百两金皂苷类似物的化学与药理学研究进展
2012-12-23蔡佳仲胡英杰
蔡佳仲,胡英杰
广州中医药大学热带医学研究所,广州510405
百两金皂苷属于齐墩果烷型五环三萜类,代表化合物是百两金皂苷A 和B(ardisiacrispin A/B),苷元均为西克拉明皂苷元A(cyclamiretin A)。百两金皂苷结构类似物主要来自紫金牛科(Myrsinaceae)紫金牛属(Ardisia)、铁仔属(Myrsine),报春花科(Primulaceae)报春花属(Primula)、珍珠菜属(Lysimachia)、仙客来属(Cyclamen)和点地梅属(Androsace)植物[1-4]。已有报道,百两金皂苷A 和B(ardisiacrispin A/B)对白血病细胞HL-60、肝癌细胞Bel-7402、口腔上皮癌细胞KB、宫颈癌细胞HeLa、卵巢癌细胞SKOV-3、胃癌细胞BGC-823、乳腺癌细胞MCF-7 等均具有较强的细胞毒作用,机理与诱导细胞凋亡、抑制增殖有关[5,6]。不仅如此,结构类似西克拉明皂苷元A 的一些化合物也表现出一定的细胞毒活性,如Ardipusilloside III 在体外能浓度依赖性地抑制非小细胞肺癌NCI-H460 和胶质瘤细胞U251MG 的生长而诱导其凋亡[7,8];而Saxifragifolin A、C 和D 体外对卵巢癌细胞SKOV-3、肺腺癌细胞A549、黑色素瘤细胞SK-MEL-2、平滑肌瘤细胞MESSA、结直肠腺癌细胞HCT-15 均显示了较强的细胞毒,其IC50在0.19~2.37 μM 之间[9]。显示这类化合物具有潜在的抗肿瘤药物研究开发价值。迄今发现的百两金皂苷类似物约有56 个,对该类化合物的研究似乎成为植物药研究的一个热点,因此我们将百两金皂苷类似物的化学和药理学研究情况作一综述。
1 化学结构
百两金皂苷三萜母核结构的重要特征有:(1)共有取代基13β,28-环氧醚,(2)共有取代基30-氧化甲基,(3)共有取代基3β-OH 或3β-O-糖链(由β-D-吡喃葡萄糖(β-D-glucopyranose),α-L-吡喃鼠李糖(α-L-rhamnopyranose),β-D-吡喃木糖(β-D-xylopyranose),α-L-吡喃阿拉伯糖(α-L-arabinopyranose)和β-D-吡喃葡萄糖醛酸(β-D-glucopyranuronic acid)等构成)[1-11],以及(4)16α-羟基等。根据30-氧化甲基氧化程度的不同,结构涉及醛/缩醛、醇、羧酸等。
1.1 30-醛/缩醛
这类化合物30 位是一个醛基,具有西克拉明皂苷元A 的基本结构(图1)。根据苷元16 位或30 位取代基氧化情况可再划分成:(I)16α-羟基-30-醛;(II)16-羰基-30-醛;(III)30-缩醛等(表1)。

图1 具有30-醛/缩醛结构的西克拉明皂苷元A 类似物Fig.1 Structures of cyclamiretin A analogues with 30-aldehyde
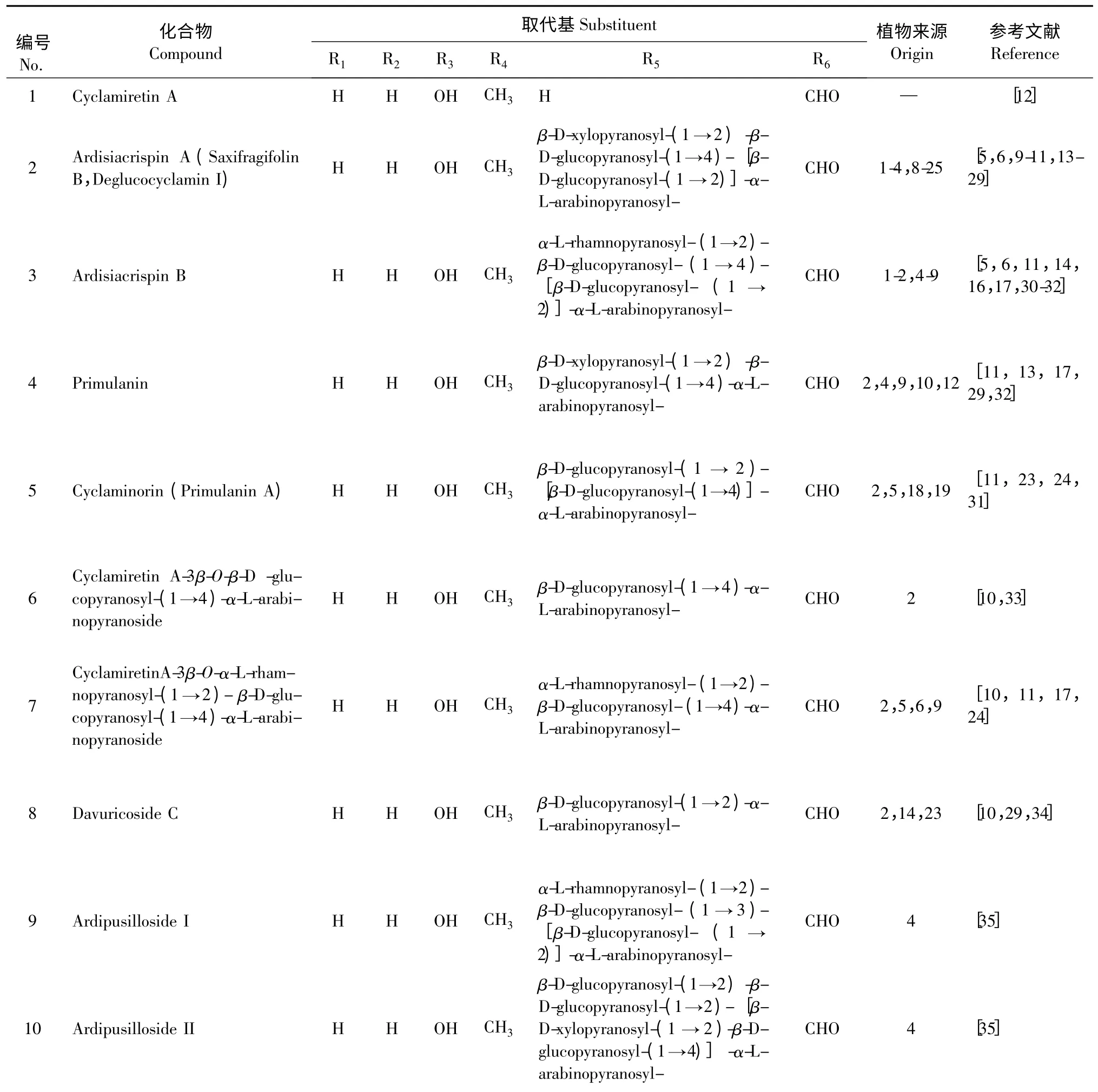
表1 具有30-醛/缩醛结构的西克拉明皂苷元A 类似物Table 1 Derivatives of cyclamiretin A with 30-aldehyde structure

11 Lysichriside A H H OH CH3 β-D-xylopyranosyl-(1 →2)-β-D-glucopyranosyl-(1→4)-[β-D-6-acetyl-glucopyranosyl- (1→2)]-α-L-arabinopyranosyl-CHO 12 [13]12 Paridiformoside H H OH CH3 α-L-rhamnopyranosyl-(1 →2)-β-D-glucopyranosyl-(1 →2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-CHO 