Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
2023-10-23WangYingjieLiuSiyuanWangXueningLiuZhihaoChenHongyuanQiuKui
Wang Yingjie; Liu Siyuan; Wang Xuening; Liu Zhihao; Chen Hongyuan; Qiu Kui
(School of Chemistry and Chemical Engineering, Chongqing University of Science and Technology,Chongqing 401331)
Abstract: A copper-based non-aqueous-phase desulfurization agent is prepared by adding CuCl2 to the solvent N,Ndimethylformamide (DMF). Static desulfurization experiments show that the agent has high efficiency. However, the desulfurization reaction leads to the formation of a copper sulfide precipitate. It is found that the addition of chloride ions in the form of hydrochloric acid or potassium chloride prevents the formation of copper sulfide, and elemental sulfur is precipitated instead. The efficient absorption of H2S by the Cu/HCl–DMF agent relies on the rapid coordination of Cu2+ with DMF, Cl-, and H2S molecules to form a [Cu(DMF)n-p(HS-)p(Cl-)m](2-p-m)+ complex. The desulfurization agent has a sulfur capacity of up to 9.81 g/L when used in static bubble desulfurization at atmospheric pressure. The system has low viscosity and good chemical and thermal stability. It can be rapidly regenerated through continuous oxidation. After five repetitions of the regeneration procedure, the sulfur capacity reaches more than 91% of the initial capacity, indicating the potential of the system for commercial applications.
Key words: hydrogen sulfide removal; wet oxidation; DMF; CuCl2
1 Introduction
Natural gas contains hydrogen sulfide (H2S), which is toxic and reactive, not only causing corrosion to metal pipeline equipment, but also posing a serious threat to human life[1]. Therefore, for effective and safe utilization of natural gas, it is imperative to thoroughly eliminate all traces of H2S during the gas extraction process.Desulfurization technology can be divided into two categories, dry desulfurization and wet desulfurization,and it is the latter that is considered in this paper. In wet desulfurization, H2S is dissolved in solution, with the formation of HS-and other sulfide species. These sulfides are then oxidized by a catalyst to form sulfur and water. The catalyst is regenerated by air or oxygen and can then be reused. The catalysts typically used in this wet oxidation process have V5+/V4+[2]and Fe3+/Fe2+[3-4]as their primary active components. With its simple operating procedure, wide range of applicable concentrations, high cleanliness, and low cost[5-6], wet desulfurization is widely used for dealing with low to medium concentrations of H2S in natural gas. However,this method inevitably suffers from low sulfur capacity,high energy consumption, poor quality of the sulfur byproduct, complex solvent formulations, easy degradation of the solution, and high effluent discharge[7]. Therefore,there is an urgent need to develop new desulfurization processes and techniques to address these problems.
The LO-CAT process is one of the most widely used wet oxidation desulfurization processes[8]. However, it has some disadvantages, including the need to ensure that the desulfurization agent is alkaline to promote the rapid absorption of H2S, the addition of extra chelating agents to prevent the hydrolysis of Fe3+, and the tendency to generate sulfate, sulfite, and thiosulfate by-products during solution regeneration, which results in low sulfur purity[9].
Owing to the unique physical and chemical properties of ionic liquids, their characteristics can be modified by altering the type and structure of the cation. This versatility allows for a wide range of applications, making these liquids potentially effective and environmentally friendly solvents for desulfurization[10-14]. Guo et al.[15]proposed a new non-aqueous oxidative desulfurization system based on an iron-based ionic liquid (Fe-IL),which can achieve a theoretical sulfur capacity of 66 g/L.However, its actual sulfur capacity is much lower, at only 0.23 g/L. Ionic liquids typically encounter problems associated with their high viscosity[16]. In the case of Fe-IL, its high viscosity can potentially affect the rate of oxidation transfer from Fe3+to H2S. Wang and Zhu[17]proposed that the dual function of ionic liquids as both solvent and catalyst, combined with their strong acidity, leads to a lower actual sulfur capacity of the desulfurization agent compared with the theoretical value.Considering the numerous challenges associated with aqueous-phase wet oxidation and some ionic liquid desulfurization methods, non-aqueous-phase desulfurization systems employing organic solutions have attracted considerable attention. Hua et al.[18]reported a non-aqueous-phase desulfurization system based on an organic solution of FeCl3. This desulfurization process was able to efficiently catalyze the oxidation of H2S to sulfur, giving a high-purity product, although the H2O2generated in the system promoted degradation of the organic solvent. The low solubility of FeCl3in the solvent made it difficult to meet the desulfurization requirements of high-sulfur-content natural gas. To deal with these problems, our group has focused on adding hydrochloric acid to the desulfurization agent in this reaction system to enable regeneration.
