大白口蘑子实体的化学成分研究
2014-11-08孙程亮李正辉王元忠刘吉开
孙程亮,李正辉,冯 涛,王元忠,刘吉开,王 刚
(1.安徽中医药大学药学院 现代中药安徽省重点实验室 安徽省中医药科学院药物化学研究所,安徽 合肥 230031;2.中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室,云南 昆明 650201;3.云南省农业科学院药用植物研究所,云南 昆明 650200)
大白口蘑(TricholomagiganteumMassee),隶属于真菌门(Eumycota)担子菌亚门(Basidimycotina)层菌纲(Hymenomycetes)伞菌目(Agaricales)口蘑科(Tricholomataceae)口蘑属(Tricholoma),别名长柄口蘑、巨大口蘑、洛巴口蘑等,在中国、日本、韩国等地均有发现,并且是一种营养价值很高的可食用菌。文献报道大白口蘑具有广泛的生物活性,如抗肿瘤、降压降糖、抗氧化等[1]。国内外关于大白口蘑的研究多集中在其药理活性方面,而关于其化学成分的相关报道极少,为了进一步弄清大白口蘑中的有效活性成分,笔者对大白口蘑子实体进行了系统的化学成分研究,从其乙酸乙酯提取物中分离得到12个已知化合物,经波谱解析,鉴定为:亚油酸甲酯(1)、亚油酸(2)、麦角甾-4,6,8(14),22-四烯-3-酮(3)、麦角甾-5,7,22-三烯-3β-醇(4)、过氧麦角甾醇(5)、麦角甾-7,22-二烯-3β,5α,9α-三羟基-6-酮(6)、麦角甾-7,22-二烯-3β,5α-二羟基-6-酮(7)、麦角甾-7,22-二烯-3β,5α,6α,9α-四醇(8)、麦 角 甾-7,22-二烯-3β,5α,6β,9α-四 醇 (9)、麦 角 甾-7,22-二 烯-3β,5α,6β-三 醇 (10)、3β-O-glucopyranosyl-5α,6β-dihydroxy-ergosta-7,22-diene(11)、脑 苷 脂 D(12)。除化合物4和5外其余10个化合物均为首次从该种真菌中分离得到。
1 仪器与材料
Waters AutoSpec Premier P776和Xevo TQ-S质谱仪:美国 Waters公司生产;Bruker AvanceⅢ-600、AV-400和DRX-500核磁共振仪:德国 Bruker公司生产,以TMS为内标;Agilent 1100型和Agilent 1200型高效液相色谱仪:美国安捷伦公司生产,检测器为DAD检测器,分析型泵为四元泵,制备型泵为二元泵,进样器为自动进样器,分析型色谱柱为Agilent Zorbax SB-C18,色谱柱粒径为5μm,柱长150mm,内径4.6mm,制备型色谱柱同为Agilent Zorbax SB-C18,色谱柱粒径为5μm,柱长150 mm,内径9.4mm;正相柱层析硅胶(80~100目和200~300目)以及GF254薄层层析色谱(thin layer chromatography,TLC)预制硅胶板:均由山东省青岛海洋化工厂生产;Rp-18反相硅胶(40~75μm):日本Fuji silysia化学公司生产;Sephadex LH-20:瑞典Amersham Biosciences公司产品;显色方法为荧光灯下波长254nm和365nm处观察荧光,碘蒸汽显色,10%硫酸香草醛处理后加热显色。大白口蘑(TricholomagiganteumMassee)于2013年8月采自中国云南玉溪,由昆明植物研究所杨祝良研究员鉴定,标本存放于中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室高等真菌化学组(标本号:L20130812)。
2 提取与分离
