Synthesis of Sustainable Sulfur-Rich Copolymers as Mercury Sorbents at 130 °C Using Tung Oil as an Activator
2023-10-23LyuYaZhangSai
Lyu Ya; Zhang Sai
(School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China)
Abstract: Sulfur-rich copolymers made through inverse vulcanization exhibit a wide range of potentially valuable applications, for example as adsorbents to capture mercury pollution. Among the diverse second monomers of the copolymers, vegetable oil is a renewable resource, and recycled cooking oils have an important role in saving natural products. However, they need relatively high temperatures (160–180 °C) to react with sulfur. To develop a low-temperature(130 °C) reaction process for non-conjugated vegetable oil, we incorporate a small amount of tung oil, which contains conjugated trienes that can produce highly active free radicals during reactions. A variety of analytical techniques(proton nuclear magnetic resonance and Fourier-transform infrared spectroscopy, differential scanning calorimetry,thermogravimetric analysis, X-ray diffraction, and dynamic mechanical analysis) are used to characterize the chemical structures and physical properties of the copolymers. The addition of tung oil is found to significantly improve the thermal stability and mechanical properties of the copolymers. We also investigate the effect of different ratios of raw materials on the gel time, free sulfur content, glass transition temperature Tg, and degradation temperature of the copolymers. We find that increasing the amount of tung oil in the raw material mixture decreases the gel time and free sulfur content, but increases Tg and the degradation temperature. The copolymers exhibit a high adsorption capacity for mercury ions up to 33 mg Hg2+ per gram of adsorbent. These results demonstrate the feasibility of using sulfur-rich copolymers as effective mercury removal adsorbents, with the potential for further improvement by foaming the copolymers into porous materials.
Key words: sulfur; vegetable oil; polymer; mercury; reaction mechanism
1 Introduction
The importance of natural gas with regard to the need to reduce greenhouse gas emissions and optimize energy resources cannot be overstated. However, the risk of mercury contamination of natural gas increases as exploration and production move into deeper zones.This poses a significant challenge for the industry, since mercury can lead to catalyst poisoning, equipment corrosion, and mercury embrittlement, which can cause catastrophic incidents in liquid natural gas plants that use aluminum cryogenic heat exchangers[1]. To eliminate these risks, mercury removal systems are employed,including those based on physical adsorption, chemical adsorption, and reactive absorption. However, the most commonly used mercury adsorbents, such as activated carbons and metal-loading zeolites, are expensive[2-3].Therefore, there is a need to investigate cost-effective and recycled adsorbents for mercury removal.
The reaction between mercury and sulfur is well established. Hsi et al.[2]demonstrated that the deposition of sulfur onto the surface of an adsorbent material can significantly increase mercury adsorption capacity.Elemental sulfur is abundant, oversupplied, and inexpensive[4-5], making it an attractive option for use in mercury removal materials, in alignment with the principles of green chemistry[6]. Recently, sulfur-rich copolymers synthesized via inverse vulcanization[7]have been explored as mercury adsorbents. The second monomers used in this process include benzoxazines[8-9],divinyl benzene[10], diisopropenylbenzene[11-12],farnesene[13], dicyclopentadiene[14], polyethylenimine/1,4-diethynylbenzene[15], and plant oils[16-18], such as canola oil,sunflower oil (SFO)[19], and tung oil (TO)[20]. However,some of these vegetable oils require high reaction temperatures of up to 180–200 °C, which can result in the formation of toxic hydrogen sulfide, hazardous autoacceleration, and increased energy costs[21].TO, which contains a unique conjugated triene, can react with elemental sulfur at a lower temperature of 130 °C,just above the melting point of sulfur[20]. However, TO is a relatively scarce natural resource[22]. Non-conjugated vegetable oils, such as canola oil and SFO, are widely used and are similar in fatty acid constitution to recycled cooking oils from the food industry. In the work described in this article, by combining elemental sulfur with mixed oils with different proportions of SFO and TO, we synthesize sulfur-rich copolymers, using for activation inverse vulcanization at a temperature of 130 °C. The resulting material is a rubber that captures mercury ions in an aqueous phase, which aligns with the principles of green chemistry and provides a solution to environmental problems associated with mercury.