Zhang and Tong[19]used a CuCl2solution to adsorb H2S,with the formation of CuS that could subsequently be separated. They then used a variety of copper complexes containing chloride ions in a high-concentration CuCl2solution to dissolve CuS and produce CuCl32-and sulfur. Leaching kinetic studies showed that the leaching efficiencies were close to 100%. These results show that Cu2+has the ability not only to rapidly bind H2S but also to catalyze its oxidation to sulfur under specific conditions. However, a significant amount of CuS is produced, and the need to separate it during the desulfurization process requires a complex operation that substantially increases production costs. Our group has investigated the possibility of removing H2S and dissolving CuS to obtain sulfur using only CuCl2solutions.
Anhydrous CuCl2can dissolve in certain organic solvents to form complexes, particularly in solvents that exhibit Lewis basicity, such as dimethyl sulfoxide (DMSO) andN,N-dimethylformamide (DMF)[20]. Organic Lewis base solvents are considered excellent choices as solvents for desulfurization agents owing to their strong absorption of H2S and the absence of any production of polluted water. Considering environmental protection and economic efficiency, our group has developed an excellent desulfurization method by combining organic solvents with CuCl2. Based on the relevant literature and preliminary experiments with six potential desulfurization solvents,we selected DMF. Also, to introduce additional Cl-ions, a small amount of concentrated hydrochloric acid was added.The excellent stability and desulfurization performance at ambient temperature and pressure of this Cu/HCl–DMF system demonstrate its feasibility for desulfurization.
During the desulfurization process, a yellow intermediate product appears, and this is then transformed into sulfur after the introduction of oxygen for regeneration.This suggests a distinct mechanism compared with conventional wet oxidation. There have been few reports of studies of the formation of dissolved copper complexes in organic solvents for the efficient absorption of H2S,and the mechanism of action is still unknown. Therefore,we conducted static desulfurization experiments to investigate the factors that influence the performance of the desulfurization agent. We have also proposed a potential mechanism for the process based on a series of characterization analyses of the desulfurization agent and its solid products.
2 Experimental
2.1 Materials
DMF (99.5%), was purchased from Tianjin Damao Chemical Reagent Factory (Tianjing, China). Anhydrous copper(II) chloride (99%) and anhydrous iron(III)chloride (99%), were purchased from Chengdu Kelon Chemical Co. Hydrochloric acid (37%) was purchased from Chongqing Chuandong Chemical Co. All the chemicals used were of analytical grade and were used without further purification. Both H2S and standard gas (molar fraction: H2S 5.01%, N294.99%, i.e., an H2S concentration of 50100 μL/L) were purchased from Rio Tinto Gas Company (Chongqing, China)for desulfurization and regeneration experiments,respectively.
2.2 Characterization
Fourier-transform infrared (FTIR) spectra were obtained by scanning the samples over the range from 4000 to 650 cm-1on a Thermo Fisher Nicolet Is5 infrared spectrometer. The thermal stability of the desulfurization fluid was tested using a NETZSCH STA 449C thermogravimetric analyzer (NETZSCH, Germany)under nitrogen and at a temperature gradient of 10 °C/min from room temperature to 500 °C. The dynamic viscosity of the desulfurization fluid was measured with a rotational viscometer (DV-II+, Brookfield). The structure of the sulfur produced by the regeneration process was examined using a Nihon Rei SmartLab-9 X-ray diffractometer. The test conditions were as follows:Cu Kαradiation source, operating voltage 40.0 kV,operating current 30.0 mA, scan rate 5 (°)/min, scan range 10°-80°. The different forms of the elements in the intermediate desulfurization products were determined by X-ray photoelectron spectrometry (XPS) on a Thermo Fisher ESCALAB 250Xi instrument with Al Kαradiation(hν= 1486.6 eV). The binding energy (BE) was calibrated using the C1sline (284.8 eV). A Gauss-Lorentz peak was fitted to the XPS curve using the Advantage program.
2.3 Selection of desulfurization solvent
To choose the best solvent, desulfurization agents were prepared by dissolving 1 g of CuCl2in 40 mL of six different solvents, namely, ethylene carbonate (EC),sulfolane, polyethylene glycol dimethyl ether (NHD),DMSO,N,N-dimethylacetamide (DMAC), and DMF.These agents were then used in static desulfurization experiments to determine which was the most effective.
2.4 Preparation of desulfurization agent
Once DMF had been selected as the solvent, CuCl2was dissolved in 40 mL of DMF, and varying volumes of HCl were added to create different desulfurization agents.