大白口蘑子实体(干质量1.5kg)用氯仿-甲醇1∶1混合溶剂提取3次。浓缩合并提取液后,将浓缩物(78g)用水溶解,用乙酸乙酯萃取4次。回收乙酸乙酯得浸膏30g。粗提物经正相硅胶柱色谱粗分,以石油醚-丙酮(100∶0→0∶100)梯度洗脱得5个组分(A、B、C、D、E)。组分A(石油醚∶丙酮为20∶1)经石油醚-氯仿(100∶0→0∶100)梯度洗脱得到个5亚组分,A2硅胶柱色谱以石油醚-乙酸乙酯(50∶1)反复洗脱得到化合物1(17mg)。A3经以石油醚-乙酸乙酯(50∶1)反复洗脱得化合物2(8mg),组分B(石油醚∶丙酮为10∶1)经石油醚-丙酮(20∶1→5∶1)梯度洗脱得到4个亚组分,其中B1以石油醚-丙酮(20∶1)反复洗脱得到化合物3(10mg),B2经正相硅胶柱色谱以石油醚-丙酮(10∶1)洗脱再重结晶得到化合物4(50mg),B3正相硅胶柱色谱以石油醚-丙酮(8∶1)洗脱再重结晶得到化合物5(30mg)。组分D(石油醚∶丙酮为2∶1)经反相RP-18以甲醇-水(50∶50→100∶0)梯度洗脱得到5个亚组分,其中 D3经硅胶柱色谱以三氯甲烷-丙酮(8∶1)反复洗脱得化合物6(3mg)和化合物7(1mg)。组分E(石油醚∶丙酮为1∶1)以氯仿-丙酮(6∶1)反复洗脱得到5个亚组分,各亚组分分别重结晶得化合物8(5.2mg)、化合物9(3.6mg)、化合物10(5mg)、化合物11(3 mg)、化合物12(20mg)。
3 结构鉴定
化合物1:亚油酸甲酯,C19H34O2,淡黄色油状物;EI-MSm/z:294[M]+;1H-NMR (CDCl3,400 MHz)δ:2.30(2H,t,J=7.2Hz,H-2),1.61(2H,m,H-3),1.25~1.37(m,H-4~H-7和H-15~H-17),2.04(4H,m,H-8和 H-14),5.35(4H,m,H-9,H-10,H-12,H-13),2.77(2H,t,J=6.8Hz,H-11),0.88 (3H,t,J=6.8Hz,H-18),3.66(3H,s,-OCH3);13C-NMR(CDCl3,100MHz)δ:174.3 (s,C-1),34.0 (t,C-2),29.7~22.5(t,C-3~C-7和 C-15~C-17),31.9 (t,C-8),130.1 (d,C-9),130.0 (d,C-10),25.6 (t,C-11),128.0(d,C-12),127.8(d,C-13),31.5(t,C-14),14.0(q,C-18),51.4(q,-OCH3)。以上数据与文献[2]数据报道一致,确定该化合物为亚油酸甲酯(见图1)。
化合物2:亚油酸,C18H32O2,无色油状物;1HNMR(CDCl3,400MHz)δ:2.34(2H,t,J=7.6 Hz,H-2),1.63(2H,m,H-3),1.31(14H,m,H-4~H-7,H-15~H-17),2.04 (4H,m,H-8,H-14),5.35(4H,m,H-9,H-10,H-12,H-13),2.75(2H,m,H-11),0.87(3H,t,J=7.2Hz,H-18);13C-NMR(CDCl3,100MHz)δ:179.5(s,C-1),33.9(t,C-2),24.6(t,C-3),29.0~29.6(t,C-4~C-7,C-15),27.2 (t,C-8),130.1 (d,C-9),130.0 (d,C-10),25.6 (t,C-11),128.0(d,C-12),127.8 (d,C-13),27.1 (t,C-14),31.9(t,C-16),22.7(t,C-17),14.1(q,C-18)。以上数据与文献[3]数据报道一致,确定该化合物为亚油酸(见图1)。