2 Experimental
2.1 Materials
Elemental sulfur (reagent grade, 99.9%) was used as received from Alfa Aesar. TO was purchased from Shandong Lucheng chemicals Ltd. SFO was purchased from Shanghai Rongs. High-oleic-acid oil (HOO) was kindly provided by Croda China Trading Co. (Shanghai).Iodine standard titration solution was provided by Quanzhou YiDa Technology. Anhydrous sodium sulfite,acetic acid, starch solution, and all solvents were obtained from Aladdin Biochemical Technology and were used as received. All these compounds were of reagent grade,except for the natural vegetable oils. The fatty acid compositions of SFO, TO, and HOO are listed in Table 1, from which it can be seen that linoleic acid is the dominant fatty acid in SFO, eleostearic acid in TO, and oleic acid in HOO.
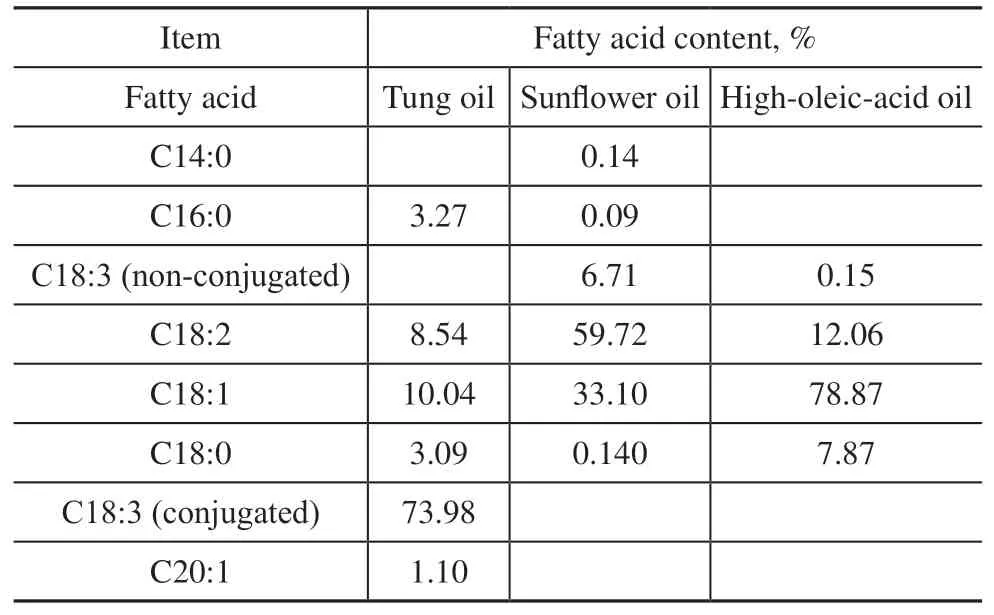
Table 1 Composition and distribution of fatty acids in triglyceride oils
2.2 Preparation of sulfur-rich copolymers based on vegetable oils
In a typical procedure, the synthesis of sulfur-rich copolymers involved mixing 2 g of vegetable oil and 2 g of elemental sulfur in a 20 mL glass vial with a magnetic stirrer bar. The mixture was stirred vigorously in an oil bath at 130 ℃ to obtain a light yellow emulsion. The color of the emulsion changed from yellow to deep brown as the reaction proceeded. Gel time was recorded when the stirrer bar stopped owing to the extremely viscous paste that was formed. A series of sulfur-rich copolymers with various proportions of TO in mixed oils were synthesized and named S50-TOx-VO, wherexrepresents the weight percent of TO used in the feed oil and VO stands for “vegetable oil.” The reproducibility of the gel times was verified by repeating the experiments,with errors of 3%–5% due to the heating rate and stirring intensity of the instrument. The accuracy of the bath temperature was crucial for the reproducibility of the gel times.
