Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
2023-10-23WuHaoWeiHongyuanLiWangliang
Wu Hao; Wei Hongyuan; Li Wangliang
(1. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China; 2. SINOPEC Research Institute of Petroleum Processing Co., Ltd., Beijing 100083, China; 3. CAS Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China)
Abstract: As one of the important aspects of upgrading coal tar, the ultra-deep removal of metal ions via the complexation method was investigated by screening four complexing agents and performing density functional theory (DFT) simulations.Analysis of the compositions and contents of the metallic compounds in the coal tar revealed that the main components were iron and calcium naphthenates. Direct filtration reduced the mechanical impurity content from 0.24% to 0.0752%,indicating that most of the large particles could be easily removed. Among the four complexing agents, namely, acetic acid,oxalic acid, citric acid, and ethylenediaminetetraacetic acid, oxalic acid exhibited the best demetallization performance.The DFT simulations suggested that the high performance of oxalic acid originated from its 1:1 coordination mode, rigid dicarboxyl structure, and greater binding energy.
Key words: complexation, metallic compounds, coal tar, upgrading, density functional theory
1 Introduction
Coal is the main energy resource in China, accounting for over 60% of the total energy supply. However, more than half of the coal consumed in China is low-rank coal. To realize clean and effective utilization, low-rank coal can be converted into high-value-added products such as gas,tar, and char by pyrolysis, dry distillation, and gasification.The production of clean fuel oil from coal tar could significantly alleviate oil and energy shortages, which would be of great value for sustainable development. In China, the annual output of medium-low-temperature coal tar, as the main liquid product of coal pyrolysis technology, has reached 15 million metric tons[1]. Coal tar is a complex mixture of condensed hydrocarbons,including monocyclic and polycyclic aromatics, oxygencontaining hydrocarbons, and heterocyclic compounds.The coal type and composition and the pyrolysis operation parameters exert significant influences on the composition and properties of the resulting coal tar[2]. Coal tar contains numerous metallic heteroatoms (e.g., Na, Fe, and Ca)and nonmetallic heteroatoms (e.g., S and N), which are difficult to remove and can hinder the subsequent processing into high-value-added products[3]. From the perspectives of comprehensive resource utilization and environmental protection, it is crucial to develop more efficient methods for upgrading, processing, and cleanly utilizing coal tar.
Researchers have developed a diverse variety of coal tar upgrading processes for heteroatom removal, such as hydrogenation[4], distillation[5], extraction[6], and adsorption[7]. In particular, catalytic hydrogenation and catalytic cracking processes have been extensively used to upgrade coal tar[8-9]. However, most industrial facilities adopt the process route of first cutting out the light and heavy components of coal tar by distillation and then upgrading the light components via hydrogenation, during which approximately 20%–40% of the heavy components are not effectively utilized. This greatly reduces the comprehensive utilization value of coal tar. For the distillation and acid washing method, large quantities of wastewater are generated during the acid/base refining process, which can result in severe environmental pollution. Therefore, whether from the perspective of environmental protection or that of comprehensive resource utilization, it is necessary to realize effective processing strategies to improve the quality of coal tar.To date, the removal of heteroatoms using organic solvents extraction has been thoroughly investigated for crude oil[10].Density functional theory (DFT) is one of the main quantum chemical calculation methods and has been widely applied to predict molecular properties and reaction characteristics in a variety of fields, including chemistry, materials, and catalysis[11-13]. For example, DFT has been used to investigate the structures and energetics of iron carbonyl complexes as well as the extraction of metal ions[14-16].
The main objectives of this work are to identify a highefficiency complexing agent for metallic species in coal tar and perform a computational study of the most promising complexing agents for this application. To this end, the composition and contents of the metallic components in coal tar were thoroughly analyzed and several complexing agents were screened with respect to their complexation performance. Then, the interactions between the complexing agents and metal ions as well as the corresponding binding energies were evaluated via ab initio DFT simulations.
2 Experimental
2.1 Materials
The coal tar sample was obtained from the pyrolysis of coal at 600 °C by Xinjiang Wanshunfa New Energy Co.,Ltd. The main metal constituents present in the coal tar sample are listed in Table 1. Complexing agents including acetic acid (≥99%), oxalic acid (≥99%), citric acid(≥99%), and ethylenediaminetetraacetic acid (EDTA,≥99%) were obtained from Sinopharm Chemical Reagent Co., Ltd.

