Immune responses induced by BCG prime and single dose of recombinant adenovirus Ad5-CEAB boosted strategy in mice
2017-06-28LIWuDENGGuangcunLIMinLIUXiaomingWANGYujiong
LI Wu, DENG Guang-cun, LI Min, LIU Xiao-ming, WANG Yu-jiong
(Key Laboratory of Ministry of Education for Conservation and Utilization of Special Biological Resources in the Western, Ningxia University, Yinchuan 750021, China)
Immune responses induced by BCG prime and single dose of recombinant adenovirus Ad5-CEAB boosted strategy in mice
LI Wu, DENG Guang-cun, LI Min, LIU Xiao-ming, WANG Yu-jiong
(KeyLaboratoryofMinistryofEducationforConservationandUtilizationofSpecialBiologicalResourcesintheWestern,NingxiaUniversity,Yinchuan750021,China)
Tuberculosis (TB) remains an enormous health burden worldwide. To date,MycobacteriumbovisBacillusCalmette Guerin (BCG) is the unique anti-TB vaccine available for humans, which provides an important but limited protection from theMycobacteriumtuberculosis(Mtb) infection. It is therefore an urgent need to develop better vaccines and vaccination strategies to prevent the spread of Mtb infection. Heterologous prime-boost vaccination strategies using both BCG and novel anti-TB vaccines have been demonstrated to induce robust immune responses than BCG alone. We have previously demonstrated that a recombinant adenoviral vector Ad5-CEAB co-expressing CFP10, ESAT6, Ag85A and Ag85B of Mtb was able to induce robust antigen-specific immune responses in mice. In the present study, we examined immunological effects of Ad5-CEAB in the mice primed with BCG and boosted with a single dose of the virus via an intranasal route. Results demonstrated that this vaccination strategy could effectively induce strong antigen-specific mucosal and humoral immune responses. These immune responses were characterized with an increased productions of cytokines (IL-12 and IFN-γ), increased concentration of secretary IgA (sIgA) in bronchoalveolar lavage fluid (BALF) and serum IgG in mice in comparison with mice in BCG group. These data suggested that the regimen of BCG prime-single dose of Ad5-CEAB boosted strategy was novel for inducing antigen-specific immune responses in response to Mtb antigensinvivo, which warrants for further development of adenoviral-based vaccine against Mtb infection.
Mycobacteriumtuberculosis; prime-boost; recombinant adenovirus; vaccine
Tuberculosis (TB) is caused byMycobacteriumtuberculosis(Mtb), remains a major global public health problem with an estimated incidence of 9 million new cases per year[1].MycobacteriumbovisBacille Calmette-Guerin (BCG) is the only licensed TB vaccine for protection of human beings from Mtb infection worldwide. However, the protective efficacy of BCG is highly varied from different trials with complications (0-80%)[2-3]. Great efforts have been made in developing new TB vaccines and various types of vaccines have been developed in recent years, including DNA vaccines[4-5], subunit vaccines[6], the recombinant BCG (rBCG)[7-8], nanoparticle vaccines[9-10], immunotherapeutic vaccine[11]and recombinant adenoviral-based vaccines[12-14]. Among them, the adenoviral vaccines have gained increased attention in TB vaccine development. Recombinant adenoviral vectors expressing mycobacterial antigens have been extensively tested in different animal models[12-13]and in humans[14]. Previous studies have demonstrated that vaccination with heterologous prime-boost strategies show a great promise for TB prevention[15-16]. As a consequence, feasible vaccination strategies that able to boost BCG-primed immunity have gained increasing attention, taking into account that billions of people in the world have already vaccinated with BCG.
Given the facts of TB is primarily a respiratory infectious disease caused by the infection of intracellular pathogen Mtb, and both of the mucosal immunity and the cellular immunity thus have been suggested to play pivotal roles in protection from Mtb infection, vaccination via a mucosal route is thus believed to be superior to other vaccination routes[17]. In this regard, a vaccine candidate that able to be delivered via a mucosal route and elicit strong mucosal and cellular immune responses may be ideal for TB vaccine development. The recombinant adenoviral vector is an ideal antigen delivery system for TB vaccine development, owing to their properties of type 1 immune adjuvant activity, excellent safety record in humans, restrained high levels of antigen release, suitability for both parenteral and intranasal mucosal delivery, as well as high efficacy at eliciting a robust cellular immunity in experimental animals[18-20]. Increasing attention has thus been paid to adenoviral vector in TB vaccines development[21-23].
