云南烤烟型烟叶中糖苷类化学成分的研究
2012-12-23陈永宽王昆淼刘春波刘志华
陈永宽,王 燕,王昆淼,何 沛,刘春波,刘志华
云南省烟草化学重点实验室,云南烟草科学研究院,昆明650106
在烟叶烘烤和陈化过程中,烟叶糖苷类化合物会转化成香味成分,并已有大量综述报道了烟叶化学成分的鉴定[1,2]。最近几年,烟叶糖苷类化合物也有深入研究的报道,主要集中在烟草糖苷类化合物作为香味前体所发生的作用[3,4]。然而,系统地分离、鉴定烟草糖苷类化合物仍非常有限,烤烟型烟叶中糖苷类化合物研究困难在于缺少有效的纯化、分离技术。本研究从云南烤烟型烟叶中分得7 个糖苷类化合物:东莨菪甙(1)、槲皮素-3-β-D-芸香糖甙(2)、山柰酚-3-O-新橙皮糖苷(3)、3-氧代-α-紫罗兰醇-β-D-吡喃葡萄糖甙(4)、7,8-二氢-3-氧代-α-紫罗兰醇-β-D-吡喃葡萄糖甙(5)、异秦皮定-6-O-β-D-吡喃葡萄糖甙(6)、异香豆素萄糖甙(7)。化合物3、5、6 和7 为首次从烟草中分离得到。
1 仪器与材料
Bruker AR 500 型核磁共振仪波谱仪(TMS 为内标);1100 型高效液相色谱仪(包括二元泵,自动进样器,二极管阵列检测器,柱温箱,Chemstation 色谱工作站,美国Agilent 公司);YMC-pack ODS-AQ柱(250 ×10 mmL,S-5 μm,12 nm,日本YMC 公司);旋转蒸发仪(瑞士BUCH 公司);电子天平(中山市金利电子衡器有限公司);高速中药粉碎机(WND-500A 型);电磁式空气压气机(广东海利集团有限公司);超声波清洗器(SK 25H/086A023,美国KUDOS)。XAD-2 大孔吸附树脂(美国Amberlite 公司);50 μm 反相C18球形硅胶(日本YMC 公司);XAD-2 吸附柱1(Φ10 cm ×100 cm);反相C18吸附柱2(Φ4 cm×60 cm);0.22 μm 有机滤膜;甲醇为色谱纯(Mallinckrodt Baker,Inc. ),其他试剂均为分析纯。
烤烟型烟叶KC2F 于2010 年7 月15 日采自红塔集团,经鉴定为Nicotiana tabacum L. 复烤烟叶。标本收藏在云南烟草科学研究院烟草化学重点实验室;样品号:No.20100715-3。
2 提取与分离
烟叶KC2F 2.5 Kg,粉碎,过40 目筛,加入10 L甲醇,常温下浸泡60 h 后,用超声萃取5 h,过滤,滤渣用5 L 甲醇重复以上操作2 次。合并滤液,用旋转蒸发仪减压浓缩至浸膏状654 g。上Amberlite XAD-2 吸附柱,先用2000 mL 乙醚正己烷(v/v =1/1)溶液洗去游离香味物质和油脂、蜡质成分。再用800 mL 水洗脱,最后分别用500 mL10%、30%、50%、70%和100%甲醇水溶液(v/v)梯度洗脱,分段收集。减压回收溶剂后分别得到糖甙粗提物A—E。粗提物A—E 分别用适量水溶解,分别过反相C18吸附柱,分别用40%、50%、60%、70%、80% 和100% 的甲醇水溶液(v/v)反复洗脱;半制备型HPLC 纯化。分离得到化合物1(20.1 mg)、化合物2(18.5 mg)、化合物3(22.0 mg)、化合物4(45 mg)、化合物5(15.3 mg)、化合物6(7.8 mg)、化合物7(13.8 mg)。
3 结构鉴定
化合物1:黄色固体(CH3OH);mp.224~225℃;MF:C16H18O9,MW:354;TOF MS FD+m/z:354;1H NMR[C5D5N,400 MHz]δ:3.71 (3H,s,6-OMe),4.16(1H,m,H-5'),4.33 (1H,m,H-4'),4.35 (1H,m,H-2'),4.37 (1H,m,H-3'),4.38(1H,m,H-6a'),4.52 (1H,brd,J= 2.0 Hz,H-6b'),5.79 (1H,d,J = 7.2 Hz,H-1'),6.31 (1H,d,J =9.6 Hz,H-3),7.02 (1H,s,H-5),7.51(1H,s,H-8),7.63 (1H,d,J = 9.6 Hz,H-4);13C NMR[C5D5N,100 MHz]δ:56.6 (6-OMe),63.7 (CH2,C-6'),71.6(CH,C-4'),75.2 (CH,C-2'),78.9 (CH,C-3'),79.6 (CH,C-5'),102.3 (CH,C-1'),104.6 (CH,C-8),110.0(CH,C-5),113.3(CH,C-10),114.5(CH,C-3),144.1(CH,C-4),147.5(CH,C-6),150.1(CH,C-9),151.2(CH,C-7),161.8(C =O,C-2);元素分析结果为:C54.24%;H5.12%。