15 [36]13 Ardicrenin H H OH CH3 α-L-rhamnopyranosyl-(1 →4)-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl-CHO 2,13 [21,33]14 Cyclacoumin H H OH CH2OH β-D-xylopyranosyl-(1→2)-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranosyl-CHO 18,19 [23,24]15 Cyclamin H H OH CH3 β-D-glucopyranosyl-(1 →3)-[β-D-xylopyranosyl-(1 →2)]-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl-CHO 6,19,20 [24,25,32,37]16 Isocyclamin H H OH CH3 β-D-glucopyranosyl -(1 →6)-[β-D-xylopyranosyl-(1 →2)]-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl-CHO 19 [24,37]17 Saxifragifolin D H H OH CH3 β-D-xylopyranosyl -(1 →2)-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl -(1 →4)-β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranosyl-CHO 22,23 [26,29]18 Cyclamiretin A-3β-O-α-L- rhamnopyranosyl-(1 →4)-β -Dglucopyranosyl-(1→2)H H OH CH3 α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl- (1 →2)-[β-D-glucopyranosyl-CHO 6 [38,39]-[β-D-glucopyranosyl-(1 →4)]-α-L-arabinopyranoside (1→4)]-α-L-arabinopyranosyl-19 Cyclamiretin A-3β-O-β-D- xylopyranosyl-(1 →2)-β-D- glucopyranosyl-(1 →2)-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranoside H H OH CH3 β-D-xylopyranosyl -(1 →2)-β-D-glucopyranosyl-(1 →2)-[β-D-glucopyranosyl -(1 →4)]-α-L-arabinopyranosyl-CHO 16 [40]20 Cyclamiretin A-3β-O-β-D- glucopyranosyl uronic acid-(1 →2)-β-D-xylopyranoside H H OH CH3 β -D-glucopyranosyl uronic acid-(1→2)-β-D-xylopyranosyl- CHO 14 [34]21 Cyclamiretin A-3β-O-β-D-glucopyranosyl-(1→2)-{β-D-xylopyranosyl-(1→2)-[β-D-glucopyranosyl-(1 →3)-β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)}-α-L-arabinopyranoside H H OH CH3 β-D-glucopyranosyl- (1 →2)-{β-D-xylopyranosyl- (1 →2)-[β-D-glucopyranosyl-(1 →3)-β-D-glucopyranosyl-(1 →3)]-β-D-glucopyranosyl-(1 →4)}-α-L-arabinopyranosyl-CHO 4 [16]22 Cyclamiretin A-3β-O-α-L- rhamnopyranosyl-(1 →3)[β-Dxylopyranosyl-(1 →2)] -β-Dglucopyranosyl-(1→4)-[β-Dglucopyranosyl-(1 →2)]-α-Larabinopyranoside H H OH CH3 α-L-rhamnopyranosyl-(1 →3)[β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl-CHO 3 [15]
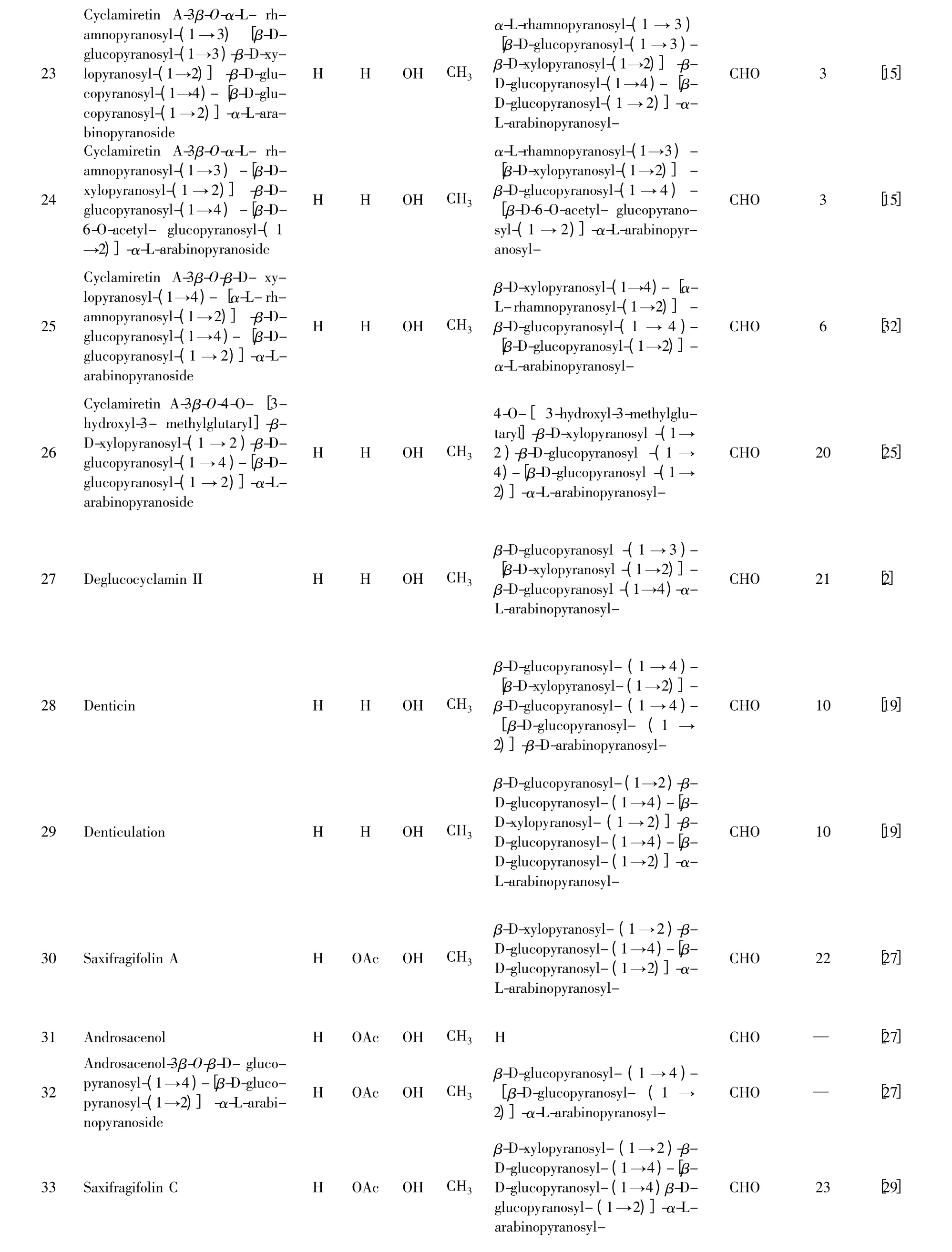
23 Cyclamiretin A-3β-O-α-L- rhamnopyranosyl-(1 →3)[β-Dglucopyranosyl-(1→3)-β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-[β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranoside H H OH CH3 24 Cyclamiretin A-3β-O-α-L- rhamnopyranosyl-(1→3)-[β-Dxylopyranosyl-(1 →2)] -β-Dglucopyranosyl-(1→4)-[β-D-6-O-acetyl- glucopyranosyl-(1→2)]-α-L-arabinopyranoside H H OH CH3 25 Cyclamiretin A-3β-O-β-D- xylopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1 →2)]-β-Dglucopyranosyl-(1→4)-[β-Dglucopyranosyl-(1 →2)]-α-Larabinopyranoside H H OH CH3 26 Cyclamiretin A-3β-O-4-O-[3-hydroxyl-3- methylglutaryl]-β-D-xylopyranosyl-(1 →2)-β-Dglucopyranosyl-(1 →4)-[β-Dglucopyranosyl-(1 →2)]-α-Larabinopyranoside H H OH CH3 27 Deglucocyclamin II H H OH CH3 28 Denticin H H OH CH3 29 Denticulation H H OH CH3 30 Saxifragifolin A H OAc OH CH3 α-L-rhamnopyranosyl-(1 →3)[β-D-glucopyranosyl-(1 →3)-β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-[β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranosyl-CHO 3 [15]α-L-rhamnopyranosyl-(1→3)-[β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl-(1 →4)-[β-D-6-O-acetyl- glucopyranosyl-(1 →2)]-α-L-arabinopyranosyl-CHO 3 [15]β-D-xylopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl-CHO 6 [32]4-O-[ 