We have recently developed a novel recombinant adenoviral vector Ad5-CEAB, which was capable of co-expressing a mixture of the above four most studied Mtb antigens in a single adenoviral vector, and induce stong antigen-specific immune responses in mice[20]. The aim of this study was to investigate the effect of this vector in mice with a BCG prime-single dose of virus boosted strategy. Our data demonstrated that single dose of the recombinant adenovirus Ad5-CEAB was capable of boosting BCG primed immunity.The immunization strategy could induce robust antigen-spencific immune responses in mice. Our data suggest that the BCG prime- recombinant adenovirus boost strategy may be a novel vaccination strategy in the future of anti-TB vaccine development, which warrants further investigation.
Materials and methods
Animals, bacterial strains andMycobacteriumtuberculosisantigens
Female ICR mice between 6 and 8 weeks of age were purchased from the Animal facility of Ningxia Medical University (Yinchuan, China). The mice were raised at the Experimental Animal Center of Ningxia University (Yinchuan, China). All experiments using animals were conducted following the guidelines of the Chinese Council on Animal Care and approved by the Ethic Committee for the Conduct of Animal Research of Ningxia University. BCG vaccine was produced by Chengdu Institute of Biological Products (Chengdu, China). The his-tagged Mtb CFP10, ESAT6, Ag85A and Ag85B proteins were prepared and purified with AKTA protein purification system per manufacturer’s instruction (GE Healthcare, USA), as previously described[20]. Endotoxins in the purified proteins were removed using ToxinEraserTM Endotoxin Removal Kit (GenScript, USA). The antigenic proteins used in this study had a purity of greater than 85%.
Recombinant adenovirus Ad5-CEAB preparation and immunization
The recombinant adenovirus Ad5-CEAB expressing Mtb genes of CFP10, ESAT6, Ag85A and Ag85B were prepared in our lab as previous described[20]. This vector expressed these four interest genes as a mixture of proteins rather than a fusion protein[20]. For immunization, mice were randomly divided into four groups (8 mice/group). (1) PBS group: mice were treated intranasally with 100 μL of PBS three times with two-week intervals; (2) BCG group: mice were injected subcutaneously with a single dose of 1×106colony-forming units (CFU) of BCG vaccine; (3) BCG /Ad5-2 group: mice were injected subcutaneously with 1×106CFU of BCG and then intranasal boosted with two doses of 100 μL of 1×109plaque-forming units (PFU) of Ad5-CEAB with two-week intervals. (4) BCG /Ad5-1 group: mice were injected subcutaneously with 1×106CFU of BCG and then intranasal boosted with a single dose of 100 μL of 1×109PFU of Ad5-CEAB two weeks post the BCG injection. Six weeks after the first immunization, animals were euthanized for analysis of immune responses.
Secretory IgA (sIgA) measurement
Bronchoalveolar lavage fluid (BALF) was collected as described previously[23]. The supernatants of BALF were harvested and stored at -20 ℃ till the sIgA measurement. The sIgA was determined with an Enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA, USA) using above purified bacterial expressed His-tagged Mtb proteins (5 μg/mL of each purified Mtb CFP10, ESAT6, Ag85A and Ag85B) as antigens for coating the plates.
Cytokines induction and measurement
Splenocytes at a density of 5×105/well were seeded into 24-well plates and cultured in 5% FCS RPMI-1640 medium with stimuli (5 μg/mL of each purified Mtb CFP10, ESAT6, Ag85A and Ag85B) for 72 h. Culture supernatants were then collected and stored at -80 ℃ till use. The concentrations of IFN-γ, TNF-α and IL-2 were determined using an ELISA cytokine detection system (RayBiotech, Norcross, GA, USA) per manufacture’s instruction. All experiments were performed in triplicate.
ELISA assay for antigen-specific IgG
The sera were collected 6 weeks after the first immunization for the antibody detection. The concentration of antigen-specific IgG was measured by a mouse ELISA Ready-SET-Go! Kit according to manufacturer’s instructions with minor modifications (eBioscience, San Diego, CA, USA). Briefly, the ELISA plates were pre-coated with Mtb antigens (5μg/ml of each purified Mtb CFP10, ESAT6, Ag85A and Ag85B proteins) at 4 ℃ overnight, rather than directly coated with the capturing antibodies provided in the kit.