2.5 Desulfurization experiment
The static desulfurization test rig (Figure 1) consisted of a standard gas bottle containing 5% volume fraction H2S, a gas flow meter, a constant-temperature water bath, a bubbling reactor, an H2S detector, and a saturated NaOH absorption solution. The bubbling reactor had a diameter of 3 cm and a height of 15 cm, with a volume of 40 mL for the desulfurization agent. The water bath was used to control the reaction temperature within the range 20–60 °C. The gas flow meter regulated the flow rate of the H2S standard gas. The H2S was introduced into the bubbling reactor to undergo reaction with the desulfurization agent. The exhaust gas from the reaction was passed through the H2S detector and then absorbed by the saturated NaOH solution. When the mass concentration of H2S in the exhaust gas reached 20 μL/L(a standard upper limit for safety), the supply of H2S was halted, and the duration of time during which the H2S gas had passed through the device was taken as the desulfurization time.
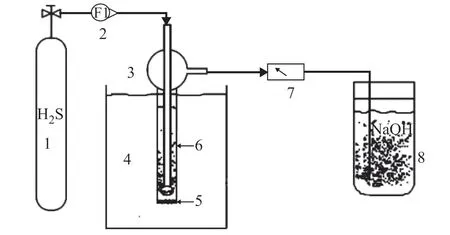
Figure 1 Experimental device for H2S desulfurization
Taking into account the presence of physically dissolved and chemically absorbed H2S in the desulfurization process, the sulfur capacitya(g/L) is calculated as follows[21]:
whereQ(mL/min) is the flow rate of raw gas,t1(min)is the desulfurization time,t2(min) is the time from the first detection of H2S in the exhaust gas until its mass concentration reached 20 μL/L,MH2S(g/mol) is the molar mass of H2S,Vmis the molar volume of the gas (taken to be 22.4 L/mol in this paper),V(mL) is the volume of desulfurization agent,Cis the volume fraction of H2S in the raw gas (taken to be 5% in this paper), andC'is the volume fraction of H2S in the reaction exhaust gas. As the maximum mass concentration of H2S in the reaction exhaust gas in this study was set at 20 μL/L, the conversion results in a maximumC'of 0.04%. Therefore,C'is significantly smaller thanCand can be considered negligible.
3 Results and Discussion
3.1 Selection of desulfurization solvent
Experiments were conducted on the desulfurization agents based on the six different solvents at a temperature of 313.15 K and a feed gas flow rate of 40 mL/min. The results of the static desulfurization experiments with the six solvents are depicted in Figure 2.

Figure 2 Breakthrough curves and sulfur capacities of desulfurization agents based on different solvents
EC is a crystalline solid at room temperature (20 °C) and needs to be melted by heating in a water bath before being used to prepare the desulfurization agent. Sulfolane is a liquid at room temperature, but CuCl2has a low solubility in it: 1 g of CuCl2still remains partially undissolved even after 30 min of stirring. As depicted in Figure 2,for both the EC-based and sulfolane-based agents, the concentration of H2S in the purified gas exceeded 20 μL/L at a desulfurization time of only 5 min. The solubility of CuCl2in NHD is moderate, and heating is required for it to dissolve completely. With the NHD-based agent, the H2S concentration in the purified gas exceeded 20 μL/L when the desulfurization time was only 10 min. When DMSO was used as the solvent for the desulfurization agent, the H2S concentration in the purified gas remained below 20 μL/L up to a desulfurization time of 20 min.However, further investigation is needed to determine the feasibility of DMSO as a solvent for desulfurization in an industrial environment, owing to its hygroscopic and flammable properties. Among the solvents examined here, the solubility of CuCl2is highest in DMAC and DMF, which have similar molecular structures. As Lewis bases, both effectively induce ionization of H2S in the desulfurization agent[22], resulting in a good desulfurization effect. With the agents based on DMAC and DMF, the desulfurization time reached 25 min and 30 min, respectively, before an H2S concentration of 20 μL/L was exceeded.
From these experimental results, it can be seen that the efficiency of a desulfurization agent is influenced by the solubility of CuCl2in the solvent that is used, as well as the physical properties of that solvent. Given its low economic cost, environmental friendliness, and high desulfurization efficiency, DMF was chosen as the optimal solvent in the rest of the investigation.
3.2 Characterization of desulfurization agents
The chemical structures of DMF and the prepared desulfurization agents were analyzed using FTIR spectroscopy, with the results shown in Figure 3. DMF exhibits a distinct absorption peak at a wavenumber of 1660 cm-1, which corresponds to stretching vibration of the C=O bond[23]. The absorption peak does not change after the addition of HCl, but it shifts to 1645 cm-1after the addition of CuCl2. The presence of coordination bonds between oxygen and metal has previously been shown to result in alterations in the electron energy band and a decrease in the wavenumber corresponding to C=O vibration[24]. The redshift of DMF is thought to be caused by the formation of a C=O–Cu coordination bond between the oxygen atom in C=O and Cu2+. The characteristic peak of C=O–Cu disappears after the absorption of H2S by the desulfurizing agent, indicating that the H2S disrupts the coordination of Cu2+with DMF.This phenomenon shows that the main active ingredient of these desulfurization agents is Cu2+.