2.3 Measurements
The samples were characterized using various techniques.Proton nuclear magnetic resonance (1H-NMR) spectra were obtained using a Bruker Ascend 400 spectrometer with an average number of transients of 64. The samples were dissolved in deuterated chloroform(CDCl3; purchased from Shanghai Macklin Biochemical Technology Co., Ltd.). Fourier-transform infrared(FTIR) spectra were recorded using a Thermo Nicolet IR 200 spectrometer at a resolution of 4 cm-1. Sulfurrich copolymers were pressed into a KBr pellet, and TO was prepared as a thin film on a KBr pellet. Differential scanning calorimetry (DSC) was performed using a TA Instruments Model 2920 differential scanning calorimeter,with a heating rate of 10 ℃/min and a nitrogen flow rate of 60 mL/min. Thermogravimetric analysis (TGA) was performed on a TA Instruments TGA-Q4000 apparatus from 50 °C to 800 °C at a heating rate of 20 °C/min under a nitrogen atmosphere. The samples were crushed into 100-mesh granules and mixed thoroughly before DSC and TGA. X-ray diffraction (XRD) and dynamic mechanical analysis (DMA) were carried out using a Bruker D8-Advance X-ray polycrystalline diffractometer and a TA Instruments Model DMA 2980, respectively.The storage modulusE' and tanδwere determined by sweeping the temperature from -40 °C to 140 °C at a rate of 5 °C/min and a frequency of 1 Hz in the bending mode with an amplitude of 5 mm. Heat-pressed samples with dimensions of 60 mm × 12 mm × 3 mm were used for XRD and DMA measurements.
3 Results and Discussion
3.1 Preparation of sulfur-rich copolymers using sunflower oil and tung oil
To prepare sulfur-rich copolymers, we used mixtures of SFO or HOO and TO at various ratios as raw materials.Table 2 shows the gel times for each mixture. It was observed that SFO and HOO did not reach the gel state even after 8 h of reaction at 130 °C. At the end of the reaction, the products of SFO and HOO were black viscous slurries with sulfur crystals precipitating on cooling. However, when TO was added, the gel time decreased significantly. In the case of SFO, only 5% TO was needed to activate polymerization, whereas 10%TO was required for HOO. The resulting products were brown pastes from which sulfur did not separate at room temperature (Figure 1). These pastes were soft and could be easily transferred to a hot-press machine for shaping and curing. On curing at 140 °C, they turned into black rubber solids.

Figure 1 Reaction phenomena of mixed vegetable oils reacting with elemental sulfur
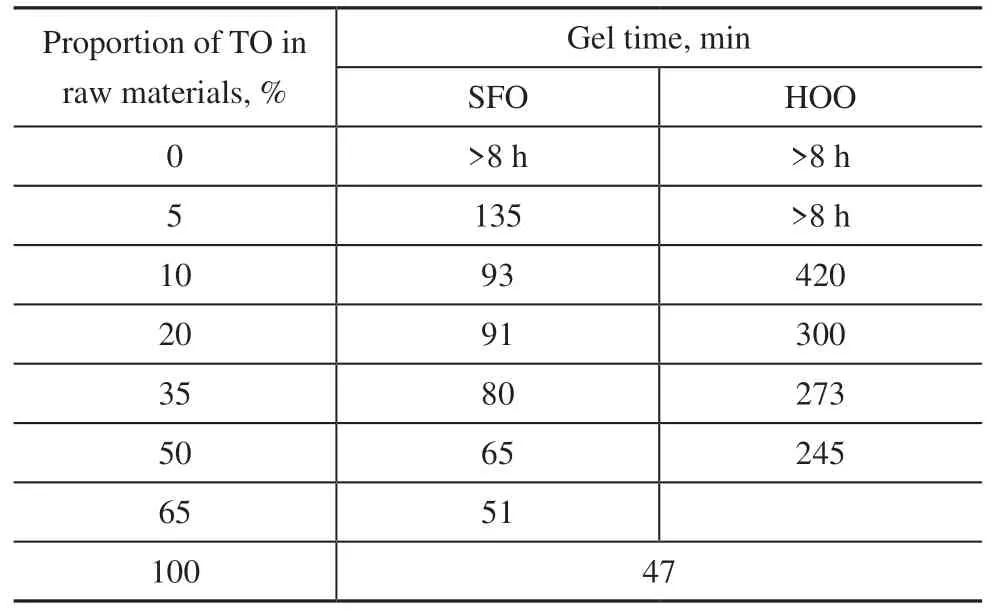
Table 2 Gel times of sulfur copolymerization with TO,SFO, and HOO
For a given amount of TO in the mixture, TO-SFO combinations required less time to reach the gel state than TO-HOO combinations. This difference in gel time could be attributed to the difference in the content of polyunsaturated fatty acids, including linoleic acid and linolenic acid. SFO contains a larger proportion of these acids (66.43%) compared with HOO (12.21%), which contains mainly oleic acid (78.87%). The non-conjugated polyunsaturated fatty acids in SFO can be attacked by free radicals through hydrogen abstraction, with the formation of conjugated double bonds. These free radicals L· are produced from the conjugated trienes in TO and exhibit high reactivity. They also react with cyclooctasulfur to produce LS8·, addition