以上数据与文献报道的东莨菪甙(Scopolin)完全一致[5]。由此推断化合物1 为的东莨菪甙(Scopolin),其结构简式如图1。
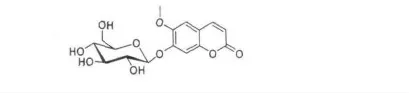
图1 化合物1 的化学式结构Fig.1 The chemical structure of compound 1
化合物2:淡黄色结晶(CH3OH);mp.176-178℃;MF:C27H30O16,MW:610;TOF MS FD+m/z:610;1H NMR[DMSO-d6,400 MHz]δ:7.57 (1H,d,J= 2.0,8.8 Hz,H-6'),7.52 (1H,d,J= 1.6 Hz,H-2'),6.79 (1H,d,J= 8.4 Hz,H-5'),6.30 (1H,d,J= 2.0 Hz,H-8),6.11 (1H,d,J =1.6 Hz,H-6),5.02 (1H,d,J = 7.6 Hz,H-1''),3.72 (1H,d,J =10.8 Hz,H-1'''),3.54 (1H,m,H-2''),3.53~3.27(9H,m,H-5,H-3''',H-3'',H-2''',H-4''',H-6a''',H-5''',H-6b''',H-4'',H-5''),1.03(3H,d,J = 6.0 Hz,H-Me'');13C NMR [DMSO-d6,100 MHz] δ:179.43 (C-4),166.11 (C-7),163.00 (C-5),159.35 (C-9),158.54 (C-2),135.64 (C-3),105.63 (C-10),104.73 (C-1'''),102.44 (C-1''),99.98 (C-6),94.89 (C-8),78.20 (C-3''),77.24(C-3'''),75.24(C-5'''),73.98 (C-2'''),72.25 (C-4''),72.12 (C-4'''),71.41 (C-2''),69.72 (C-5''),68.57 (C-6'''),17.90 (C-6'');元素分析结果为C53.12%,H4.95%。
以上数据与文献报道的槲皮素-3-β-D-芸香糖甙(Rutin)完全一致[6]。由此推断,化合物2 为芦丁(Rutin),其结构简式如图2。
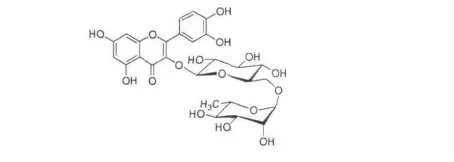
图2 化合物2 的化学式结构Fig.2 The chemical structure of compound 2
化合物3:淡黄色针状物(CH3OH);MF:C27H30O15,MW:594;TOF MS ESI m/z:594(M-,1);1H NMR[DMSO-d6,400 MHz]δ:8.00 (2H,d,J = 8.8 Hz,H-2',H-6'),6.90 (2H,d,J =8.8 Hz,H-3',H-5'),6.43 (1H,d,J = 1.6 Hz,H-8),6.23 (1H,d,J= 2.0 Hz,H-6),5.32 (1H,d,J =7.2Hz,H-1''),5.02 (1H,d,J = 7.6 Hz,H-1''),4.39 (1H,brs,H-1'''),3.43(1H,d,J =1.6Hz,H-6''),3.708~3.057(8H,m,H-2'',H-3'',H-4'',H-5'',H-2''',H-3''',H-4''',H-5'''),0.99 (3H,d,J =6.0 Hz,H-Me''');13C NMR[DMSO-d6,100 MHz]δ:Rha 100.70 (CH,C-1'''),70.29 (CH,C-2'''),70.54 (CH,C-3'''),70.54 (CH,C-4'''),68.19 (CH,C-5'''),17.6(CH3,C-6'''),Glu103.85 (CH,C-1''),71.77 (CH,C-2''),74.12 (CH,C-3''),76.30 (CH,C-4''),75.66 (CH,C-5''),69.87 (CH,C-6''),156.48 (C-2),133.14 (C-3),177.30 (C=O,C-4),120.83 (C-5),101.30 (CH,C-6),161.12 (CH,C-7),93.76(CH,C-8),159.84 (C-9),103.85 (C-10),120.83(C-1'),130.83 (CH,C-2'),115.07 (CH,C-3'),156.81 (CH,C-4'),115.07 (CH,C-5'),130.83(CH,C-6');元素分析结果为C 54.55%;H 5.09%。