Table 1 Main metal constituents present in the coal tar sample
2.2 Complexation procedure
The complexation experiments to assess the removal of metal ions were performed in a custom-built jacketed glass vessel with a volume of 50 mL connected to a thermostated water supply and equipped with a magnetic stirrer bar. Suitable amounts of the coal tar sample and complexing agent were added to the glass vessel and maintained at a constant temperature within ±0.1 °C.During the experiment, the magnetic stirrer was set at 300 r/min at certain temperature for 30 min at room temperature. Then, the mixture was allowed to settle for 1 h at the same temperature. During the settling process,any external disturbance was avoided until the phase equilibrium state had been reached. Because the density of coal tar is slightly higher than that of water, it was not possible to use the conventional electric desalination method for separation.
2.3 Metallic compound analysis
The coal tar samples were heated to 50–70 °C. The oil sample (ca. 5 g, accurately weighed to 0.01 g), xylene(10 mL), and pure water (30 mL) were added to a centrifuge tube. The resulting mixture was placed in a shaker maintained at 50 °C and operating at a speed of 300 r/min for 3 h. Finally, the sample was centrifuged at 5000 r/min for 5 min. The concentrations of calcium,iron, and sodium ions in the water and oil phases were determined by inductively coupled plasma atomic emission spectroscopy (Optima 7300 DV or Optima 3300 DV, PerkinElmer).
2.4 Simulation methods
DFT simulations were performed using the DMol3module in the Materials Studio software. The corresponding molecules were subjected to geometry optimization using the Becke-Perdew functional (BP) with the generalized gradient approximation. Restricted and unrestricted algorithms were adopted for the molecules without iron and with iron on account of their closed-shell and open-shell structures, respectively. For the molecules containing calcium or iron, the effective core potentials were used. Quantum chemical methods rely on the theory of quantum mechanics to deal with chemical problems and use the wave functionψ(r,t) of the system to describe the molecular properties. In particular, when attempting to elucidate the ground-state properties of molecules, the steady-state assumption can be made, that is, only the steady-state wave functionψ(r) needs to be examined by solving the Schrödinger equation without a time term:
In quantum chemical simulations, molecular orbital(MO) theory is often used to approximate polyatomic systems, such as the complexes in coal tar in this case.MO theory holds that (i) after atoms form molecules,the electrons belong to the entire molecular system; (ii)MOs are formed by the linear combination of atomic orbitals; (iii) this linear combination of atomic orbitals must satisfy the principles of symmetry matching and energy approximation; and (iv) the filling of electrons in orbitals needs to satisfy the Pauli exclusion principle,minimum energy principle, and Hund’s rule. In addition,the valence electrons contribute more to the MOs, while the inner-shell electrons contribute less, that is, they are in a “localized” state. Therefore, for a multi-electron system,the steady-state Schrödinger equation can be expressed as follows:
The Hamiltonian of this equation includes five terms,namely, the nuclear kinetic energy, electron kinetic energy, electron-nucleus potential energy, electronelectron potential energy, and nucleus-nucleus potential energy. The presence of cross terms makes this equation extremely difficult to solve. Therefore, three major assumptions are often introduced: the Born-Oppenheimer approximation, the single-electron approximation, and the non-relativistic approximation. The Born-Oppenheimer approximation holds that the nucleus is stationary and the electrons outside the nucleus move around the “skeleton”formed by the nucleus. The single-electron approximation holds that each electron moves in the average field of the nucleus and other electrons. The non-relativistic approximation holds that the rate of electron motion is below relativistic velocity. The rate of electron motion in molecules is much greater than that of nuclei, such that the Born-Oppenheimer approximation is reasonable.
However, the single-electron approximation introduces large errors because the motion of electrons in space is affected by the electron correlation phenomenon,meaning that electron correlation correction is usually required during quantum chemical calculations, such as in configuration interaction theory, microperturbation theory, and coupled-cluster theory. The non-relativistic approximation is reasonable when dealing with elements belonging to the first three periods, but for heavier elements the velocity of electrons close to the nucleus more closely approaches the speed of light, such that relativistic effects cannot be neglected. This typically necessitates relativistic corrections, such as all-electron relativity, the zeroth order regular approximation, and the relativistic effective core potential.