of which to the conjugated double bonds results in a cross-linked structure[23-25].Without TO, the system has low free radical content and activity, which makes it difficult for effective hydrogen abstraction to take place. Consequently, the nonconjugated double bonds in SFO cannot be converted into conjugated systems, or, at most, only a small portion can be converted. This conversion can also occur at higher temperatures, possibly 180 °C. Therefore, an oil such as SFO that contains only non-conjugated unsaturated fatty acids needs high temperatures to react with elemental sulfur. A small amount of TO with its unique triene constituents allows non-conjugated vegetable oils to be copolymerized with elemental sulfur at low temperatures.At the same time, non-conjugated polyunsaturated fatty acids play an indispensable role in the reaction of sulfur and vegetable oils too.
3.2 Structural analysis of copolymers by 1H-NMR and FTIR
Because of the importance of polyunsaturated fatty acids in the copolymerization, and the similarity of the fatty acid distribution of SFO to that of recycled cooking oil, copolymers from SFO-TO mixtures were investigated. The1H-NMR spectra of the copolymers formed by polymerizing different ratios of TO and SFO mixed with sulfur are presented in Figure 2. All peaks in the spectra are weak compared with the reference tetramethylsilane (TMS) peak, indicating that the samples have low solubility in deuterated chloroform and crosslinked structures. Comparison of the NMR spectra of the copolymers with those of TO and SFO shows that the peaks in the 5.00–6.50 range decrease and almost disappear, suggesting that most of the double bonds have been saturated and have reacted with sulfur. However,a small amount of unreacted C=C remains. Zooming in on the 1.70–2.82 range, peaks between 1.90 and 2.25 are observed, which represent the protons in allyl groups, also decreased in intensity but still visible, indicating that a small fraction of –C=C– bonds have remained unreacted.The weak peaks at 2.70–2.80, which correspond to the protons in –CH2- groups between two non-conjugated–C=C– bonds, also decrease, demonstrating that some non-conjugated double bonds have been converted to conjugated or saturated ones. Additionally, the newly occurring peaks between 1.31 and 1.61 are assigned to protons in the –CHS group, suggesting that C–S bonds have been generated during the reaction.
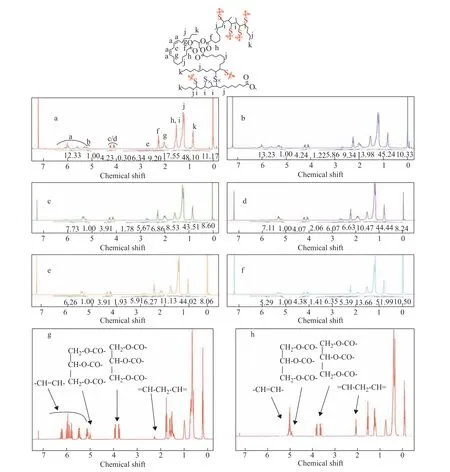
Figure 2 1H-NMR spectra of copolymers
Figure 3 displays the FTIR spectra of the TO–SFO–sulfur copolymers at different TO ratios. The vibrational absorptions at 1640 cm-1for –C=C– and 3018 cm-1for vinylic C–H in TO are used to identify changes after the reaction. The absorption peaks at these two sites are dramatically reduced in the spectra of the polymers,indicating that addition of sulfur to the double bonds has been achieved. Furthermore, the peaks at 1740 cm-1and 3465 cm-1are attributed to the stretching of C=O and O–H bonds in the ester groups of triglycerides. An absorption band at 467 cm-1, attributed to S–S stretching,appears in the product spectra, which is another indicator of the successful conversion[18,26-27]. Thus, both1H-NMR and FTIR have shown that most of the double bonds in the vegetable oils have been saturated, reacted with elemental sulfur, and formed C–S bonds.