以上数据与文献报道的山柰酚-3-O-新橙皮糖苷(Kaempferol-3-O-neohesperidoside)完全一致[7]。由此推断,化合物3 为山柰酚-3-O-新橙皮糖苷(Kaempferol-3-O-neohesperidoside)。其结构简式如图3。
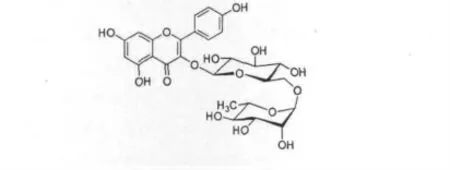
图3 化合物3 的化学式结构Fig.3 The chemical structure of compound 3
化合物4:黄色油状物;MF:C19H30O7,MW:370;TOF MS ESI m/z:370(M-,1);1H NMR[CD3OD,400 MHz]δ:5.80 (1H,s,H-4),5.71 (1H,dd,J = 16,6.4 Hz,H-7),5.67 (1H,dd,J =16,6.4 Hz,H-8),4.29 (1H,dd,H-2'),4.19 (1H,d,J = 7.6 Hz,H-1'),3.60 (2H,m,H-6'),3.12 (1H,m,H-9),3.10~3.01(3H,m,H-4',H-5',H-3'),2.62(1H,d,J =8.8 Hz,H-6),2.38 (1H,s,H-2),2.07 (1H,s,H-2),1.85(3H,s,H-10),1.20 (3H,dd,J = 6.4 Hz,H-13),0.95 (6H,dd,J= 15.2 Hz,H-11,H-12);13C NMR [CD3OD,100 MHz]δ:20.72 (CH3,C-13),22.90 (CH3,C-10),26.72 (CH3,C-11),27.39(CH3,C-12),35.61 (C-1),47.11 (CH2,C-2),54.55 (CH,C-6),60.91 (CH2,C-6'),69.87(CH,C-4'),73.68(CH,C-3'),74.62 (CH,C-2'),76.77(CH,C-9),76.79(C-3),100.86(CH,C-1'),124.35(CH,C-4),127.24(CH,C-8),136.62(CH,C-7),162.00(C-5),117.39(C=O,C-3);元素分析结果为C 61.60%;H 8.16%。
以上数据与文献报道的3-氧代-α-紫罗兰醇-β-D-吡 喃 葡 萄 糖 甙(3-oxo-α- ionol-β-D-glucopyranoside)完全一致[8,9]。由此推断,化合物4 为(3-oxoα-ionol-β-D-glucopyranoside),其结构简式如图4。

图4 化合物4 的化学式结构Fig.4 The chemical structure of compound 4
化合物5:不规则粉末(CH3OH);MF:C19H32O7,MW:372;TOF MS FD+m/z:372;1H NMR[CD3OD,400 MHz] δ:5.71 (1H,s,H-4),4.29(1H,dd,H-2'),4.24 (1H,d,J = 7.6 Hz,H-1'),3.79~3.76 (2H,m,H-6'),3.57~3.53 (2H,d,H-7,H-8),3.20 (1H,m,H-9),3.17~3.02 (3H,m,H-4',H-5',H-3'),2.39(1H,s,H-6),2.34 (1H,s,H-2),2.31 (1H,s,H-2),1.88 (3H,s,H-10),1.09(3H,dd,J = 6.0 Hz,H-13),0.99 (3H,s,H-11,H-12),0.91 (3H,s,H-12);13C NMR [CD3OD,100 MHz]δ:19.90 (CH3,C-13),25.00 (CH3,C-10),26.34 (CH2,C-7),27.54 (CH3,C-11),29.09(CH3,C-12),37.34 (CH2,C-8),37.82 (C-1),48.11 (CH2,C-2),52.42 (CH,C-6),62.25 (CH2,C-6'),71.37 (CH,C-4'),75.13 (CH,C-3'),75.55(CH,C-2'),77.90 (CH,C-9),78.28 (C-3),102.14(CH,C-1'),125.41 (CH,C-4),170.16 (C-5),200.46 (C=O,C-3);元素分析结果为C 61.27%;H 8.66%。