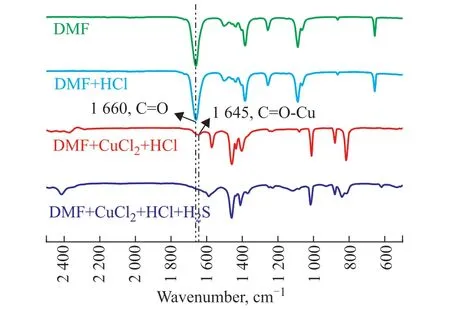
Figure 3 IR spectra of DMF, DMF + HCl, DMF + CuCl2+ HCl, DMF + CuCl2 + HCl + H2S, DMF + CuCl2 + FeCl3 +HCl, DMF + CuCl2 + FeCl3 + HCl + H2S
To investigate the thermal stability of a Cu/HCl–DMF desulfurization agent, 1 g of CuCl2and 0.5 mL of HCl were added to 40 mL DMF solvent. A thermogravimetric analysis was then carried out, and the resulting curve is shown in Figure 4. It is evident that the desulfurization agent experienced mass loss between 40 °C and 120 °C caused by the evaporation of water in the HCl solution,water absorbed by the desulfurization agent, and the DMF solvent[25]. Above 200 °C, only about 12% of the mass of the solid form of the coordination compound of DMF and Cu2+remained unchanged[26]. On the basis of the results of the thermogravimetric analysis, it is recommended that the temperature of the desulfurization procedure be maintained below 60 °C to avoid evaporative losses and high-temperature decomposition during the desulfurization process.
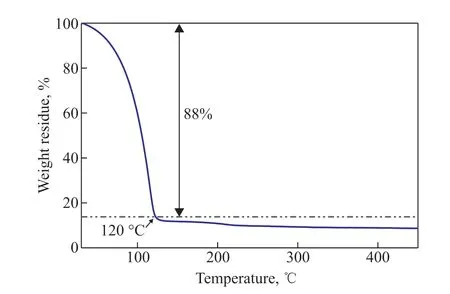
Figure 4 TGA curve of desulfurization agent
The lower the mass transfer resistance of the gas in a low-viscosity solution, the higher is the mobility, the greater is the absorption rate, and the more effective does the desulfurization process become. The actual sulfur capacities of conventional ionic liquids are lower than the theoretical values owing to their high viscosities[11].To investigate the impact of metal salts on the viscosity and density of a desulfurization agent based on DMF as solvent, 4 g CuCl2and 0.5 mL of HCl were added to 40 mL of DMF to prepare the agent. Viscosity and density were measured at various temperatures and compared with those of pure DMF. The results are shown in Table 1. As can be seen, DMF has a low viscosity and density, making it an excellent solvent for preparing desulfurization agents. The addition of CuCl2and HCl increased the viscosity to approximately 4 mPa·s. Thus the addition of the metal salt increased the viscosity of the solution by five times, as well as increasing the density by 13%. In comparison, the viscosity of the desulfurization agent was lower than that of the normal ionic liquids reported in the literature[27]. This suggests that the organic desulfurization agent still possesses properties of low viscosity and low density.

Table 1 Dependence of viscosity η and density ρ on temperature of desulfurization
3.3 Performance evaluation of desulfurization agents
3.3.1 Factors affecting H2S absorption
The effects of the composition of the desulfurization agent, the amounts of CuCl2and HCl, the temperature,and the flow rate on the efficiency of desulfurization were investigated through static desulfurization experiments were in a controlled indoor environment with a humidity level of 20%. The initial amount of H2S in the raw gas was set at 5.01%, and the flow rate was set at 40 mL/min.The H2S standard gas was desulfurized using four different agents, namely, DMF alone, DMF + CuCl2,DMF + CuCl2+ HCl, and DMF + CuCl2+ KCl, at a temperature of 313.15 K and a feed gas flow rate of 40 mL/min. The concentration of H2S in the purified gas was then recorded. The results are shown in Figure 5(a).DMF had almost no desulfurization effect, because it can only physically absorb H2S. The concentration of H2S in the purified gas reached 20 μL/L after only 5 min of continuous absorption at atmospheric pressure.However, when a DMF solution containing CuCl2was used for desulfurization, the concentration of H2S remained below 20 μL/L until 30 min, indicating the positive effect of CuCl2on the absorption of H2S. With the addition of HCl to the DMF solution of CuCl2, the desulfurization performance was improved still further,with an H2S concentration in the purified gas not being reached until 40 min. Unexpectedly, with the DMF +CuCl2+ HCl desulfurization agent, the absorption of H2S led to the immediate formation of a yellow precipitate,which quickly turned black on the addition of water.We believe that this precipitate contains copper, but that the mechanism of its formation is distinct from that responsible for the formation of CuS precipitates from the reaction between Cu2+and H2S in an aqueous-phase desulfurization system[28]. With the DMF + CuCl2+ KCl solution, the concentration of H2S in the purified gas reached 20 μL/L after 20 min, the same time as for the solution without KCl. The same experimental phenomena were observed in the desulfurization process using this agent as those occurring with the DMF + CuCl2+HCl system. Therefore, we believe that the additional Cl-altered the reaction mechanism of desulfurization.Overall, these results demonstrate the superiority as a desulfurization agent of the solution containing HCl.Therefore, this DMF + CuCl2+ HCl formulation (Cu/HCl–DMF) was used for the subsequent experiments.