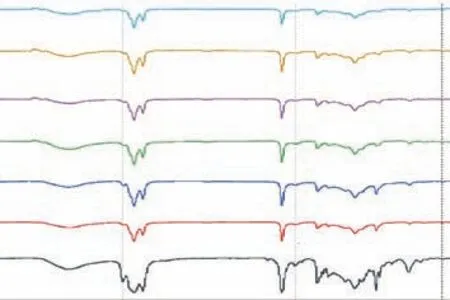
Figure 3 FTIR spectra of copolymers
3.3 Measurements of free sulfur content in copolymers
To examine the microstructure of the samples, XRD scanning of the copolymers was performed. Figure 4 shows the XRD patterns of sulfur-rich copolymers with varying ratios of TO, which are compared with the S8standard pattern[18](bottom). The peaks detected at 2θ= 23°, 31°, and 44° in all samples coincide with those of sulfur, confirming the presence of free sulfur in the samples. Additionally, a low and broad band between 2θ= 15° and 30° is observed in each pattern, indicating the presence of non-crystalline amorphous structures in the samples[28-30]. The S50-TO20-SFO copolymer has a sharp peak at 2θ= 16°, which is presumed to be attributable to crystallization of Sα[31]. The crystallinity of the samples was calculated to obtain the free sulfur contents[32], which are shown in Table 3.
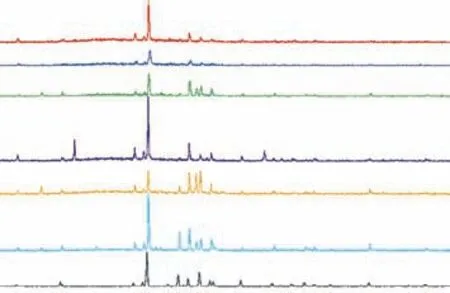
Figure 4 XRD patterns of copolymers
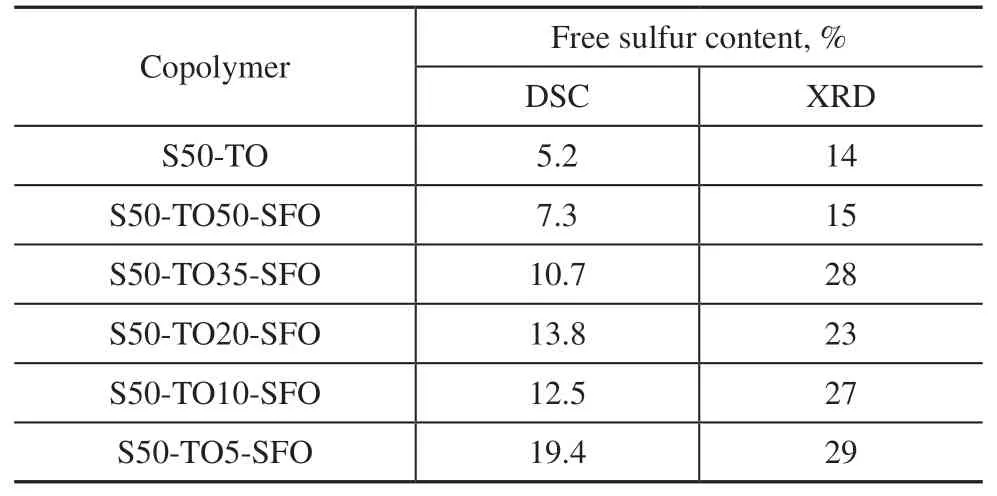
Table 3 Free sulfur contents of copolymers synthesized using mixed oil
The DSC curve of a copolymer can also reflect its content of free sulfur[19]. DSC curves of the copolymers were obtained, as shown in Figure 5. A melting peak at 119 °C is observed in each curve, attributable to melting of Sβ[33-34].The curves of S50-TO5-SFO and S50-TO10-SFO also show peaks around 108 °C, indicating the endothermic behavior of Sαin these two samples. The DSC curves suggest that the free sulfur in the sulfur-rich copolymers is mainly Sβ, with a minor presence of Sα. The stable form of sulfur at STP is orthorhombic sulfur Sα, which converts to monoclinic sulfur Sβat 95 °C. During the reaction,unreacted sulfur in tiny particles is dispersed in the sticky products and becomes Sβupon cooling without converting to Sα. However, when a copolymer contains a significant amount of free sulfur, a small fraction is in the form of Sα, probably because microcrystals of sulfur can approach one another to form a larger crystal that is more likely to convert into Sα.