3-hydroxyl-3-methylglutaryl]-β-D-xylopyranosyl -(1 →2)-β-D-glucopyranosyl -(1 →4)-[β-D-glucopyranosyl -(1 →2)]-α-L-arabinopyranosyl-CHO 20 [25]β-D-glucopyranosyl -(1 →3)-[β-D-xylopyranosyl -(1→2)]-β-D-glucopyranosyl -(1→4)-α-L-arabinopyranosyl-CHO 21 [2]β-D-glucopyranosyl- (1 →4)-[β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl- (1 →4)-[β-D-glucopyranosyl- (1 →2)]-β-D-arabinopyranosyl-CHO 10 [19]β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1 →4)-[β-D-xylopyranosyl- (1 →2)]-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranosyl-CHO 10 [19]β-D-xylopyranosyl-(1 →2)-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranosyl-CHO 22 [27]31 Androsacenol H OAc OH CH3 H CHO — [27]32 Androsacenol-3β-O-β-D- glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranoside H OAc OH CH3 33 Saxifragifolin C H OAc OH CH3 β-D-glucopyranosyl- (1 →4)-[β-D-glucopyranosyl- (1 →2)]-α-L-arabinopyranosyl-CHO — [27]β-D-xylopyranosyl-(1 →2)-β-D-glucopyranosyl-(1 →4)-[β-D-glucopyranosyl-(1→4)β-Dglucopyranosyl-(1 →2)]-α-Larabinopyranosyl-CHO 23 [29]
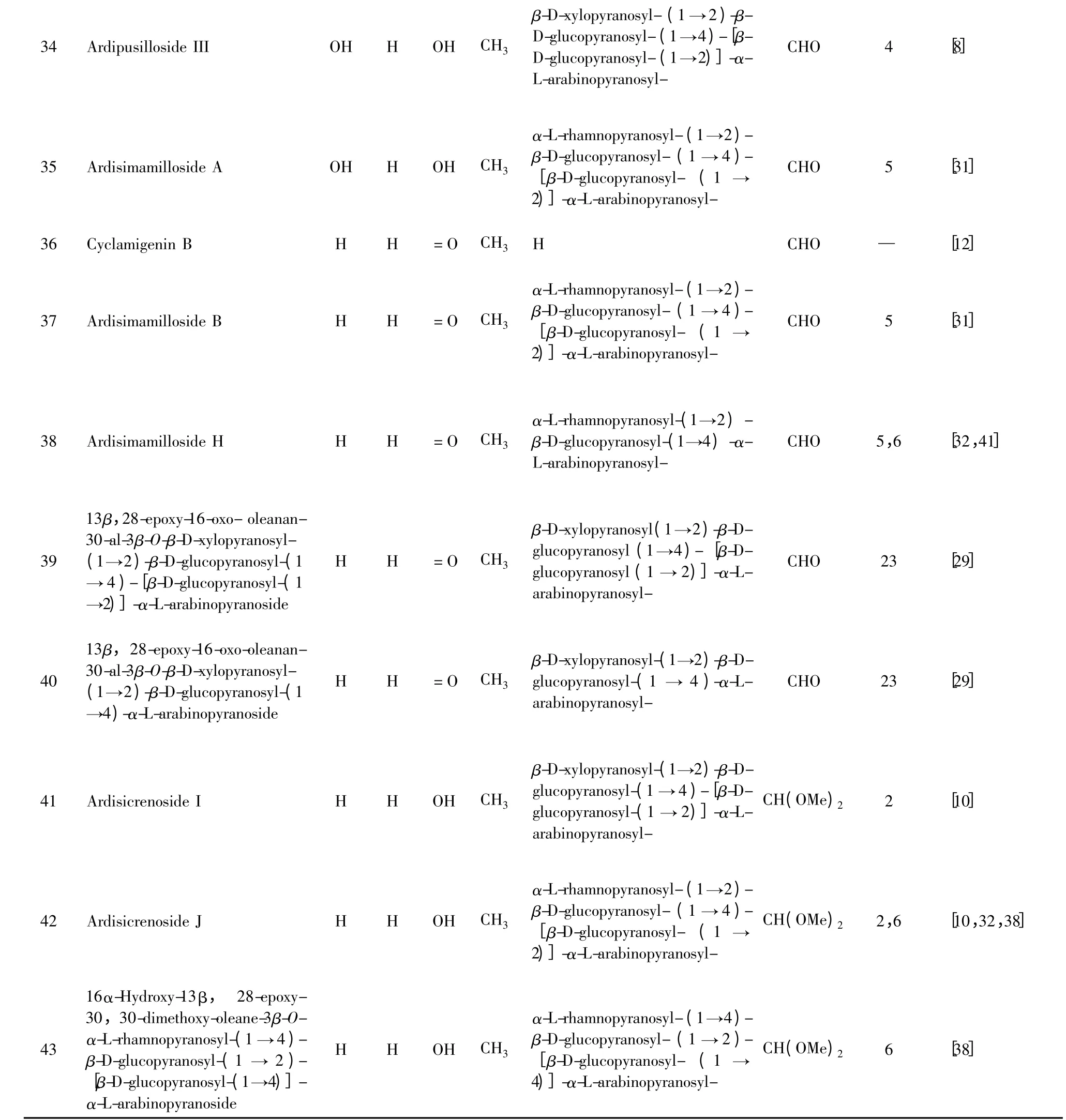
注:植物来源(Origin):1.Ardisia crispa 2. Ardisia crenata 3. Ardisia gigantifolia 4. Ardisia pusilla 5. Ardisia mamillata 6. Ardisia japonica 7. Ardisia punctate 8.Myrsine australis 9.Myrsine pellucida 10. Primula denticulate 11.Lysimachia thysiflora 12.Lysimachia christinae 13. Lysimachia patungensis 14.Lysimachia davurica 15.Lysimachia paridiformis 16. Lysimachia microcarpa 17. Cyclamen repandum 18. Cyclamen coum 19. Cyclamen mirabile 20.Cyclamen trocopteranthum 21.Cyclamen europaeum 22.Androsace saxifragaefolia 23.Androsace umbellate 24.Remusatia vivipara 25.Eupatorium chinense.
这些化合物中除了cyclamiretin A、androsacenol、androsacenol-3β-O-β-D-glucopyranosyl -(1 →4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranoside 和cyclamigenin B 等苷元和次生苷外,都是来源于紫金牛科和报春花科植物的天然产物。上述苷元和次生苷可以通过酸水解或高碘酸钠水解或酶解法获得[12,27,42]。其中,cyclamiretin A 是ardisiacrispin A的原生苷元,而cyclamiretin A 继续酸水解缩合则生成次生苷元cyclamiretin D。Androsacenol 和androsacenol-3β-O-β-D-glucopyranosyl-(1→4)-[β-D-glucopyranosyl-(1 →2)]-α-L-arabinopyranoside 是saxifragifolin A 的原生苷元和次生皂苷。而cyclamigenin B则是第二亚类化合物的原生苷元。
1.2 30/29-醇,以及30/29-羧酸
这两类化合物,29 或30 位碳为羟甲基(30/29-醇),或为羧基(30/29-羧酸)(图2)(表2)。
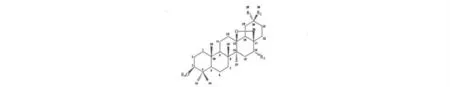
图2 具有30/29-醇或羧酸结构的西克拉明皂苷元A 类似物结构Fig.2 Structures of cyclamiretin A analogues with 30/29-alcohol,or with 30/29-carboxylic acid
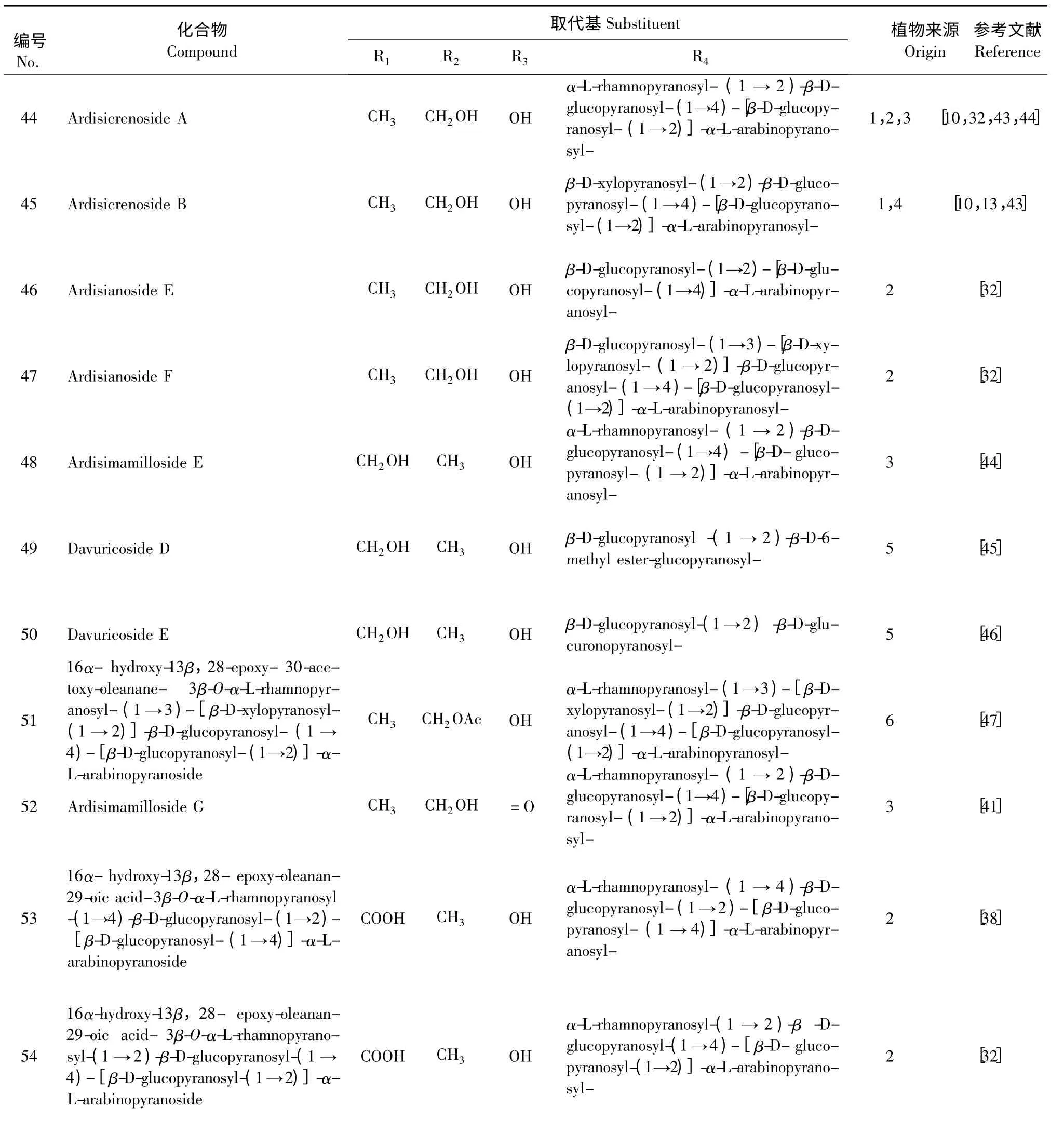
表2 具有13β,28-环醚-30/29-醇结构的西克拉明皂苷元A 类似物Table 2 Derivatives of cyclamiretin A with 13β,28-epoxy-30/29-alcohol structure
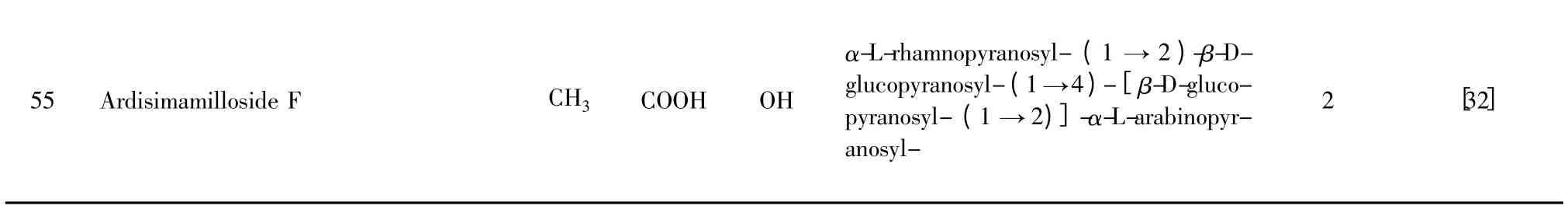
注:植物来源(Origin):1.Ardisia crenata 2. Ardisia japonica 3.Ardisia mamillata 4.Lysimachia christinae 5.Lysimachia davurica 6. Ardisia gigantifolia.
刘岱琳等[10,48]还从朱砂根(Ardisia crenata)中分得一个30 位甲基缺失的罕见三萜类似物朱砂根皂苷L。
百两金皂苷类似物中的13β,28-环氧醚结构和30 位含氧基团的核磁共振波谱数据是鉴定其苷元母核结构的重要特征。由于13β,28-环醚的存在,百两金皂苷类似物的核磁共振波谱具有相应特征[10,49]:往往出现化学位移值为86.6 的13 位连氧季碳信号和化学位移值为77.5 的28 位连氧亚甲基碳信号;如果28 位碳再有一个羟基取代的话,28 位不再出现亚甲基信号,而是在低场形成一个连氧次甲基碳信号,其化学位移值为99.0;苷元结构中30位如果是醛基,其碳-13 谱化学位移值为207 左右。
2 药理活性
2.1 细胞毒作用
研究报道较多的是百两金皂苷A 和B、九节龙皂苷Ⅰ、П 和Ш 等少数几个化合物。
其中,朱砂根皂苷A、B、I 和J、百两金皂苷A 和B 对人乳腺癌细胞MCF-7、人非小细胞肺癌NCIH460 和人神经胶质瘤细胞SF-268 均有一定的细胞毒作用,半数抑制浓度IC50在2.0~13.0 μM 之间,其中以百两金皂苷A 和B 的作用较强,而且百两金皂苷A 体外对人肾胚正常细胞293 的毒性较弱[10]。有研究显示,百两金皂苷A 对肝癌细胞HepG 2 的细胞毒作用,是通过阻滞细胞于亚G1 期,从而激活caspase-8 和caspase-9,使线粒体膜电位下降,释放细胞色素C,并使磷脂丝氨酸外化和PARP 降解机理诱导细胞凋亡[28]。有学者报道了百两金皂苷A体外抗人黑色素瘤细胞HTB-140 和对人人正常皮肤成纤维细胞HSFs 的毒性实验研究。结果显示百两金皂苷A 能明显影响HTB-140 细胞的活力、增殖、形态和细胞骨架,具有时间浓度依赖性地抑制肿瘤细胞的活力,通过使肌动蛋白细胞空泡化并解聚而影响肿瘤细胞的转移和增殖。但同时也能降低人正常皮肤成纤维细胞HSFs 的活力和生长[50]。由此可见,百两金皂苷A 在体外对多种肿瘤细胞具有较强的细胞毒活性,但对一些正常人体细胞也具有一定程度的毒性。虽然百两金皂苷A、B 的体外抗肿瘤研究有较多报道,但体内抗肿瘤实验结果仍未见报道。
对九节龙皂苷Ⅰ、П 和Ш 的体内外药理研究也有一些报道。Zhang 等研究报道了九节龙皂苷I 体外对人非小细胞肺癌NCI-H460 具有明显的抑制作用,药物作用细胞后,使细胞阻滞于亚G1 期,并使核固缩,染色质浓集而形成凋亡小体,结果表明能时间、浓度依赖性地抑制肿瘤细胞的生长,从而诱导其凋亡[7]。林洪等也发现了九节龙皂苷Ш 能通过阻滞人胶质瘤细胞U251MG 于G2/M 期,引起时间浓度依赖性地使BAD 去磷酸化,并激活caspase-8 和caspase-3,从而抑制细胞增殖[8]。对于体内抗肿瘤研究,李伟芳等报道了九节龙皂苷Ⅰ和П 经腹腔注射,对小鼠肉瘤和艾氏腹水瘤的生长有一定的抑制作用,使瘤体生长缓慢,抑瘤率为16%~39%,而九节龙皂苷Ⅰ对小鼠黑色素瘤抑瘤率为27.3%,九节龙皂苷П 对小鼠肝癌的抑制率为23.3%[51]。
也有学者研究了davuricoside C(苷元结构中有16α-OH)体外对人卵巢癌细胞A2780 的增殖抑制作用,并提出16 位碳上有α-羟基比没有α-羟基的显示出更强的细胞毒作用[34];刘岱琳等也通过对比百两金皂苷A 和B、朱砂根皂苷I 和J 这四种皂苷体外对多种肿瘤细胞和人肾胚正常细胞293 的细胞毒作用,探讨了结构与细胞毒活性之间的关系,得出一些见解:①16 位α-羟基和13β,28-环氧醚结构对活性起着决定性作用;②碳3-O-连接的糖链越长,其活性也越强;③苷元中碳30 的取代基对活性影响也很大,其对活性影响顺序为:-CHO > -CH(OCH3)2>-CH2OH >-COOH[10]。
2.2 抗炎
有实验显示,用100 μM 百两金皂苷A 作用于人巨噬细胞THP-1 能明显抑制脂多糖诱导的IL-8和TNF-α 的释放,并降低mRNA 转录水平,从而发挥其抗炎作用[22]。
2.3 收缩子宫
Chaweewan 等(1987)研究报道了百两金皂苷A和B 具有收缩子宫的作用,在8 μg/mL 的皂苷溶液中小鼠子宫的收缩程度相当于0.2 μg/mL 乙酰胆碱所起的作用[14]。在此基础上,Calis 等也对百两金皂苷A、primulanin A、cyclamin 和cyclacoumin 进行了体外小鼠子宫收缩反应试验,结果显示,化合物的浓度分别为7.5 ×10-6、8.6 × 10-6、7.4 ×10-6和6.5 ×10-6μM 时,作用效果分别相当于10-5.2、10-4.9、10-5.2和10-4.9M 的乙酰胆碱的作用[24]。作用机理目前尚不明确。
2.4 抗真菌
据研究[24],百两金皂苷A、primulanin A、cyclamin、cyclacoumin 和isocyclamin 均具有一定的抗真菌作用,前三个化合物的最小抑菌浓度MIC 在80-160 μg/mL 之间,作用要强于后两者。
2.5 其他药理作用
百两金皂苷类似物在其他方面的药理活性,还包括抗cAMP 磷酸二酯酶作用等[52]。
3 结语
百两金皂苷类似物的结构和数目具有一定的多样性,其药理作用特别是抗肿瘤活性研究也已发现一些有意义的苗头。因此,进一步从中筛选具有抗肿瘤活性且对人体毒性较小的活性类似物并作为结构优化的基础,对发现抗肿瘤药物先导化合物具有重要意义。