Figure 5 (a) Effect of composition of the desulfurization agent at 40 °C. (b) Effect of dosages of CuCl2 and HCl at 40 °C. (c)Effect of temperature on desulfurization efficiency. (d) Effect of gas flow rate on desulfurization efficiency at 40 °C and an inlet H2S concentration of 50, 100 μL/L
To investigate the effect of CuCl2and HCl addition on the efficiency of desulfurization, we conducted static desulfurization experiments using different amounts of HCl and CuCl2. The results are presented in Figure 5(b).It can be seen from the black, red, and blue curves that at for constant concentration of CuCl2, the time taken for the H2S concentration in the purified gas to reach 20 μL/L decreased as more HCl was added. Thus, the desulfurization performance decreased with increasing HCl dosage. On the other hand, the green, purple and orange curves show that for a constant concentration of HCl, the time taken to reach an H2S concentration of 20 μL/L increased with increasing dosage of CuCl2.There was a clear linear correlation due to the strong coordination effect of DMF on Cu2+, which results in the high solubility of CuCl2in DMF[29]. For a desulfurization agent containing 0.5 mL of HCl and 2 g of CuCl2, the concentration of H2S in the purified gas remained below 20 μL/L for a duration of nearly 70 min. When the amount of CuCl2was increased to 4 g, at the same HCl concentration, this time increased dramatically to more than 115 min. The sulfur capacity of this system was found to be 9.81 g/L, which is much higher than that reported for ionic liquid wet oxidation desulfurization[30].However, with a further increase in the amount of CuCl2to 6 g, although the CuCl2remained soluble in the DMF and prolonged the duration of desulfurization to 145 min,this was a much less significant increase. This is because the addition of the salt gradually increased the viscosity of the solution, hindering the transfer and absorption of gas and liquid. The use of additional CuCl2would also increase production costs.
As shown in Figure 6(a), for the desulfurization agent containing 0.5 mL HCl and 8 g CuCl2, a black precipitate was formed during the reaction, and, even after the injection of oxygen into the system at a flow rate of 100 mL/min for oxidative regeneration, this precipitate did not dissolve. Figure 6(b) shows the yellow precipitate formed by the desulfurization agent containing 0.5 mL of HCl and 4 g of CuCl2. The generation of these different precipitates by the two agents suggests that the desulfurization reaction mechanism changes when the amount of CuCl2is increased from 4 g to 8 g. It also seems that the products cannot be regenerated when the larger amount of CuCl2is used. Therefore, although increasing the concentration of CuCl2can enhance the efficiency of the desulfurization agent, it prevents its regeneration. In conclusion, the optimal dosage of HCl is 0.5 mL, and the recommended dosage of CuCl2is 4 g.
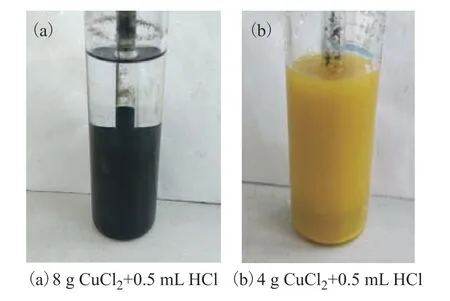
Figure 6 Precipitates formed during desulfurization reaction for different agents
From the above experimental results, we believe that Cu2+is the key component ensuring the efficient absorption of H2S. Although the addition of HCl improves the effectiveness of desulfurization, excessive amounts increase the acidity of the solution, which is detrimental to the dissolution and removal of H2S.
