumcampestre[J].Frontiers in Plant Science,2016,7: 2032.
[19] DORDAS C,RIVOAL J,HILL R D.Plant haemoglobins,nitric oxide and hypoxic stress[J].Annals of Botany,2003,91(2): 173-178.
[20] DAKORA F D.A functional relationship between leghaemoglobin and nitrogenase based on novel measurements of the two proteins in legume root nodules[J].Annals of Botany,1995,75(1): 49-54.
[21] BERGERSEN F J,BRIGGS M J.Studies on the bacterial component of soybean root nodules:Cytology and organization in the host tissue[J].Journal of General Microbiology,1958,19(3):482-490.
[22] DOWNIE J A.Legume haemoglobins:Symbiotic nitrogen fixation needs bloody nodules[J].Current Biology,2005,15(6): 196-198.
[23] PARSONS R,DAY D A.Mechanism of soybean nodule adaptation to different oxygen pressures[J].Plant Cell and Environment,1990,13(6): 501-512.
[24] OTT T,VAN DONGEN J T,GÜNTHER C,et al.Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development[J].Current Biology,2005,15(6): 531-535.
[25] SHIMODA Y,SHIMODA-SASAKURA F,KUCHO K I,et al.Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity betweenMesorhizobiumlotiandLotusjaponicus[J].Plant Journal for Cell and Molecular Biology,2009,57(2): 254-263.
[26] FUKUDOME M,CALVOBEGUERIA L,KADO T,et al.HemoglobinLjGlb1-1 is involved in nodulation and regulates the level of nitric oxide in theLotusjaponicus-Mesorhizobiumlotisymbiosis[J].Journal of Experimental Botany,2016,67(17): 5275-5283.
[27] LI X,SUN X D,YANG S H,et al.Molecular cloning and functional analysis of a novel phytoglobin gene from the alpine plantStipapurpurea[J].Plant Ecology and Diversity,2017,10(1):17-27.
[28] OHWAKI Y,KAWAGISHI-KOBAYASHI M,WAKASA K,et al.Induction of class-1 non-symbiotic hemoglobin genes by nitrate,nitrite and nitric oxide in cultured rice cells[J].Plant and Cell Physiology,2005,46(2):324-331.
[29] LEIVAERIKSSON N,PIN P A,KRAFT T,et al.Differential expression patterns of non-symbiotic hemoglobins in sugar beet(Betavulgarisssp.vulgaris)[J].Plant and Cell Physiology,2014,55(4): 834-844.
[30] WEI L,DERRIEN B,GAUTIER A,et al.Nitric oxide-triggered remodeling of chloroplast bioenergetics and thylakoid proteins upon nitrogen starvation inChlamydomonasreinhardtii[J].Plant Cell,2014,26(1): 353-372.
[31] BOSCARI A,MEILHOC E,CASTELLA C,et al.Which role for nitric oxide in symbiotic N2-fixing nodules:Toxic by-product or useful signaling/metabolic intermediate?[J].Frontiers in Plant Science,2013,4(18): 384-384.
[32] BLANQUET P,SILVA L,CATRICE O,et al.Sinorhizobiummeliloticontrols nitric oxide-mediated post-translational modification of aMedicagotruncatulanodule protein[J].Molecular Plant Microbe Interactions,2015,28(12): 1353-1363.
[33] VINOGRADOV S N,MOENS L.Diversity of globin function:Enzymatic,transport,storage,and sensing[J].Journal of Biological Chemistry,2008,283(14): 8773-8777.
[34] WALLY O S D,MIRA M M,HILL R D,et al.Hemoglobin regulation of plant embryogenesis and plant pathogen interaction[J].Plant Signaling and Behavior,2013,8(8): e25264.
[35] MINAEVA E,ZALUTSKAYA Z,FILINA V,et al.Truncated hemoglobin 1 is a new player inChlamydomonasreinhardtiiacclimation to sulfur deprivation[J].PLoS ONE,2017,12(10): e0186851.
[36] BOSCARI A,DEL GLUDICE J,FERRARINI A,et al.Expression dynamics of theMedicagotruncatulatranscriptome during the symbiotic interaction withSinorhizobiummeliloti:Which role for nitric oxide?[J].Plant Physiology,2013,161(1): 425-439.
[37] BERGER A,BROUQUISSE R,PATHAK P K,et al.Pathways of nitric oxide metabolism and operation of phytoglobins in legume nodules:Missing links and future directions[J].Plant Cell and Environment,2018.Doi:10.1111/pce.13151.
[38] GUPTA K J,HEBELSTRUP K H,MUR L A J,et al.Plant hemoglobins:Important players at the crossroads between oxygen and nitric oxide[J].FEBS Letters,2011,585(24): 3843-3849.
[39] MEUWLY M,DAS A K.Kinetics and structural interpretation of competitive ligand binding for NO dioxygenation in truncated hemoglobin N[J].Angewandte Chemie,2018,57(33):3509-3513.
[40] BUSTAMANTE J P,RADUSKY L,BOECHI L,et al.Evolutionary and functional relationships in the truncated hemoglobin family[J].PLoS Computational Biology,2016,12(1):e1004701.
[41] ASCENZI P,PESCE A.Peroxynitrite scavenging byCampylobacterjejunitruncated hemoglobin P[J].Journal of Biological Inorganic Chemistry,2017,22(8): 1141-1150.
[42] JOKIPII-LUKKARI S,KASTANIOTIS A J,PARKASH V,et al.Dual targeted poplar ferredoxin NADP+oxidoreductase interacts with hemoglobin 1[J].Plant Science,2016,247: 138-149.
[43] CHAMIZOAMPUDIA A,SANZLUQUE E,LLAMAS A,et al.Nitrate reductase regulates plant nitric oxide homeostasis[J].Trends in Plant Science,2017,22(2): 163-174.