化合物 3:麦角甾-4,6,8(14),22-四烯-3-酮,C28H40O,浅黄色晶体;EI-MSm/z:392[M]+;1HNMR(CDCl3,400MHz)δ:5.70(1H,s,H-4),6.00(1H,d,J=9.4Hz,H-6),6.58(1H,d,J=9.4Hz,H-7),0.93 (3H,s,H-18),0.96(3H,s,H-19),1.03 (3H,d,J= 6.8Hz,H-21),5.19(1H,dd,J=15.2,7.2Hz,H-22),5.25(1H,dd,J=15.2,7.2Hz,H-23),0.78(3H,d,J=6.8Hz,H-26),0.82(3H,d,J=6.8 Hz,H-27),0.90 (3H,d,J=6.8Hz,H-28),1.21~2.53 (18H,m, 甾 体 母 核 );13C-NMR(CDCl3,100MHz)δ:34.1 (t,C-1),19.0 (t,C-2),199.3(s,C-3),123.0(d,C-4),164.3(s,C-5),124.5(d,C-6),133.8(d,C-7),124.3(s,C-8),44.1 (d,C-9),36.8 (s,C-10),25.4 (t,C-11),34.2(t,C-12),44.0(s,C-13)156.0(s,C-14),35.7(t,C-15),27.7(t,C-16),55.8(d,C-17),16.7(q,C-18),18.9(q,C-19),39.2(d,C-20),21.2(q,C-21),135.0(d,C-22),132.6(d,C-23),42.9(d,C-24),33.1(d,C-25),19.7(q,C-26),20.0(q,C-27),17.6(q,C-28)。以上波谱数据与文献[4]数据报道一致,确定该化合物为麦角甾-4,6,8(14),22-四烯-3-酮(见图1)。
化合物4:麦角甾-5,7,22-三烯-3β-醇,C28H44O,白 色 晶 体;EI-MSm/z:396 [M]+;1H-NMR(CDCl3,400MHz)δ:3.63(1H,m,H-3),5.57(1H,m,H-6),5.38(1H,m,H-7),0.62(3H,s,H-18),0.93(3H,s,H-19),1.03(3H,d,J=6.8Hz,H-21),5.14~5.25(2H,m,H-22和H-23),0.81(3H,d,J=6.8Hz,H-26),0.84(3H,d,J=6.8Hz,H-27),0.91(3H,d,J=6.8Hz,H-28);13C-NMR (CDCl3,100MHz)δ:38.4 (t,C-1),32.0 (t,C-2),70.4 (d,C-3),40.8(t,C-4),139.8(s,C-5),119.6(d,C-6),116.3(d,C-7),141.3(s,C-8),46.2(d,C-9),37.0(s,C-10),21.1(t,C-11),39.1(t,C-12),42.8(s,C-13),54.6(d,C-14),23.0(t,C-15),28.3(t,C-16),55.7(d,C-17),12.0(q,C-18),16.3(q,C-19),40.4(d,C-20),21.1(q,C-21),135.6 (d,C-22),132.0 (d,C-23),42.8 (d,C-24),33.1(d,C-25),19.6(q,C-26),19.9(q,C-27),17.6(q,C-28)。以上波谱数据与文献[5]数据报道一致,确定该化合物为麦角甾-5,7,22-三烯-3β-醇(见图1)。
化合物5:过氧麦角甾醇,C28H44O3,无色针晶;EI-MSm/z:428 [M]+;1H-NMR (CDCl3,400 MHz)δ:3.97(1H,m,H-3),6.24(1H,d,J=8.4Hz,H-6),6.50(1H,d,J=8.4Hz,H-7),0.82(3H,s,H-18),0.88(3H,s,H-19),0.99(3H,d,J=6.8Hz,H-21),5.22(1H,dd,J=15.2,7.4Hz,H-22),5.13(1H,dd,J=15.2,7.4Hz,H-23),0.80 (3H,d,J=6.8Hz,H-26),0.83(3H,d,J=6.8Hz,H-27),0.90(3H,d,J=6.8Hz,H-28);13C-NMR (CDCl3,100MHz)δ:34.7 (t,C-1),30.1 (t,C-2),66.4(d,C-3),36.9 (t,C-4),82.1 (s,C-5),135.4(d,C-6),130.7(d,C-7),79.4(s,C-8),51.0(d,C-9),36.9(s,C-10),23.4(t,C-11),39.3(t,C-12),44.5(s,C-13),51.6(d,C-14),20.6(t,C-15),28.6(t,C-16),56.2(d,C-17),12.8(q,C-18),18.1(q,C-19),39.7(d,C-20),20.8 (q,C-21),135.2 (d,C-22),132.3 (d,C-23),42.7(d,C-24),33.0(d,C-25),19.6(q,C-26),19.9(q,C-27),17.5(q,C-28)。以上波谱数据与文献[6]数据报道一致,确定该化合物为过氧麦角甾醇(见图1)。
化合物6:麦角甾-7,22-二烯-3β,5α,9α-三羟基-6-酮,C28H44O4,无色针晶;1H-NMR (CDCl3,600 MHz)δ:4.07(1H,m,H-3),5.66(1H,d,J=2.0Hz,H-7),0.62(3H,s,H-18),1.02(3H,s,H-19),1.04(3H,d,J=6.3Hz,H-21),5.16(1H,dd,J=15.2,8.2Hz,H-22),5.24(1H,dd,J=15.2,7.6Hz,H-23),0.82(3H,d,J=6.8Hz,H-26),0.84 (3H,d,J=6.8Hz,H-27),0.92 (3H,d,J=6.7Hz,H-28);13CNMR (CDCl3,150MHz)δ:25.4 (t,C-1),30.1(t,C-2),67.2(d,C-3),37.1(t,C-4),79.7(s,C-5),197.8 (s,C-6),119.8 (d,C-7),164.4(s,C-8),74.6 (s,C-9),41.8 (s,C-10),28.8(t,C-11),34.8(t,C-12),45.3(s,C-13),51.8(d,C-14),22.3(t,C-15),27.9(t,C-16),55.9(d,C-17),12.2(q,C-18),20.4(q,C-19),40.3 (d,C-20),21.1 (q,C-21),135.0 (d,C-22),132.4 (d,C-23),42.8 (d,C-24),33.0(d,C-25),19.6(q,C-26),20.0(q,C-27),17.6(q,C-28)。以上波谱数据与文献[7]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α,9α-三羟基-6-酮(见图1)。
化合物7:麦角甾-7,22-二烯-3β,5α-二羟基-6-酮,C28H44O3,白色结晶;ESI-MSm/z:427 (MH)-;1H-NMR(CDCl3,600MHz)δ:4.03(1H,m,H-3),5.66(1H,br.s,H-7),0.61(3H,s,H-18),0.96(3H,s,H-19),1.03(3H,d,J=6.6Hz,H-21),5.16(1H,dd,J=15.2,8.4Hz,H-22),5.24(1H,dd,J=15.2,7.6Hz,H-23),0.84(3H,d,J=7.6Hz,H-26),0.83(3H,d,J=7.2Hz,H-27),0.91(3H,d,J=7.1Hz,H-28);13C-NMR(CDCl3,150MHz)δ:30.4(t,C-1),30.2 (t,C-2),67.5 (d,C-3),38.8 (t,C-4),77.8(s,C-5),198.2(s,C-6),119.7(d,C-7),165.2(s,C-8),43.9 (d,C-9),40.3(s,C-10),22.0(t,C-11),36.5(t,C-12),44.7(s,C-13),55.8(d,C-14),22.5(t,C-15),27.9(t,C-16),56.0(d,C-17),12.7(q,C-18),16.4(q,C-19),40.0(d,C-20),21.1(q,C-21),135.0(d,C-22),132.5 (d,C-23),42.8 (d,C-24),33.0(d,C-25),19.6(q,C-26),19.9(q,C-27),17.6(q,C-28)。以上波谱数据与文献[8]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α-二羟基-6-酮(见图1)。