To investigate the impact of temperature on desulfurization efficiency, we conducted measurements of the desulfurization efficiency of the Cu/HCl-DMF agent at different temperatures. The changes in H2S concentration in the purified gas over time are depicted in Figure 5(c). The purified gas maintained a consistent level of purity for 120 min at a temperature of 20 °C.The corresponding times at 40 °C and 60 °C were both 115 min. It can be seen that the desulfurization performance was better at the lowest temperature within the range of temperatures tested in the experiment. With increasing temperature, the desulfurization performance decreased slightly, but remained consistent. According to thermogravimetric analysis, the decrease in performance was caused by loss of components during desulfurization as the temperature increased. Above 40 °C, evaporation of DMF led to a decrease in the absorption of H2S. However,the main component of desulfurization, Cu2+, was not lost.Therefore, the desulfurizing effect did not decline further when the temperature was increased.
Figure 5(d) illustrates the time variation in the concentration of H2S in the purified gas at various gas flow rates. With increasing gas flow rate, the absorption capacity of the desulfurization agent gradually decreases.This is because an excessive gas flow rate shortens the time available for the interaction between the active component of the desulfurization agent and the H2S. The sulfur capacities of the desulfurization agent were 10.24 g/L, 9.81 g/L, and 9.39 g/L for gas flow rates of 20 mL/min, 40 mL/min, and 60 mL/min, respectively, and thus it can be seen that the decline in capacity with increasing flow rate is slow.
3.3.2 Study of regeneration performance
Good regeneration performance of a desulfurization agent can reduce the time required for regeneration,increase the efficiency of solution circulation, and decrease energy consumption and solution loading. To investigate regeneration performance of a CuCl2/HCl–DMF solution that had already absorbed H2S, 0.5 g of FeCl3was added to it, and oxygen was then injected at a flow rate of 100 mL/min for oxidative regeneration.Changes in oxidation-reduction potential (ORP) were used to assess the effectiveness of regeneration of the desulfurization agent. These changes were measured and recorded as a function of oxygen regeneration time. As can be seen from Figure 7, the initial ORP of the solution was 187 mV. After continuous oxygenation for 2 h, the ORP had risen to 490 mV. This shows that most H2S can be oxidized to S within 2 h under appropriate conditions,enabling complete regeneration. Over time, the color of the solution gradually changed from a cloudy orange to a clear dark green. A yellow precipitate was also formed,indicating the generation of sulfur.
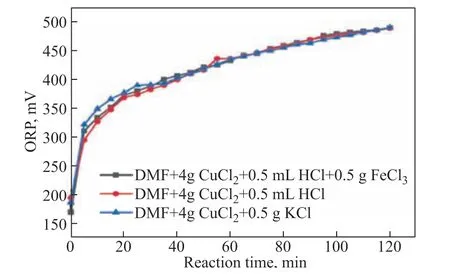
Figure 7 Variation of ORP with time during the oxidative regeneration of different formulations of desulfurization agent
In addition, surprisingly, we found that the desulfurization agent could be regenerated without the use of FeCl3.Instead, we achieved regeneration by directly feeding 100 mL/min of oxygen into a bubble reactor containing the H2S-rich desulfurization mixture. As illustrated in Figure 7, even when Fe3+was not introduced for catalytic regeneration, the ORP of the desulfurization agent still recovered to the same level as in the presence of FeCl3and within the same reaction time.
We believe that the presence of additional Cl-in the desulfurization agent plays an important role in the regeneration process. With the aim of validating this hypothesis, a small amount of KCl was chosen as a substitute for HCl to create a Cu/KCl–DMF desulfurization agent for the desulfurization/regeneration experiment. It should be noted that KCl is almost insoluble in DMF, and therefore, after the addition of 0.5 g of KCl, the mixture was heated to 40 °C to ensure complete dissolution. The excess insoluble KCl was filtered out to obtain the Cu/KCl-DMF desulfurization agent. As can be seen in Figure 7, when HCl was replaced with KCl, the ORP of the desulfurization agent still recovered to the same level after oxidative regeneration,thus confirming our hypothesis.
XPS and XRD analyses were conducted to investigate the nature of the desulfurization product. We obtained a yellow solid product immediately after the desulfurization experiment, but the analysis revealed that it was distinct from the product obtained during the regeneration process.