3 Results and Discussion
3.1 Composition and content of metallic compounds
The types and contents of metallic compounds present in coal tar vary with the coal feedstock and pyrolysis conditions. As such, the metal species in the coal tar sample used in this study were determined as shown in Table 1. It can be seen that the main metallic species were iron and calcium. The proportion of inorganic metallic compounds was low, and the metals were mainly present in the form of naphthenates. No porphyrins and non-porphyrins were detected. As reported, the iron naphthenate and calcium naphthenate could be rapidly and easily removed.
3.2 Screening of complexing agents
Oxalic acid, acetic acid, citric acid, and EDTA were screened as complexing agents to remove metal ions from coal tar. Each complexing agent was separately added in a mass ratio of 0.25% with respect to the coal tar and the resulting mixtures were stirred at 80 °C for 10 min then filtered through filter paper with a pore diameter of 5 μm.Table 2 shows the obtained results. It can be seen that direct filtration reduced the mechanical impurity content of the coal tar from 0.24% to 0.0752%, indicating that most of the large particles were removed by the filter paper. Upon the addition of the complexing agents, the mechanical impurities were not further reduced compared with direct filtration, which means that the small particles of solid impurities in the coal tar were not further removed by adding the complexing agents. The results presented in Table 2 also show that oxalic acid exhibited the best demetallization performance.

Table 2 Demetallization performance of various complexing agents
3.3 Influence of complexing agent dosage on metal removal
Table 3 shows the effects of various oxalic acid dosages on the demetallization performance. It can be seen that oxalic acid dosages of 0.15% or greater afforded a total metal content of less than 20 μg/g. In addition, the metal
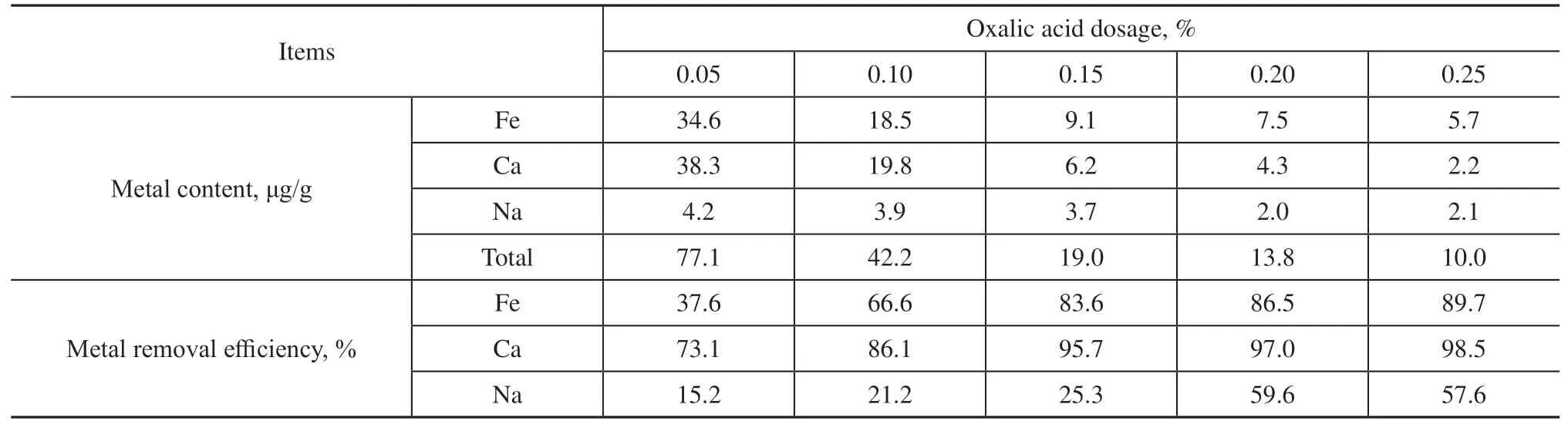
Table 3 Influence of various oxalic acid dosages on demetallization performance
removal efficiency remained relatively stable at oxalic acid dosages of 0.15% or greater, which was attributable to the fact that the metals had already been reduced to relatively low levels and the removal efficiency was already high.
The experimental results also show that oxalic acid displayed better performance in removing calcium than in removing iron, which is consistent with the simulation results presented later. The metal content of less than 20 μg/g obtained using an oxalic acid dosage of 0.15%or greater meets typical feed requirements for a coal tar hydrogenation unit.
3.4 DFT simulations of the interactions between metal ions and complexing agents
In aqueous solution, Ca(II) and Fe(II) ions exist in the form of the hydrated species [Ca(H2O)6]2+and[Fe(H2O)6]2+. Significant levels of naphthenic acids are presented in coal tar, leading to the formation of metal naphthenates. Cycloalkylcarboxylic acids are formed by linking carboxyl groups and cycloalkyl groups, which have the characteristics of cycloalkyl and acid. Therefore,cyclohexanecarboxylic acid was used as a model compound in this study. Cyclohexanecarboxylic acid loses a proton to form the cyclohexanecarboxylate ion, the optimized structure of which is presented in Figure 1(a).The calculated lengths of the two carbon-oxygen bonds were 1.265 and 1.269 Å and the OCO angle was 129.4°,which indicates that the two bonds were essentially equivalent after the deprotonation of the carboxyl group,thus forming a conjugated structure. Mulliken population analysis of the cyclohexanecarboxylate ion indicated that the oxygen end possessed a negative charge, where the two oxygen atoms had charges of -0.615e and-0.612e, such that they could interact and coordinatewith positively charged Ca(II) and Fe(II) ions. Further analysis showed that the highest occupied molecular orbital (HOMO) of the cyclohexanecarboxylate ion had a significant 2p orbital of oxygen atom, as depicted in Figure 1(b), with a suitable orientation to allow coordination with the valence orbitals of the metal ions.
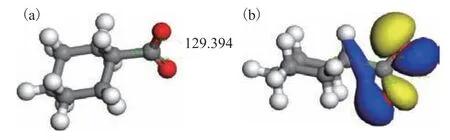
Figure 1 Geometry of the cyclohexanecarboxylate ion
As stated above, the Ca(II) and Fe(II) ions in coal tar primarily exist in the form of naphthenates. The overall valence of cycloalkanoate is -1, therefore, Ca(II) and Fe(II) ions can coordinate with two cycloalkanoate ions,which are coordinated with the carboxyl oxygen atom and the metal ions, respectively. The optimized geometric structures of the calcium and iron cyclohexanecarboxylate complexes are presented in Figure 2. It can be seen that the octahedral Ca(II) and Fe(II) centers were surrounded by six oxygen atoms derived from the two cyclohexanecarboxylate ligands and two water molecules.In the case of Ca(II), the coordination bond lengths for the four oxygen atoms of the cyclohexanecarboxylate ligands were 2.451, 2.467, 2.502, and 2.511 Å, while those for the two oxygen atoms of the water molecules were 2.504 and 2.507Å. In the case of Fe(II), the coordination bond lengths for the four oxygen atoms of the cyclohexanecarboxylate ligands were 2.057, 2.057,2.060, and 2.065 Å, while those for the two oxygen atoms of the water molecules were 2.089 and 2.088 Å.
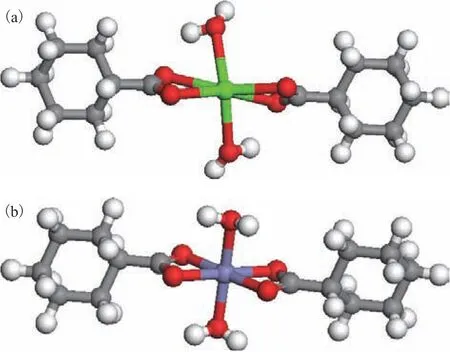
Figure 2 Geometric structures of the cyclohexanecarboxylate complexes of (a) calcium and (b) iron
To achieve the effective removal of calcium and iron ions from coal tar, four commercial complexing agents,namely, acetic acid, oxalic acid, citric acid, and EDTA, all of which contain carboxylic acid groups, were examined.The most suitable complexing agent was determined by a combination of experimental tests and computational simulations, and the underlying factors were analyzed by theoretical means. Specifically, the energy change associated with the following reaction was evaluated:
M(CyC)2(H2O)2+nHxY +mH2O → M(Y)n(H2O)y+ 2HCyC The structures of the complexes formed between Ca(II)and the four organic acids are presented in Figure 3.Two acetate anions were found to coordinate with Ca(II)through their carboxyl oxygen atoms to form a neutral Ca(acetate)2(H2O)2complex. Careful analysis of the Ca–O bond distances revealed values of 2.462 Å for both water molecules and values of 2.409, 2.429, 2.430, and 3.388 Å for the acetate groups. Therefore, one acetate anion adopted a bidentate coordination mode with Ca(II) while the other adopted a monodentate coordination mode,which indirectly indicates that the interaction between the acetate anion and Ca(II) was not strong enough for both acetate anions to engage in bidentate coordination.Oxalate coordinated with Ca(II) through two carboxyl oxygen atoms in a bidentate manner to form a neutral Ca(oxalate)(H2O)4complex. The Ca–O bond lengths for the four water molecules were 2.379, 2.417, 2.499,and 2.517 Å, while those for the oxalate ligand were 2.475 and 2.604 Å. Citric acid underwent deprotonation of two of its three carboxyl groups to afford the doubly charged anion, which formed a 1:1 complex with the metal ion, i.e., Ca(citrate)(H2O)2. During the coordination process, the citrate anion became significantly distorted compared with the free state to sterically accommodate the Ca(II) ion. The Ca–O distances for the two water molecules were 2.349 and 2.357 Å, while those for the four nearest oxygen atoms of the citrate ligand were 2.347, 2.350, 3.270, and 3.303 Å. Therefore, although citric acid underwent secondary dissociation to yield two deprotonated carboxylate groups, essentially only two of the oxygen atoms were able to coordinate directly with Ca(II), and coordination was not feasible for the other two carboxylate oxygen atoms. Therefore, the citrate anion and Ca(II) adopted a bidentate rather than tetradentate coordination mode, which indicates that the interaction strength between the two species was not sufficiently high for tetradentate coordination. EDTA has four carboxyl groups and usually loses two protons to form the doubly charged anion, thus leading to a 1:1 Ca(EDTA)(H2O)2complex. The EDTA ion was also significantly distorted compared with the free state to allow it to coordinate with the metal ion. The Ca–O distances for the two water molecules were 2.394 and 2.398 Å, while those for the EDTA ligand were 2.432, 2.446, 2.540, and 2.568 Å.
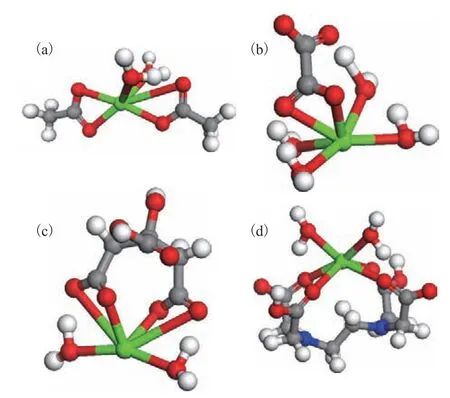
Figure 3 Structures of the complexes between calcium and(a) acetate, (b) oxalate, (c) citrate, and (d) EDTA
The structures of the complexes formed between Fe(II) and the four organic acids are shown in Figure 4. In the neutral Fe(acetate)2(H2O)2complex, the Fe–O distances were 2.041 Å for both water molecules and 1.986, 2.001, 2.120, and 3.092 Å for the acetate groups. The coordination mode was similar to that of the Ca(acetate)2(H2O)2complex, with one acetate anion in the bidentate coordination mode and the other in the monodentate coordination mode, again indicating that the interaction between the acetate ions and Fe(II) was not strong enough for both acetates to engage in bidentate coordination. As in the case of Ca(II), oxalate coordinated with Fe(II) through two carboxyl oxygen atoms in a bidentate manner to afford a neutral Fe(oxalate)(H2O)4complex. The Fe–O bond lengths for the four water molecules were 2.012, 2.092, 2.095, and 2.097 Å, while those for the oxalate ligand were 2.043 and 2.044 Å. In the 1:1 Fe(citrate)(H2O)2complex, the Fe–O distances for the two water molecules were 1.921 and 1.934 Å,while those for the four nearest oxygen atoms of the citrate ligand were 1.881, 1.910, 3.022, and 3.064 Å.Thus, the coordination mode was similar to that of the Ca(citrate)2(H2O)2complex with essentially only two of the oxygen atoms able to directly coordinate with Fe(II), which again indicates that the interaction between citrate and Fe(II) was not strong enough for tetradentate coordination. In the 1:1 Fe(EDTA)(H2O)2complex, the Fe–O distances were 2.043 and 2.118 Å for the two water molecules and 1.977, 2.005, 2.095, and 2.113 Å for the EDTA ligand.