Data analysis
Experimental data were expressed in the mean±SD. Data were analyzed by one-way ANOVA, the differences between groups were assessed by the Tukey’s post hoc test using SPSS 13.0 software (SSPS Inc, Chicago, USA), and aP<0.05 was considered as statistical significance.
Results
BALF sIgA detection in mice
sIgA is the most characteristic component of the mucosal immunity and plays indispensable role in the host defense against pathogens in mucosal surfaces[24]. Figure 1 showed that sIgA levels in BALF were remarkably elevated in both BCG primed mice and BCG primed-Ad5CEAB boosted mice. The sIgA concentration in mice immunized with the BCG/Ad5-2 (1 151.57±170.67 μg/mL), BCG/Ad5-1 group (993.64±179.52 μg/mL) and BCG priming alone (950.81±72.78, μg/mL) was significantly higher as compared to the mice in PBS group (815.13±73.65 μg/mL) (P<0.05). Importantly, a single dose of Ad5-CEAB boost led to greater elevation of antigen-specific sIgA secretion in the BALF, and was significantly greater than the PBS and BCG-treated group (Figure 1), an indication that single mucosal boost with Ad5-CEAB could induce strong mucosal immune responsesinvivo.
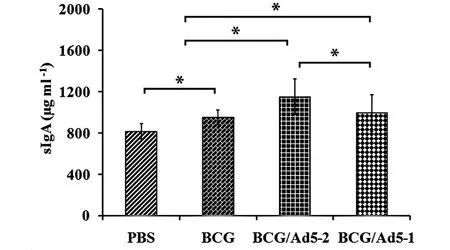
Six weeks after the first immunization, BALF was collected and BALF supernatants were harvested for analyzing antigen-specific sIgA concentrations by ELISA. Data are represented as mean values±SD from three independent triplicate experiments (n=8). *:P<0.05.Fig.1 Antigen-specific sIgA levels in bronchoalveolar lavage fluid (BALF) of immunized mice
Cytokines responses
Cytokines are key effectors in the host defense against Mtb infection[25]. Thereby, the concentrations of cytokines IL-12 and INF-γ in culture supernatants of lymphocytes stimulated with Mtb antigensinvitrowere detected using respective ELISA kits (Figure 2). The results showed that significantly elevated antigen-induced cytokines IL-12 (Figure 2A) and INF-γ (Figure 2B) were found in the BCG prime-Ad5-CEAB boosted group than the BCG and PBS group, an indication that both of the BCG prime-Ad5-CEAB boost strategies could evoke stronger antigen-specific immunity in mice than a strategy using BCG alone. Noticeably, there was no difference in the production of tested cytokines between the BCG/Ad5-2 and the BCG/Ad5-1 group, suggesting that a single dose of mucosal Ad5-CEAB boost is sufficient to stimulate stronger antigen-specific cytokines responses in mice as compared to the BCG alone.
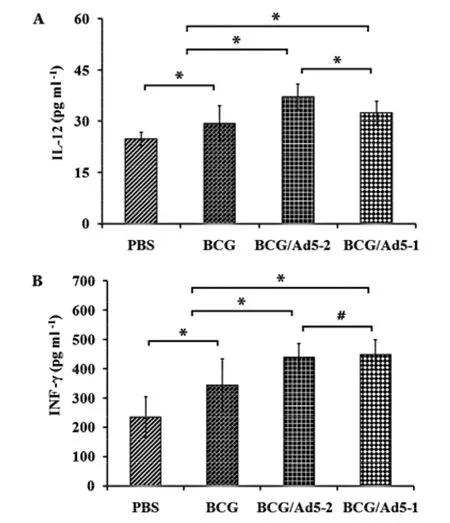
Six weeks after the first immunization, the mice were sacrificed and the splenic mononuclear cells from individual mice were stimulated with the Mtb antigens and the supernatants were harvested. The concentrations of IL-12 (A) and INF-γ (B) were then quantitatively analyzed by ELISA. Data are represented as mean values±SD from three independent triplicate experiments (n=8). *: P<0.05; #: P>0.05.Fig.2 Levels of antigen-stimulated cytokines production
Serum antibody levels
To evaluate the IgG antibody responses in mice primed with BCG and boosted with Ad5-CEAB, we examined the titers of IgG in mice at 6 weeks after the BCG immunization. Figure 3 showed that antigen-specific IgG levels in the BCG immunized group and the BCG primed/Ad5CEAB boosted groups were significantly higher than the PBS group. Moreover, the IgG level in the BCG primed and Ad5-CEAB boosted groups was superior to BCG alone group, which indicates that the prime-boosted strategy could elicit stronger antigen-specific humoral responses.