Figure 8 shows XPS spectra of the intermediate desulfurization product. The full spectrum reveals the presence of elements including Cu, Cl, S, N, O, and C.Specifically, the presence of C, N, and O elements can be attributed to the inclusion of a certain amount of DMF in the product. Spectral analyses of Cu and Cl indicate the presence of Cu2+and Cl-ions in the product. A split-peak fit to the single-scan Cl spectrum reveals that the peaks at 198.22 eV and 199.82 eV can be attributed to the 2p3/2and 2p1/2orbitals of Cl-, and a split-peak fit to the singlescan Cu spectrum reveals that the peaks at 935.01 eV and 954.86 eV can be attributed to the 2p3/2and 2p1/2orbitals of Cu2+. The presence of a higher-binding-energy shoulder and a faint satellite at approximately 943 eV provides evidence that the main component is Cu2+[31]. According to the literature[32], the maximum binding energy of the Cu2+2p3/2orbital in CuCl2is 934.7 eV, which is comparable to the maximum binding energy of the Cu2+2p3/2orbital in our spectrum. However, the maximum binding energy shown in Figure 8 is greater than the value reported in the literature. This suggests that as well as binding to Cl-, Cu2+may also bind to other electronegative electron donor elements, thereby increasing the maximum binding energy. The S spectrum indicates the presence of a certain amount of sulfur in the product. The single-scan S spectrum shows split peaks at 163.44 eV and 164.44 eV. According to the literature[33-35], values of 162 eV, 164 eV, 167 eV, and 169 eV for the maximum binding energy of the S 2p3/2orbital correspond to Cu–S, –SH, –SO2-,and –SO3-, respectively. From the S spectrum, it can be observed that the maximum binding energy of the 2p3/2orbital is approximately 164 eV, indicating the presence of the –SH structure. Therefore, the double peaks in the spectrum can be attributed to the 2p3/2and 2p1/2orbitals of –SH. However, the binding energy value in this spectrum is lower than that in the literature, indicating the formation of a chemical bond between S and Cu, which decreases the maximum binding energy.
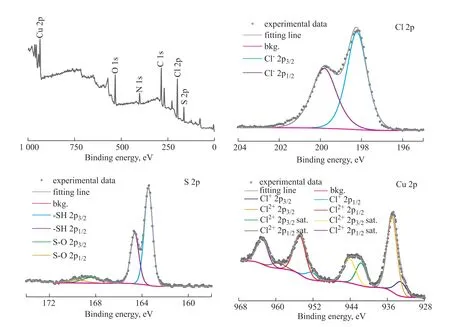
Figure 8 XPS spectra of intermediate desulfurization product
The XPS spectra shows that Cu2+and H2S form specific combinations upon the absorption of H2S. To determine the binding state, the intermediate desulfurization product was analyzed using XRD. Figure 9(a) shows the XRD spectrum of the intermediate desulfurization product. From a comparison with the standard copper sulfide pattern (PDF#06-0464), it can be seen that the characteristic peak corresponding to CuS is absent. This suggests that the intermediate desulfurization product does not contain any CuS component. The above results suggest that Cu2+and HS-are present in the product and are combined through a Cu–S coordination bond. At the same time, DMF and Cl-also coordinate with Cu2+to form a multiligand complex, thereby facilitating the efficient absorption of H2S.
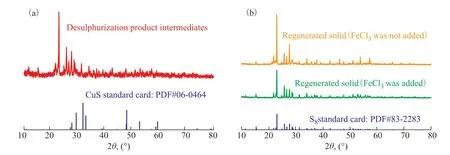
Figure 9 XRD spectra of (a) intermediate desulfurization product and (b) regenerated product
Figure 9(b) shows XRD spectra of regenerated products.Both the XRD pattern of the desulfurized product regenerated with the addition of FeCl3and the pattern of the product directly regenerated without FeCl3are consistent with the sulfur standard pattern (PDF#83-2283). This indicates that sulfur can be obtained through regeneration. Furthermore, it can be seen that the quality of the sulfur products is relatively high.
Table 2 shows the results of five repeated tests on Cu/HCl-DMF desulfurization and regeneration. Regeneration was performed without using FeCl3, and instead 100 mL/min of oxygen was fed into a bubble reactor containing the H2S-rich desulfurization solution. In the five repeated desulfurization/regeneration tests, the duration of the desulfurization process required to maintain a high level of gas purification remained relatively consistent,averaging around 105 min and without any significant fluctuations or a noticeable downward trend. The sulfur capacity was calculated to reach as high as 8.96 g/L for static bubble desulfurization under atmospheric pressure.This indicates that the sulfur capacity remains above 91% of its initial value. Thus, the organic solvent-based desulfurization system proposed here has sufficient capability of regeneration and stability to allow its reuse.