化合物8:麦角甾-7,22-二烯-3β,5α,6α,9α-四醇,C28H46O4,无色针晶;ESI-MSm/z:445[M-H]-;1HNMR(MeOD,600MHz)δ:5.06(2H,m,H-7),0.57(3H,s,H-18),1.05(3H,s,H-19),1.02(3H,d,J=6.7Hz,H-21),5.16(1H,dd,J=15.2,7.4Hz,H-22),5.24(1H,dd,J=15.2,7.4Hz,H-23),0.82 (3H,d,J=6.8Hz,H-26),0.84(3H,d,J=6.8Hz,H-27),0.92(3H,d,J=6.8Hz,H-28);13C-NMR (MeOD,150MHz)δ:28.1(t,C-1),30.3(t,C-2),67.2(d,C-3),40.1(t,C-4),74.5(s,C-5),70.2(d,C-6),122.0(d,C-7),143.2(s,C-8),77.0(s,C-9),41.0(s,C-10),27.9(t,C-11),35.0(t,C-12),43.8(s,C-13),50.5(d,C-14),22.8(t,C-15),26.5(t,C-16),55.7(d,C-17),11.7(q,C-18),21.1(q,C-19),40.4(d,C-20),20.3(q,C-21),137.0(d,C-22),133.3(d,C-23),42.8(d,C-24),33.1(d,C-25),19.7(q,C-26),20.0(q,C-27),17.6(q,C-28)。以上波谱数据与文献[9]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α,6α,9α-四醇(见图1)。
化合物9:麦角甾-7,22-二烯-3β,5α,6β,9α-四醇,C28H46O4,无 色 针 晶;ESI-MSm/z:445[M-H]-;1H-NMR (CDCl3,600MHz)δ:3.99(1H,m,H-3),3.64(1H,brd,H-6),5.32(1H,br.s,H-7),0.64(3H,s,H-18),1.11(3H,s,H-19),1.04(3H,d,J=6.5Hz,H-21),5.19(1H,dd,J=15.2,7.4Hz,H-22),5.24(1H,dd,J=15.2,7.4Hz,H-23),0.84(3H,d,J=6.8Hz,H-26),0.86 (3H,d,J=6.8Hz,H-27),0.94 (3H,d,J=7.2Hz,H-28);13CNMR(CDCl3,150MHz)δ:29.3(t,C-1),31.6(t,C-2),68.2(d,C-3),40.7(t,C-4),76.0(s,C-5),73.7(d,C-6),121.0(d,C-7),143.8(s,C-8),78.9 (s,C-9),41.9 (s,C-10),29.1 (t,C-11),36.5(t,C-12),44.8(s,C-13),51.9(d,C-14),24.0(t,C-15),28.3(t,C-16),57.3(d,C-17),12.3(q,C-18),22.2(q,C-19),41.4(d,C-20),21.6(q,C-21),137.0(d,C-22),133.3(d,C-23),44,4(d,C-24),34.4(d,C-25),20.1(q,C-26),20.5(q,C-27),18.2(q,C-28)。以上波谱数据与文献[10]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α,6β,9α-四醇(见图1)。
化合物10:麦角甾-7,22-二烯-3β,5α,6β-三醇,C28H46O3,白色粉末;EI-MSm/z:430[M]+;1HNMR(C5D5N,400MHz)δ:4.84(1H,m,H-3),4.32(1H,br.s,J=4.8Hz,H-6),5.74(1H,br.s,H-7),0.67(3H,s,H-18),1.53(3H,s,H-19),1.07(3H,d,J=6.8Hz,H-21),5.16(1H,dd,J=15.2,7.4Hz,H-22),5.24(1H,dd,J=15.2,7.4Hz,H-23),0.84(3H,d,J=6.8Hz,H-26),0.85 (3H,d,J=6.8Hz,H-27),0.94 (3H,d,J=6.8Hz,H-28);13CNMR(C5D5N,100MHz)δ:32.6(t,C-1),33.8(t,C-2),67.6(d,C-3),42.0(t,C-4),76.5(s,C-5),74.3(d,C-6),120.4(d,C-7),141.6(s,C-8),43.8 (d,C-9),38.1 (s,C-10),22.4 (t,C-11),40.1(t,C-12),43.9(s,C-13),55.2(d,C-14),23.5(t,C-15),28.2(t,C-16),56.5(d,C-17),12.3(q,C-18),18.8(q,C-19),40.7(d,C-20),21.3(q,C-21),136.2(d,C-22),132.5(d,C-23),43.0(d,C-24),33.1(d,C-25),19.9(q,C-26),20.1(q,C-27),17.6(q,C-28)。以上波谱数据与文献[11]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α,6β-三醇(见图1)。
化 合 物 11:3β-O-glucopyranosyl-5α,6β-dihydroxy-ergosta-7,22-diene,C34H56O8,白 色 结晶;1H-NMR(MeOD,600MHz)δ:5.20~5.27(3H,m,H-7,H-22,H-23),0.65 (3H,s,H-18),1.44(3H,s,H-19),1.04(3H,d,J=6.8Hz,H-21),0.84 (3H,d,J=6.4Hz,H-26),0.87(3H,d,J=6.8Hz,H-27),0.94(3H,d,J=6.8Hz,H-28),4.40(1H,d,J=7.8Hz,H-1′);13C-NMR (MeOD,150MHz)δ:34.0 (t,C-1),30.0 (t,C-2),75.1 (d,C-3),37.2(t,C-4 ),76.9 (s,C-5),74.4 (d,C-6),119.0(d,C-7),143.8(s,C-8),44.4(d,C-9),38.3(s,C-10),23.0(t,C-11),40.7(t,C-12),44.7(s,C-13),55.9(d,C-14),24.0(t,C-15),28.0(t,C-16),57.4(d,C-17),12.8(q,C-18),18.9(q,C-19),41.9(d,C-20),21.7(q,C-21),137.0 (d,C-22),133.2 (d,C-23),44.4 (d,C-24),34.4(d,C-25),20.1(q,C-26),20.5(q,C-27),18.2 (q,C-28),102.3 (d,C-1′),76.1(d,C-2′),78.1(d,C-3′),71.6(d,C-4′),77.9(d,C-5′),62.8(t,C-6′)。以上波谱数据与文献[12]数据报道一致,确定该化合物为3β-O-glucopyranosyl-5α,6β-dihydroxy-ergosta-7,22-diene (见图1)。