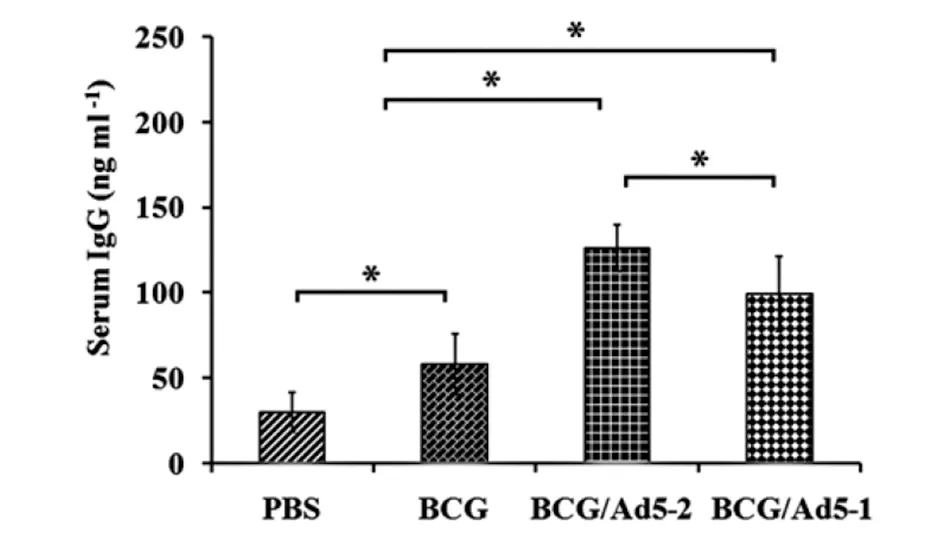
Six weeks after the first immunization, mice were sacrificed and their serum samples were collected. The antigen-specific antibody response was ascertained by measuring the antigen-specific IgG concentration. Data are represented as mean values±SD from three independent experiments (n=8). *: P<0.05. Fig.3 Antigen-specific antibody response against Mtb antigens in immunized mice
Discussion
Currently, it is generally accepted that repeated vaccination with the same vaccine results in higher levels of antibodies than following a single vaccination. This is also true in our study that the serum IgG levels in the BCG/Ad5-2 group were significant higher than that of the BCG/Ad5-1 group, suggesting that two dose of Ad5-CEAB boost is more efficient in evoking antibody responses than that of single dose boost. A recent study showed that serum antibodies had a protective effect in animal models of tuberculosis[26], despite humoral immunity has ever been thought to play a limited role in the protective immunity of vaccination against Mtb infection. Human and animal studies have demonstrated that revaccination with BCG could not confer additional protection, or even negative effect against TB[27-30], suggesting that homologous boost with the same vaccine may be is not sufficient for the intracellular pathogen Mtb infection. In contrast, a heterologous prime-boost strategy using both BCG and a novel TB vaccine candidate has displayed potentials to elicit robust protective immune responses, especially cellular immune response against Mtb infection in animal models and in humans[31-34]. Since BCG is widely used as unique anti-TB vaccine in most developing countries, employment of a second vaccine to boost BCG-primed immunity may be the most practical strategy taking into account. To some extent, a successful design of the booster vaccine for boosting BCG is essential for the successful vaccination against Mtb infection. By our knowledge, the recombinant adenovirus Ad5-CEAB was the first attempt for expression of four Mtb antigens as a mixture of individual proteins, rather than fusion protein in a single adenovirus vector, which represents a promising novel vaccine platform capable of boosting the BCG induced immunity.