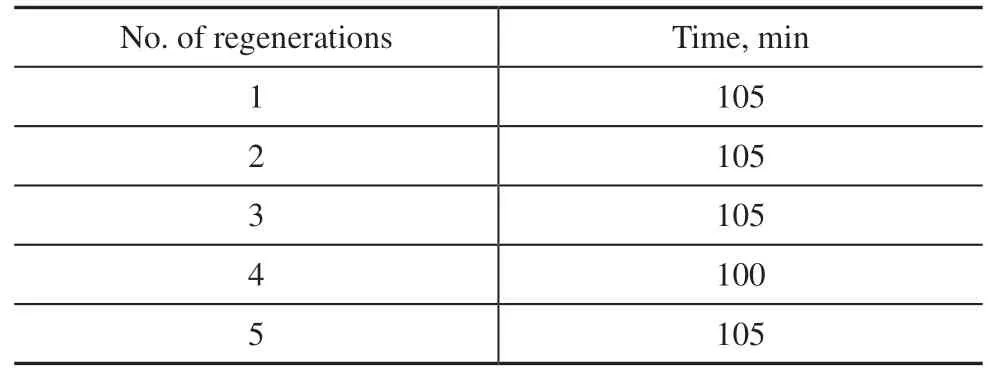
Table 2 Duration of repeated desulfurization procedures
3.4 Desulfurization mechanism
On the basis of the above experimental observations and characterization, a likely mechanism of Cu/HCl-DMF desulfurization and regeneration is proposed. Figure 10 presents a diagram of this mechanism. As a polar, aprotic solvent, DMF can dissolve large amounts of CuCl2,leading to the formation of sterically hindered complexes of CuCl2or Cu2+with DMF and Cl-, as shown in Step (1).In Step (2), H2S molecules dissolve in the liquid phase of the desulfurization agent and ionize to HS-. Because the coordination bonding between S and Cu is stronger,it replaces the coordination bonding between C=O and Cu originally formed in the DMF. This leads to the rapid formation of a more stable [Cu(DMF)n–p(HS–)p(Cl–)m](2–p–m)+coordination structure in the solution. In Step (3),this complex is subjected to rapid oxidation by oxygen,resulting in the production of sulfur. Cu2+is released and is then converted back into the soluble complex with DMF and Cl–. This process continues until all absorbed H2S has been completely converted into sulfur during the desulfurization/regeneration process.
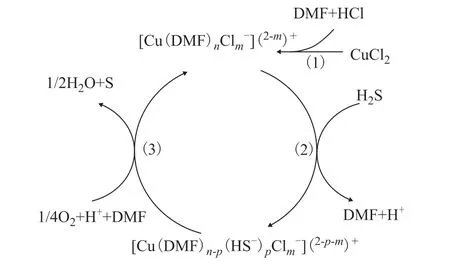
Figure 10 Suggested catalytic cycle in the non-aqueous Cu/HCl-DMF system for conversion of H2S to S
4 Conclusions
A non-aqueous phase desulfurization agent was prepared using CuCl2, DMF, and HCl. It was found to have excellent characteristics of low viscosity. By optimizing the composition of the desulfurizing agent by adjusting the quantities of CuCl2and HCl, the most effective formulation was determined to be 40 mL of DMF, 4 g of CuCl2, and 0.5 mL of HCl. The effects of temperature and gas flow rate were investigated, and it was found that the desulfurization performance was minimally affected by temperature. A sulfur capacity of 9.81 g/L was achieved at a temperature of 40 °C and a gas flow rate of 40 mL/min. Complete regeneration of the desulfurization agent was achieved by adding FeCl3and continuously introducing oxygen at a gas flow rate of 100 mL/min for 2 h. However, it was also found that complete regeneration was possible without adding FeCl3, and Cl-was shown to be the primary component in the regeneration process.
FTIR spectroscopy indicated that a C=O–Cu structure was initially formed in the solution. According to the reaction mechanism inferred from XPS and XRD analysis of the desulfurization products, the efficient absorption of H2S by Cu/HCl–DMF depends on the rapid coordination of Cu2+with DMF, Cl-, and H2S molecules.This coordination results in the formation of [Cu(DMF)n-p(HS-)p(Cl-)m](2-p-m)+complexes. In this chemisorption process, Cu2+is the active component in the capture of H2S. In the regeneration of the desulfurization agent,oxygen rapidly oxidizes the complexes, with the production of sulfur. At the same time, Cu2+is released and converted back into a soluble form, which then complexes with DMF and Cl-. This process continues until all absorbed H2S has been completely converted into sulfur. After five repeated applications of the regeneration procedure, the sulfur capacity reached 91% of its initial capacity.
Compared with conventional ionic liquid and wet desulfurization systems, the agent on which this system based is cheaper and easier to synthesize. It also does not require purification, effectively reducing the overall cost.The system is therefore a promising adsorbent for H2S in high-sulfur natural gas environments.
Acknowledgements:This work was supported by the China National Science and Technology Major Project (2016ZX05017)and the Sinopec Group Corporation 2020 Science and Technology Project “Organic Sulfur Catalytic Hydrolysis Technology Improves Quality Research” (No. 120049-1).
杂志排行
中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics