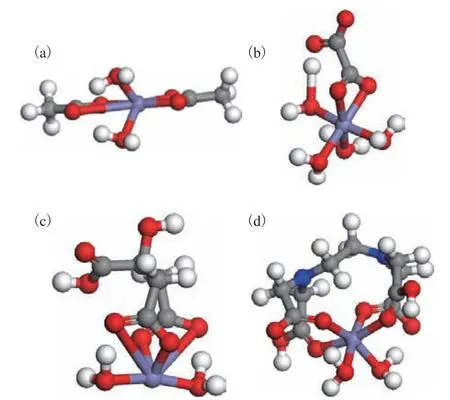
Figure 4 Structures of the complexes between iron and (a)acetate, (b) oxalate, (c) citrate, and (d) EDTA
3.5 Binding energy analysis
The basic principle of coal tar demetallization by complexation is that the complexing agent extracts the Ca(II) and Fe(II) ions from their naphthenate complexes.Therefore, the goal was to calculate the theoretical energy change for the following general reaction:metal naphthenate + complexing agent → metal complex+ naphthenic acid
The specific reactions and their corresponding energy changes are listed in Table 4. From the obtained ΔEvalues, it can be seen that oxalic acid would be expected to display the best demetallization performance, while relatively poor demetallization performances were predicted for the other three complexing agents containing one, three, or four carboxyl groups.
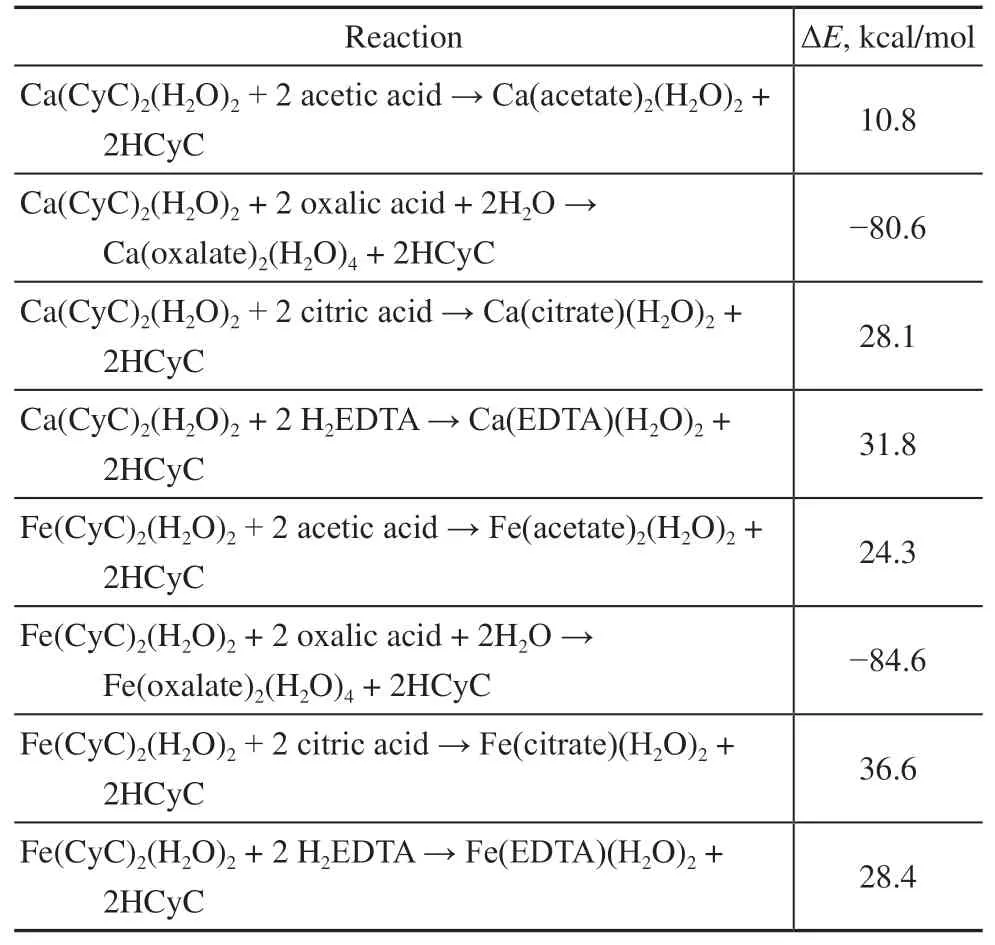
Table 4 Theoretical energy changes for the demetallization reactions
Furthermore, the differences in the complexation properties were analyzed from the perspective of molecular geometry and bonding characteristics. The molecular structures of the four complexing agents are presented in Figure 5. Acetic acid and oxalic acid are relatively small molecules with high rigidity. During complexation with the metal, the ligand structure changes little. Because acetate has only a single negative charge,two molecules are required for complexation with M(II), but the second acetate molecule cannot engage in bidentate coordination. Thus, the demetallization performance is relatively poor. By contrast, oxalate has two carboxyl groups and two negative charges after dissociation, thus allowing for coordination with M(II) in a 1:1 ratio. Both citric acid and EDTA can also dissociate to form doubly charged ions and thus coordinate with M(II) in a 1:1 ratio. However, the carboxyl groups in these molecules are relatively far away from one another, and the occurrence of cooperative coordination by multiple carboxyl groups requires the molecule to undergo structural pre-organization, that is, the molecular structure must become distorted to accommodate the metal center, resulting in high energy consumption, such that the demetallization performance is inferior to that of oxalic acid with its two carboxyl groups. In addition, as a rigid dicarboxyl ligand, oxalic acid often bridges metal ions to form a network structure or even precipitate,which greatly enhances its demetallization performance.