A better understanding of the nature of protective immunity against TB would facilitate the development of anti-TB vaccines. As a mucosally transmitted pathogen, the mucosal immunity is thought to play important roles against Mtb infection and vaccines capable of efficiently inducing mucosal responses may offer a desirable protection against Mtb. Indeed, increasing evidence does suggest that vaccination via a mucosal route is superior to vaccination with other administration routes for inducing protective immunity against Mtb infection[35-36]. Since sIgA is the most characteristic component of the mucosal immunity, and may play an important role in the host’s early defense against invading pathogens in respiratory tract[37]. We tested BALF sIgA levels to access the mucosal immunity trigged by the prime-boost strategy in this study. Our data demonstrated that the sIgA levels in BALF were remarkably elevated in the BCG/Ad5-2 group and BCG/Ad5-1 group as compared to the BCG and PBS group, indicating that the BCG prime and Ad5-CEAB boost strategy was able to strongly augment mucosal immune responses in vivo. We also found that sIgA levels in the BCG/Ad5-2 group were significant higher than that of the single dose of Ad5-CEAB boosted group. However, since sIgA levels were measured only two weeks after the last boost for the BCG/Ad5-2, and 4 weeks after the last boost for the BCG/Ad5-1, the differences between these two groups of mice are difficult for comparison and need to be understood objectively. Nevertheless, single dose of Ad5-CEAB boost via mucosal route was efficient in eliciting robust mucosal immune responses in the BCG/Ad5-1 in contrast to the BCG and PBS group.
Collectively, the results of this study show that the heterologous prime-boost strategy that subcutaneously primed with BCG and intranasally boosted with a single dose of Ad5-CEAB could elicit robust antigen-specific immune responses in mice. These results shed light on developing novel TB vaccines from recombinant adenoviral vectors and novel anti-TB vaccination strategies. An evaluation of the protection efficacy of the BCG prime Ad5-CEAB boost vaccination strategy from Mtb challenge in mice is necessary for future study.
[1] World Health Organization. Global Tuberculosis Report 2013[EB/OL]. (2014-05-17)[2016-09-25]. www.who.int/tb/publications/global_report/en/.
[2] Bolger T, O'Connell M, Menon A, et al. Complications associated with the bacille Calmette-Guerin vaccination in Ireland[J]. Arch Dis Child, 2006, 91(7): 594-597. DOI: 10.1136/adc.2005.078972
[3] Nuttall JJ, Davies MA, Hussey GD, et al. Bacillus Calmette-Guerin (BCG) vaccine-induced complications in children treated with highly active antiretroviral therapy[J]. Int J Infect Dis, 2008,12(6): e99-105. DOI: 10.1016/j.ijid.2008.06.014S1201-9712(08)01413-6
[4] Yuan W, Dong N, Zhang L, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine expressing a fusion protein of Ag85B-Esat6-HspX in mice[J]. Vaccine, 2012, 30(14): 2490-2497. DOI: 10.1016/j.vaccine.2011.06.029
[5] Lu J, Wang C, Zhou Z, et al. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein[J]. Clin Dev Immunol, 2011, 2011: 617892. DOI: 10.1155/2011/617892
[6] Brandt L, Elhay M, Rosenkrands I, et al. ESAT-6 subunit vaccination againstMycobacteriumtuberculosis[J]. Infect Immun, 2000, 68(2): 791-795.
[7] Farinacci M, Weber S, Kaufmann SH. The recombinant tuberculosis vaccine rBCG DeltaureC::hly(+) induces apoptotic vesicles for improved priming of CD4(+) and CD8(+) T cells[J]. Vaccine, 2012, 30(52): 7608-7614. DOI: 10.1016/j.vaccine.2012.10.031
[8] Rahman S, Magalhaes I, Rahman J, et al. Prime-boost vaccination with rBCG/rAd35 enhances CD8(+) cytolytic T-cell responses in lesions fromMycobacteriumtuberculosis-infected primates[J]. Mol Med, 2012, 18: 647-658. DOI: 10.2119/molmed.2011.00222
[9] Ballester M, Nembrini C, Dhar N, et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis[J]. Vaccine, 2011, 29(40): 6959-6966. DOI: 10.1016/j.vaccine.2011.07.039
[10] Bivas-Benita M, Lin MY, Bal SM, et al. Pulmonary delivery of DNA encodingMycobacteriumtuberculosislatency antigen Rv1733c associated to PLGA-PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice[J]. Vaccine, 2009, 27(30): 4010-4017. DOI: 10.1016/j.vaccine.2009.04.033
[11] Yang C, He YL, Zhang L, et al. GLS/IL-12-modifiedMycobacteriumsmegmatisas a novel anti-tuberculosis immunotherapeutic vaccine[J]. Intl J Tuberculosis Lung Dis, 2009, 13(11): 1360-1366.