1 Sparg SG,et al.Biological activities and distribution of plant saponins.J Ethnopharmacol,2004,94:219-243.
2 Foubert K,et al.Chemistry,distribution and biological activities of 13,28-epoxy-oleanane saponins from the plant families Myrsinaceae and Primulaceae.Curr Org Chem,2008,12:629-642.
3 Chang HT(常海涛),et al. Chemical and pharmacological advances of study on Lysimachia . Chin J Chin Mater Med(中国中药杂志),2004,29:295-298.
4 Huang XA(黄新安),Yang RZ(杨仁洲). Progress in the triterpenoids from the Genus Lysimachia L. J Trop Subtrop Bot(热带亚热带植物学报),2007,15:175-182.
5 Wei SY(魏少荫),et al. Study on inhibitory efects and mechanism of ardisiacrispin(A +B)on human promyeloleukemic HL-60 cells. Chin Pharm Bull(中国药理学通报),2007,23:586-590.
6 Li M,et al. Pro-apoptotic and microtubule-disassembly effects of ardisiacrispin (A +B),triterpenoid saponins from Ardisia crenata on human hepatoma Bel-7402 cells. J Asian Nat Prod Res,2008,10:729-736.
7 Zhang Y,et al.Ardipusilloside I purified from Ardisia pusilla competitively binds VEGFR and induces apoptosis in NCIH460 cells.Phytomedicine,2010,17:519-526.
8 Lin H,et al.Apoptosis induced by ardipusilloside III through BAD dephosphorylation and cleavage in human glioblastoma U251MG cells.Apoptosis,2008,13:247-257.
9 Park JH,et al.Cytotoxic effects of triterpenoid saponins from Androsace umbellata against multidrug resistance (MDR)and non-MDR cells.Arch Pharm Res,2010,33:1175-1180.
10 Liu DL(刘岱琳). Studies on the bioactive constituents of Ardisia crenat Sims and Bulbophyllum ororatissimum Lindl.Shenyang:Shenyang Pharmaceutical University(沈阳药科大学),PhD.2004.
11 Zheng ZF,et al.Cytotoxic triterpenoid saponins from the roots of Ardisia crenata.J Asian Nat Prod Res,2008,10:833-839.
12 Dorchaí Ró,Thomson JB. Triterpenoids-III:The structure of cyclamigenin B.Tetrahedron,1968,24:1377-1384.
13 Tian LJ,et al. Triterpene saponins from Lysimachia christinae.J Asian Nat Prod Res,2008,10:265-269.
14 Chaweewan J,et al. Ardsiacrispin A and B,two utero -contracting saponins from Ardisia crispa .Planta Med,1987,53:405-409.
15 Wen P,et al. Four new triterpenoid saponins from Ardisia gigantifolia Stapf. and their cytotoxic activity. J Asian Nat Prod Res,2008,10:873-880.