以上数据与文献报道的7,8-二氢-3-氧代-α-紫罗兰醇-β-D-吡喃葡萄糖甙(7,8-dihydro-3-oxo-α-ionol-β-D-glucopyranoside)完全一致[10]。由此推断,化合物5 为的结构确定为7,8-二氢-3-氧杂-α-紫罗兰醇-β-D-吡喃葡萄糖甙(7,8-dihydro-3-oxo-α-ionol-β-D-glucopyranoside),其结构简式如图5。

图5 化合物5 的化学式结构Fig.5 The chemical structure of compound 5
化合物6:黄色液体;MF:C16H18O10,MW:370;TOF MS ESI m/z:393.0 (M+Na)+;1H NMR[D2O,600 MHz]δ:7.90 (1H,dd,J = 3.9 Hz,H-4),7.29(1H,d,H-8),7.15 (1H,dd,J= 4.2 Hz,H-3),6.35(1H,d,J = 10.02 Hz,H-9),5.19 (1H,d,J =5.7 Hz,H-1'),3.49 (1H,dd,J =9.06 Hz,H-6'),1.26(3H,s,7-OMe);13C NMR[D2O,150 MHz]δ:56.03(C-OMe),60.17 (CH2,C-6'),69.02 (CH,C-4'),72.50 (CH,C-2'),75.25 (CH,C-3'),76.09 (CH,C-5'),99.72 (CH,C-1'),99.72 (C-10),103.31(CH,C-8),109.69 (CH,C-3),113.30 (C-6),145.59 (C-4),145.90 (C-5),148.88 (C-7),164.71(C-9),170.94 (C =O,C-2);元素分析结果为C 51.90%,H 4.90%。
以上数据与文献报道的异秦皮定-6-O-β-D-吡喃葡萄糖甙(Isofraxetin-6-O-β-D-glucopyranoside)一致[11]。由此推断,化合物6 的结构确定为异秦皮定-6-O-β-D-吡喃葡萄糖甙(Isofraxetin-6-O-β-D-glucopyranoside)。其结构简式如图6。
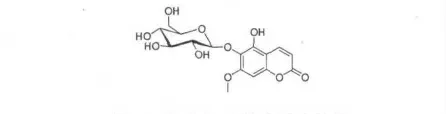
图6 化合物6 的化学式结构Fig.6 The chemical structure of compound 6
化合物7:浅黄色固体。MF:C16H18O9,MW:354;TOF MS ESI m/z:377.0 (M + Na)+;1H NMR[C5D5N,400 MHz]δ:7.97 (H,d,J =6.32 Hz,H-7),6.33 (H,d,J =6.32 Hz,H-5),4.16 (1H,m,H-5'),4.33 (1H,m,H-4'),4.61 (1H,m,H-2'),3.81(1H,m,H-3'),0.86 (3H,s,2-Me);13C NMR[C5D5N,100 MHz] δ:13.93 (CH3,C-2),60.57(CH2,C-6'),69.53 (CH,C-4'),73.00 (CH,C-2'),69.74 (CH,C-3'),76.68 (CH,C-5'),102.92 (CH,C-1'),113.26 (C-8),99.51 (CH,C-5),160.53 (C= O,C-10),109.58 (CH,C-3),112.20 (C-4),149.83 (C-6),148.87 (C-9),145.92 (CH,C-7);元素分析结果为C 45.20%,H 5.08%。
以上数据与文献报道的异香豆素萄糖甙(Delphoside)一致[12,13]。由此推断,化合物7 的结构确定为3-甲基-6-羟基-8-O-β-D-吡喃葡萄糖氧基异香豆素(3-methyl-6-hydroxy-8-O-β-D-glucopyranosyloxy isocoumarin)。其结构简式如图7。