[12] Perez de Val B, Villarreal-Ramos B, Nofrarias M, et al. Goats primed withMycobacteriumbovisBCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis[J]. Clin Vaccine Immunol, 2012, 19(9): 1339-1347. DOI: 10.1128/CVI.00275-12
[13] Xing Z, McFarland CT, Sallenave JM, et al. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis[J]. PLoS One, 2009, 4(6): e5856. DOI: 10.1371/journal.pone.0005856
[14] Smaill F, Jeyanathan M, Smieja M, et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity[J]. Sci Transl Med, 2013, 5(205): 205ra134. DOI: 10.1126/scitranslmed.3006843
[15] Siddiqui KF, Amir M, Khan N, et al. Prime-boost vaccination strategy with bacillus Calmette-Guerin (BCG) and liposomized alpha-crystalline protein 1 reinvigorates BCG potency[J]. Clin Exp Immunol, 2015, 181(2): 286-296. DOI: 10.1111/cei.12634
[16] Ji P, Hu ZD, Kang H, et al. Boosting BCG-primed mice with chimeric DNA vaccine HG856A induces potent multifunctional T cell responses and enhanced protection againstMycobacteriumtuberculosis[J]. Immunol Res, 2016, 64(1): 64-72. DOI: 10.1007/s12026-015-8674-9
[17] Davis SS. Nasal vaccines[J]. Adv Drug Deliv Rev, 2001, 51(1-3): 21-42. DOI: S0169-409X(01)00162-4
[18] Xing Z, Lichty BD. Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization[J]. Tuberculosis (Edinb), 2006, 86(3-4): 211-217. DOI: 10.1016/j.tube.2006.01.017
[19] Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors[J]. Mol Ther, 2009, 17(8): 1333-1339. DOI: 10.1038/mt.2009.130mt2009130
[20] Li W, Deng G, Li M, et al. A recombinant adenovirus expressing CFP10, ESAT6, Ag85A and Ag85B ofMycobacteriumtuberculosiselicits strong antigen-specific immune responses in mice[J]. Mol Immunol, 2014, 62(1): 86-95. DOI: 10.1016/j.molimm.2014.06.007S0161-5890(14)00138-2
[21] Wang J, Thorson L, Stokes RW, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis[J]. J Immunol, 2004, 173(10): 6357-6365.
[22] Ronan EO, Lee LN, Beverley PC, et al. Immunization of mice with a recombinant adenovirus vaccine inhibits the early growth ofMycobacteriumtuberculosisafter infection[J]. PLoS One, 2009, 4(12): e8235. DOI: 10.1371/journal.pone.0008235
[23] Santosuosso M, McCormick S, Zhang X, et al. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteralMycobacteriumbovisBCG immunization against pulmonary tuberculosis[J]. Infect Immun, 2006, 74(8): 4634-4643.
[24] McNabb PC, Tomasi TB. Host defense mechanisms at mucosal surfaces[J]. Annu Rev Microbiol, 1981, 35: 477-496. DOI: 10.1146/annurev.mi.35.100181.002401
[25] Flynn JL, Chan J. Immunology of tuberculosis[J]. Annu Rev Immunol, 2001, 19: 93-129. DOI: 10.1146/annurev.immunol.19.1.93
[26] Olivares N, Marquina B, Mata-Espinoza D, et al. The protective effect of immunoglobulin in murine tuberculosis is dependent on IgG glycosylation[J]. Pathog Dis, 2013, 69(3): 176-183. DOI: 10.1111/2049-632X.12069
[27] Rodrigues LC, Pereira SM, Cunha SS, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial[J]. Lancet, 366(9493): 1290-1295. DOI: 10.1016/S0140-6736(05)67145-0
[28] Dantas OM, Ximenes RA, de Albuquerque Mde F, et al. A case-control study of protection against tuberculosis by BCG revaccination in Recife, Brazil[J]. Int J Tuberc Lung Dis, 2006, 10(5): 536-541.