16 Tian Y,et al. Triterpenoid saponins from Ardisia pusilla and their cytotoxic activity.Planta Med,2009,75:70-75.
17 Lavaud C,et al.Triterpene saponins from Myrsine pellucida.Phytochemistry,1994,37:1671-1677.
18 Bloor SJ,Qi L.Cytotoxic saponins from New Zealand Myrsine species.J Nat Prod,1994,57:1354-1360.
19 Ahmad VU,et al.Triterpenoid saponins from Primula denticulate.Planta Med,1990,56:94-97.
20 Podolak I,et al.A triterpene saponin from Lysimachia thyrsiflora L.Acta Pol Pharm,2007,64:39-43.
21 Huang XA(黄新安),et al.Triterpenoid saponins from Lysimachia patungensis and their anti-tumor activities in vitro. J Trop Subtrop Bot(热带亚热带植物学报),2007,15:363-365.
22 Dall'Acqua S,et al.Triterpene glycosides with in vitro anti -inflammatory activity from Cyclamen repandum tubers.Carbohydr Res,2010,345:709-714.
23 Calis I,et al. Triterpene saponins from Cyclamen coum var.coum.Planta Med,1997,63:166-170.
24 Calis I,et al.Triterpene saponins from Cyclamen mirabile and their biological activities.J Nat Prod,1997,60:315-318.
25 Mihci-Gaidi G,et al.Triterpene saponins from Cyclamen trocopteranthum.Planta Med,2010,76:818-821.
26 Pal BC,Mahato SB. New triterpenoid pentasaccharides from Androsace saxifragifolia .J Chem Soc,Perkin Trans 1,1987:1963-1967.
27 Waltho JP,et al.Structure elucidation of two triterpenoid tetrasaccharides from Androsace saxifragifolia.J Chem Soc,Perkin Trans 1,1986:1527-1531.
28 Zhang DM,et al. Saxifragifolin B from Androsace umbellata induced apoptosis on human hepatoma cells.Biochem Biophys Res Commun,2007,362:759-765.
29 Wang Y,et al. Triterpenoid saponins from Androsace umbellata and their anti-proliferative activities in human hepatoma cells.Planta Med,2008,74:1280-1284.
30 Tang HF,et al. Two new triterpenoid saponins cytotoxic to human glioblastoma U251MG cells from Ardisia pusilla.Chem Biodivers,2009,6:1443-1452.
31 Huang J,et al.Triterpenoid saponins from Ardisia mamillata.Phytochemistry,2000,54:817-822.
32 Chang XL,et al. Biologically active triterpenoid saponins from Ardisia japonica. J Nat Prod,2007,70:179-187.
33 Wang MT,et al.A new triterpenoid saponin from Aridsia crenata.Planta Med,1992,58:205-207.
34 Liang B,et al.Triterpenoid saponins from Lysimachia davurica.Chem Pharm Bull,2006,54:1380-1383.
35 Zhang QH(张清华),Wang XJ(王晓娟). Studies on the saponin constituents of Jiu jielong(Ardisia Pusilia). Acta Pharm Sin(药学学报),1993,28:673-678.
36 Han DX(韩定献),et al.Studies on the paridiformoside.Acta Pharm Sin(药学学报),1987,22:746-749.
37 Reznicek G,et al.Saponins in Cyclamen species:Configuration of cyclamiretin C and structure of isocyclamin. Phytochemistry,1989,28:825-828.
38 De TN,et al.Characterization of three new triterpenoid saponins from Ardisia japonica.J Nat Prod,1993,56:1669-1675.
39 Li YF,et al.PTP1B inhibitors from Ardisia japonica.J Asian Nat Prod Res,2005,7:13-18.
40 Ding ZH(丁智慧),et al.The chemical constituents of Lysimachia microcarpa. Acta Bot Yunnan(云 南 植 物 研 究),1993,15:201-204.
41 Huang J,et al.Ardisimamillosides G and H,two new triterpenoid saponins from Ardisia mamillata. Chem Pharm Bull,2003,51:875-877.
42 Dorchaí Ró,et al. Triterpenoids-IV:The cyclamigenins A1,A2,C,and D.Tetrahedron,1968,24:5649-5654.
43 Jia Z,et al.Triterpenoid saponins from Ardisia crenata.Phytochemistry,1994,37:1389-1396.
44 Huang J,et al.Ardisimamillosides C-F,four new triterpenoid saponins from Ardisia mamillata. Chem Pharm Bull,2000,48:1413-1417.
45 Tian JK(田景奎),et al.Two new triterpenoid saponins from Lysimachia davurica. Acta Pharm Sin(药学学报),2004,39:194-197.
46 Tian JK,et al.Two New Saponins from Lysimachia davurica.Chin Chem Lett,2005,16:212-214.
47 Mu LH,et al. Triterpenoid saponins from Ardisia gigantifolia.Chem Pharm Bull,2010,58:1248-1251.
48 Liu DL,et al.Two new triterpenoid saponins from Ardisia crenata.J Asian Nat Prod Res,2007,9:119-127.
49 Liu DL(刘岱琳),et al.NMR spectroscopy features of triterpenoid saponins from Ardisia.J Shenyang Pharm Univ(沈阳药科大学学报),2004,21:394-400.
50 Galanty A,et al. The influence of LTS-4,a saponoside from Lysimachia thyrsiflora L.,on human skin fibroblasts and human melanoma cells.Cell Mol Boil Lett,2008,13:585-598.
51 Li WF(李伟芳),et al.The antitumor activity of ardipusilloside in vivo. Chin J Trad Chin Med Pharm(中国医药学报),2003,18:60-61.
52 Jia Z,et al. Triterpenoid saponins from Ardisia crenata and their inhibitory activity on cAMP phosphodiesterase. Chem Pharm Bull,1994,42:2309-2314.