[44] SANZ-LUQUE E,OCANA-CALAHORRO F,DE MONTAIGU A,et al.THB1,a truncated hemoglobin,modulates nitric oxide levels and nitrate reductase activity[J].The Plant Journal,2015,81(3):467-479.
[45] HUWALD D,SCHRAPERS P,KOSITZKI R,et al.Characterization of unusual truncated hemoglobins ofChlamydomonasreinhardtiisuggests specialized functions[J].Planta,2015,242(1): 167-185.
[46] JOHNSON E A,RICE S L,PREIMESBERGER M R,et al.Characterization of THB1,aChlamydomonasreinhardtiitruncated hemoglobin:Linkage to nitrogen metabolism and identification of lysine as the distal heme ligand[J].Biochemistry,2014,53(28): 4573-4589.
[47] CORPAS F J,BARROSO J B.Nitric oxide from a "green" perspective[J].Nitric Oxide Biology and Chemistry,2015,45: 15-19.
[48] HEMSCHEMEIER A,D NER M,CASERO D,et al.Hypoxic survival requires a 2-on-2 hemoglobin in a process involving nitric oxide[J].Proceedings of the National Academy of Sciences of the United States of America,2013,110(26): 10854-10859.
[49] KIM D Y,HONG M J,LEE Y J,et al.Wheat truncated hemoglobin interacts with photosystem I PSK-I subunit and photosystem II subunit PsbS1[J].Biologia Plantarum,2012,57(2): 281-290.
[50] HILL R D,HUANG S,STASOLLA C.Hemoglobins,programmed cell death and somatic embryogenesis[J].Plant Science,2013,211(3): 35-41.
[51] WILLIAMS B,VERCHOT J,DICKMAN M B.When supply does not meet demand-ER stress and plant programmed cell death[J].Frontiers in Plant Science,2014,5(5): 211-219.
[52] MIRA M M,WALLY O S D,ELHITI M,et al.Jasmonic acid is a downstream component in the modulation of somatic embryogenesis byArabidopsisClass 2 phytoglobin[J].Journal of Experimental Botany,2016,67(8): 2231-2246.
[53] HUANG X,STETTMAIER K,MICHEL C,et al.Nitric oxide is induced by wounding and influences jasmonic acid signaling inArabidopsisthaliana[J].Planta,2004,218(6): 938-946.
[54] YOUSSEF M S,MIRA M M,RENAULT S,et al.Phytoglobin expression influences soil flooding response of corn plants[J].Annals of Botany,2016,118(5): 919-931.
[55] MIRA M M,HUANG S,KAPOOR K,et al.Expression ofArabidopsisclass 1 phytoglobin(AtPgb1) delays death and degradation of the root apical meristem during severe PEG-induced water deficit[J].Journal of Experimental Botany,2017,68(20): 5653-5668.
[56] YAMAUCHI T,RAJHI I,NAKAZONO M.Lysigenous aerenchyma formation in maize root is confined to cortical cells by regulation of genes related to generation and scavenging of reactive oxygen species[J].Plant Signaling and Behavior,2011,6(5): 759-761.
[57] ZUPPINI A,NAVAZIO L,MARIANI P.Endoplasmic reticulum stress-induced programmed cell death in soybean cells[J].Journal of Cell Science,2004,117(Pt12):2591-2598.
[58] WATANABE N,LAM E.Bax inhibitor-1 modulates endoplasmic reticulum stress-mediated programmed cell death inArabidopsis[J].Journal of Biological Chemistry,2008,283(6): 3200-3210.
[59] PETERSSON S V,JOHANSSON A I,KOWALZYK M,et al.An auxin gradient and maximum in theArabidopsisroot apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis[J].Plant Cell,2009,21(6): 1659-1668.
[60] VIETEN A,VANNESTE S,WISNIEWSKA J,et al.Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression[J].Development,2005,132(20): 4521-4531.
[61] FRIML J,BENKOV E,BLILOU I,et al.AtPIN4 mediates sink-driven auxin gradients and root patterning inArabidopsis[J].Cell,2002,108(5): 661-673.
[62] PI L,AICHINGER E,VAN DER GRAAFF E,et al.Organizer-derivedWOX5 signal maintains root columella stem cells through chromatin-mediated repression ofCDF4 expression[J].Developmental Cell,2015,33(5): 576-588.
[63] DING Z J,FRIML J.Auxin regulates distal stem cell differentiation inArabidopsisroots[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(26): 12046-12051.
[64] JI H T,LIU L,LI K X,et al.PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat[J].Journal of Experimental Botany,2014,65(17): 4863-4872.
[65] DUAN Y,ZHANG W,LI B,et al.An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress inArabidopsis[J].New Phytologist,2010,186(3):681-695.
[66] SABATINI S,BEIS D,WOLKENFELT H,et al.An auxin-dependent distal organizer of pattern and polarity in theArabidopsisroot[J].Cell,1999,99(5): 463-472.
[67] SCHERES B.Stem-cell niches:Nursery rhymes across kingdoms[J].Nature Reviews Molecular Cell Biology,2007,8(5): 345-354.
[68] PETRICKA J J,WINTER C M,BENFEY P N.Control ofArabidopsisroot development[J].Annual Review of Plant Biology,2012,63: 563-590.
[69] MIRA M M,EL-KHATEEB E A,SAYEDAHMED H I,et al.Are avoidance and acclimation responses during hypoxic stress modulated by distinct cell-specific mechanisms?[J].Plant Signaling and Behavior,2017,12(1): e1273304.
[70] BOGUSZ D,APPLEBY C A,OUML,et al.Functioning haemoglobin genes in non-nodulating plants[J].Nature,1988,331(6152): 178-180.