化合物12:脑苷脂D,C43H81NO9,白色结晶;ESI-MSm/z:754[M -H]-;1H-NMR (MeOD,400MHz)δ:5.49(1H,dd,J=15.6,7.2Hz,H-4),5.73 (1H,d,J=15.6,5.6Hz,H-5),5.12(1H,t,J=5.6Hz,H-8),1.96 (2H,t,J=7.2Hz,H-10),0.89(6H,t,J=6.4Hz,H-18,H-18′),1.59(3H,s,H-19),4.28(1H,d,J=7.8Hz,H-1″);13C-NMR (MeOD,100MHz)δ:68.8 (t,C-1),53.8 (d,C-2),72.5 (d,C-3),129.6(d,C-4),134.5(d,C-5),33.1(t,C-6),25.6(t,C-7),123.8(d,C-8),136.4(s,C-9),40.2(t,C-10),29.8(t,C-11),176.4(s,C-1′),72.3 (d,C-2′),35.0(t,C-3′),28.5(t,C-4′),30.0~30.2(t,C-12~15,5′~15′),32.4(t,C-16,16′),23.1(t,C-17,17′),14.3 (q,C-18,18′),16.1(q,C-19),103.6(d,C-1″),73.9(d,C-2″),78.3(d,C-3″),70.5(d,C-4″),78.0(d,C-5″),61.9(t,C-6″)。以上波谱数据与文献[13]数据报道一致,确定该化合物为脑苷脂D(见图1)。
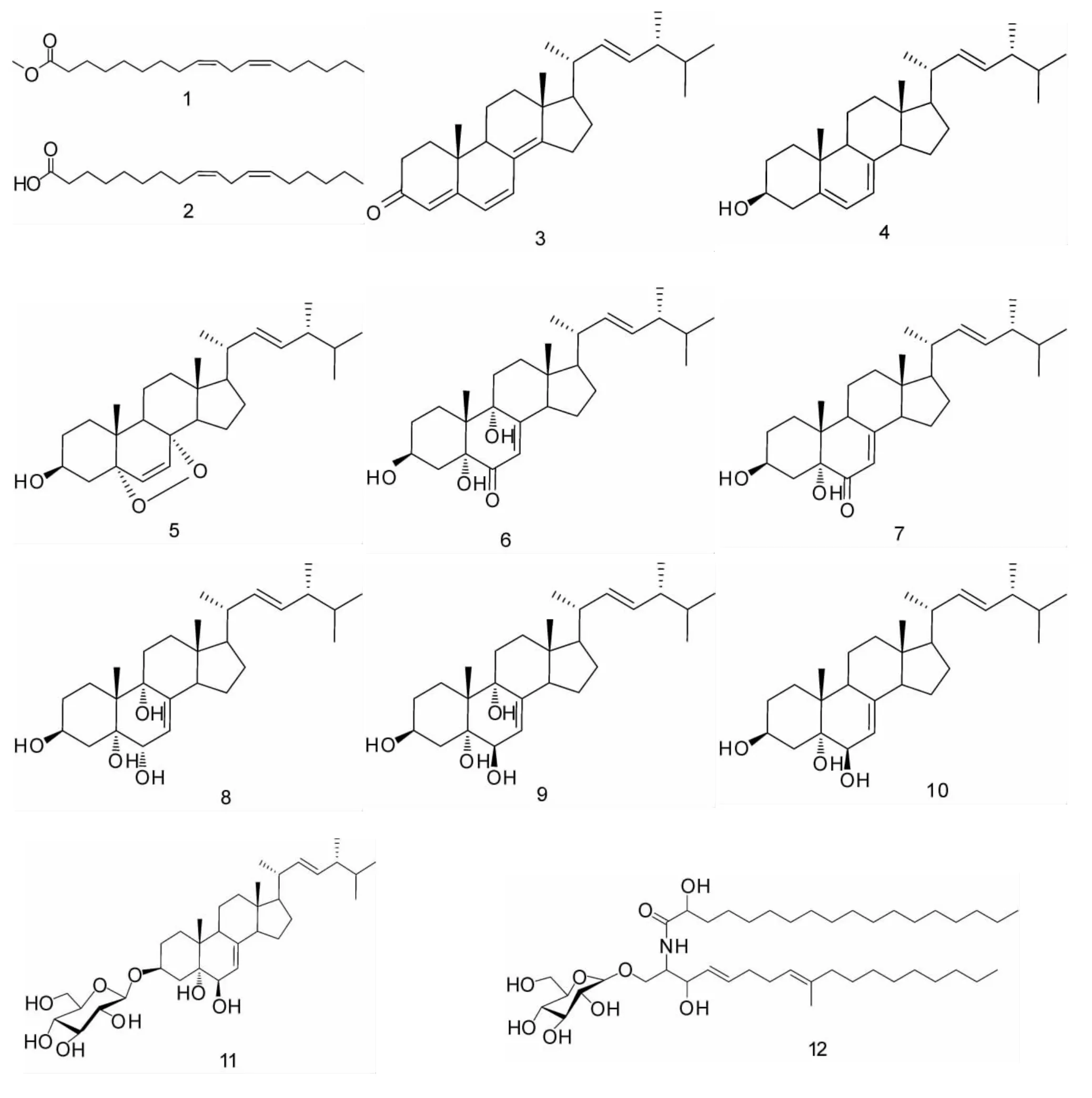
图1 化合物1—12的化学结构
[1]张婷,王元忠,李杰庆,等.大白口蘑研究进展[J].中国食用菌,2013,32(4):4-7.
[2]朱珠,马琳,朱海燕,等.民族药珠芽艾麻化学成分研究[J].中药材,2011,34(2):223-225.
[3]卢慧明,谢海辉,杨宇峰,等.大型海藻龙须菜的化学成分研究[J].热带亚热带植物学报,2011,19(2):166-170.
[4]Gao JM,Hu L,Liu JK.A novel sterol from Chinese trufflesTuberindicum[J].Steroids,2001,66(10):773-774.
[5]杜子伟,刘吉开,项晨,等.虎皮小牛肝菌的化学成分研究[J].天然产物研究与开发,2012,24(5):618-621.
[6]Rosecke J,Konig WA.Constituents of the fungiDaedalea quercinaandDaedaleopsisconfragosavar.tricolor[J].Phytochemistry,2000,54(8):757-762.
[7]雷辉,周雪峰,杨亚玲,等.南海多室草苔虫甾醇类化学成分研究[J].中药材,2011,34(2):180-183.
[8]宫俊,汤华,刘宝姝,等.中国南海黑乳海参共附生白色侧齿霉菌中的甾体类成分[J].第二军医大学学报,2013,34(3):310-314.
[9]龚庆芳,张玉梅,谭宁华,等.亚稀褶黑菇的化学成分[J].天然产物研究与开发,2007,19(3):436-438.
[10]Yue JM,Chen SN,Lin ZW,et al.Sterols from the funguslactariumvolemus[J].Phytochemistry,2001,56(8):801-806.
[11]何其伟,刘吉开,杜子伟,等.齿贝栓菌的化学成分[J].安徽中医学院学报,2011,30(2):73-75.
[12]Yoshihisa T,Minoru U,Takashi O,et al.Glycosides of ergosterol derivatives fromHericumErinacens[J].Phytochemistry,1991,30(12):4117-4120.
[13]张梅,徐良雄,薛璟花,等.一株茄病镰刀菌的代谢产物研究[J].热带亚热带植物学报,2012,20(6):585-590.