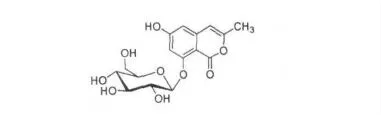
图7 化合物7 的化学式结构Fig.7 The chemical structure of compound 7
1 Stedman RL.The chemical composition of tobacco and tobacco smoke.Chem Rev,1968,68:153-207.
2 Fujimori T,Kaneko H.Studies on tobacco aroma.Nippon Nogeikagaku Kaishi,1979,53:R95-R121.
3 Ito K,et al.Glycosidic fraction of flue-cured tobacco leaves:its separation and component analysis.Biosci Biotechnol Biochem,2000,64:584-587.
4 Hisashi K,Takane F,Kunio K. Isolation of a new terpene glucoside from flue-cured tobacco. Agric Biol Chem,1981,45:941-944.
5 Teh CH,et al.2,3-Dehydro-4α-Hydroxyl Ongilactone,a novel quassinoid and two known phenyl propanoids from Eurycoma longifolia Jack.Food Chem,2010,120:794-798.
6 Termentzi A,Zervou M,Kokkalou E. Isolation and structure elucidation of novel phenolic constituents from Sorbus domestica fruits.Food Chem,2009,116:371-381.
7 Falodun A,Qadir MI,Choudhary MI.Isolation and characterization of xanthine oxidase inhibitory constituents of Pyrenacantha staudtii. Acta Pharmaceutica Sinica,2009,44:390-394.
8 Yves C,et al. Norterpenoid and sesquiterpenoid glucosides from Juniperus phoenicea and Galega officinalis. Phytochem,1999,50:1219-1223.
9 Chen ZL(陈振玲),Zhang HB(张浩博),Zhou WH(周宛虹),et al. Isolation and identification of 3-oxo-α-ionol-β-Dglucopyranoside from tobacco and analysis of its pyrolytic products.Tob Sci Tech(烟草科技),2008,7:28-31.
10 Midiwo JO,Owuor FAO,Juma BF,et al.Diterpenes from the leaf exudate of Psiadia punctulata.Phytochem,1997,45:117-120.
11 Li ZL,Li X,Li DY,et al.A new coumarin glycoside from the husks of Xanthoceras sorbifolia. Fitoterapia,2007,78:605-606.
12 Arazashvili AI,Moniava IT,Kemertelidze EP. Flavonoids of Delphinium flexudsum and Delphinium elisabethae.Khim Prir Soedin,1973,9:556-557.
13 Arazashvili AI,Moniava IT,Kemertelidze EP. Flavonoids from some species of Delphinium. Khim Prir Soedin,1974,10:251.