[29] Basaraba RJ, Izzo AA, Brandt L, et al. Decreased survival of guinea pigs infected withMycobacteriumtuberculosisafter multiple BCG vaccinations[J]. Vaccine, 2006, 24(3): 280-286. DOI: 10.1016/j.vaccine.2005.07.103
[30] Buddle BM, Wedlock DN, Parlane NA, et al. Revaccination of neonatal calves withMycobacteriumbovisBCG reduces the level of protection against bovine tuberculosis induced by a single vaccination[J]. Infect Immun, 2003, 71(11): 6411-6419.
[31] Hoft DF, Blazevic A, Stanley J, et al. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity[J]. Vaccine, 2012, 30(12): 2098-108. DOI: 10.1016/j.vaccine.2012.01.048S0264-410X(12)00086-2
[32] Perez de Val B, Villarreal-Ramos B, Nofrarias M, et al. Goats primed withMycobacteriumbovisBCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis[J]. Clin Vaccine Immunol, 2012, 19(9): 1339-1347. DOI: 10.1128/CVI.00275-12CVI.00275-12
[33] Dou J, Wang Y, Yu F, et al. Protection againstMycobacteriumtuberculosischallenge in mice by DNA vaccine Ag85A-ESAT-6-IL-21 priming and BCG boosting[J]. Int J Immunogenet, 2012, 39(2):183-190. DOI: 10.1111/j.1744-313X.2011.01066.x
[34] Dean G, Whelan A, Clifford D, et al. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines[J]. Vaccine, 2014, 32(11): 1304-1310. DOI: 10.1016/j.vaccine.2013.11.045S0264-410X(13)01566-1
[35] Chen L, Wang J, Zganiacz A, et al. Single intranasal mucosalMycobacteriumbovisBCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis[J]. Infect Immun, 2004, 72(1): 238-246.
[36] Giri PK, Sable SB, Verma I, et al. Comparative evaluation of intranasal and subcutaneous route of immunization for development of mucosal vaccine against experimental tuberculosis[J]. FEMS Immunol Med Microbiol, 2005, 45(1): 87-93. DOI: 10.1016/j.femsim.2005.02.009
[37] Lamm ME. Interaction of antigens and antibodies at mucosal surfaces[J]. Annu Rev Microbiol, 1997, 51: 311-340. DOI: 10.1146/annurev.micro.51.1.311
Received:2016-03-18 Editor:LIN Dan
BCG初免-单次重组腺病毒Ad5-CEAB加强免疫策略的免疫效应研究
李 武,邓光存,李 敏,刘晓明,王玉炯
目的 为了评价BCG初免-单次重组腺病毒Ad5-CEAB加强的免疫策略诱导小鼠产生的免疫效应。方法 在前期工作的基础上,制备并纯化获得了高滴度的重组腺病毒Ad5-CEAB,然后以小鼠为动物模型,采用BCG初免-Ad5-CEAB加强的异源初免-加强的免疫策略对动物进行免疫,通过测定小鼠淋巴细胞抗原刺激液中IL-12和IFN-γ的含量,测定小鼠血清IgG抗体含量以及小鼠肺盥洗液中sIgA的浓度来评价BCG初免-单次重组腺病毒Ad5-CEAB加强免疫策略的免疫效应。结果 BCG初免-Ad5-CEAB加强免疫后,经抗原刺激后的小鼠淋巴细胞会分泌高水平的IL-12和IFN-γ,这两种细胞因子的浓度均显著高于对照组。BCG初免-Ad5-CEAB加强后,还可以有效的刺激小鼠分泌IgG抗体,血清IgG抗体浓度显著高于对照组。另外,小鼠肺盥洗液中sIgA的浓度也显著高于对照组。结论 采用BCG初免-单次Ad5-CEAB加强的异源初免-加强的免疫策略能够有效的刺激小鼠产生较强的免疫应答反应。
结核分枝杆菌;初免-加强;重组腺病毒;疫苗
R378.9
A
1002-2694(2017)06-0501-07
王玉炯,Email:yujiongw@126.com
宁夏大学西部特色生物资源保护与利用教育部重点实验室,银川 750021
10.3969/j.issn.1002-2694.2017.06.006
Wang Yu-jiong, Email: yujiongw@126.com
国家自然科学基金项目(No. 31572494,31160515,31560678)联合资助
Supported by grant from the National Natural Science Foundation of China (Grant Nos. 31572494, 31160515, 31560678)