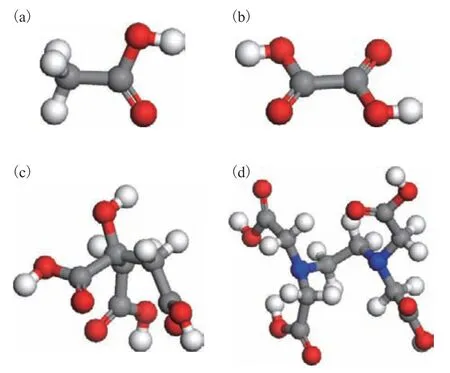
Figure 5 Geometries of the four complexing agents examined in this work (a) acetic acid, (b) oxalic acid, (c)citric acid, and (d) EDTA
Figure 6 shows the structure of oxalic acid alongside the HOMOs of oxalic acid and the Fe(oxalate)(H2O)4complex. The oxalic acid molecule contains two carboxyl groups, and its HOMO has the characteristics of remarkable 2p orbital of carbon and oxygen atoms,and exhibits certain conjugation characteristics, which is favorable for the coordination of oxalic acid and the metal. After coordination with iron, the HOMO of the complex is dominated by the iron 3d orbital.Overall, the coordination bond exhibits strong ionic characteristics.
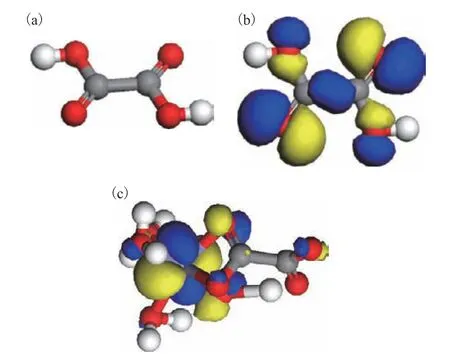
Figure 6 (a) Structure of oxalic acid, (b) HOMO of oxalic acid, and (c) HOMO of the Fe(oxalate)(H2O)4 complex
4 Conclusions
The main metal species in the coal tar samples examined in this work were found to be iron and calcium, which were primarily presented in the form of naphthenates.Direct filtration reduced the mechanical impurity content of the coal tar from 0.24% to 0.0752%. Among the four complexing agents tested, including acetic acid, oxalic acid, citric acid, and EDTA, oxalic acid exhibited the optimal demetallization performance, affording removal efficiencies of 89.7%, 98.5%, and 57.6% for iron,calcium, and sodium, respectively. The high performance observed for oxalic acid was ascribed to its coordination state, rigid dicarboxyl structure, and greater binding energy. The results obtained in this study demonstrate the feasibility of using complexation to remove metal ions from coal tar and provide a promising strategy for upgrading coal tar. Furthermore, the consistency observed between the experimental results and DFT simulations indicates the viability of using simulation results to guide experimental work in the future.
Acknowledgements:This work is financially supported by the National Energy R&D Center of Petroleum Refining Technology(RIPP, SINOPEC), the National Natural Science Foundation of China (22078347), the Key Research and Development Program of Hebei Province, China (21373303D).
杂志排行
中国炼油与石油化工的其它文章
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics