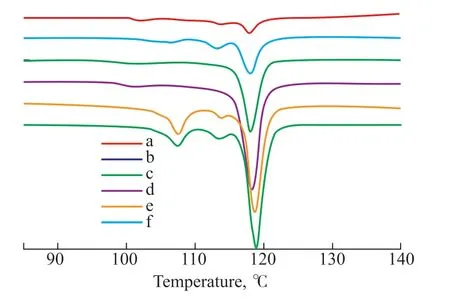
Figure 5 DSC curves of copolymers
The areas under the melting peaks can be used to calculate the free sulfur contents of the copolymers, as listed in Table 3. S50-TO has the lowest free sulfur content among the six samples, and S50-TO5-SFO has the highest. The free sulfur contents calculated by DSC are smaller than those from the XRD results. The free sulfur content decreases with increasing TO content, except for the sample S50-TO20-SFO. Adding 5% TO the raw materials significantly shortened the gel time but left a considerable amount of sulfur unreacted. However, when the raw material contained 50% TO, the unreacted sulfur content was close to that in pure TO, and the reaction degree was significantly increased.The use of activators in inverse vulcanization, such as TO in this study, has been observed in other studies[17,20]to shorten gel times, but not reduce the amount of unreacted sulfur. One possible explanation is that these activators effectively catalyze or promote crosslinking, but have little effect on the reaction between sulfur and the second monomers. However, this is not to deny the role of activators in catalyzing the reaction. In the absence of an activator, the product of sulfur and SFO remains a viscous liquid at 130 °C, even after an extended reaction time. On cooling, the unreacted sulfur crystallizes and precipitates, causing phase separation. Such a product has no practical application. By contrast, activators in inverse vulcanization can achieve a gel state in a short period of time, resulting in a uniform dispersion of sulfur in rubber-like copolymers. Unreacted sulfur then has the opportunity to react with the raw material again during the curing or molding process. Alternatively, micrometersized sulfur particles can be embedded in a crosslinking polymeric network to produce composite materials with promising applications[26,35], such as mercury absorbents. In this way, plentifully supplied SFO or ecofriendly recycled oil might be used to produce highvalue materials. In other work[24], we have observed that the conjugated system in TO can form LS8· free radicals with S8at lower temperatures. These free radicals can further react with non-conjugated double bonds. As the structure of natural lipids consists of three fatty acids attached to glycerol, these sulfur radicals intermolecularly and intramolecularly connect the fatty acids, which is the mechanism of the activation crosslinking reaction.
3.4 Properties of sulfur-rich copolymers
Before applications are found for the copolymers, their properties should be studied. The thermal stability of the sulfur-rich copolymers obtained from TO–SFO mixed oils was investigated using TGA. Figure 6 shows the TGA curves, and the degradation temperatures and char yield results are summarized in Table 4. Two degradation stages can be observed in each curve. The first stage occurs between 215 °C and 229 °C, and the second stage between 330 °C and 340 °C. The first stage corresponds to the decomposition of elemental sulfur and polysulfide components. The mass loss in the second stage is attributed to pyrolysis of the crosslinking structures in the remaining polymer and of the triglycerides, and to the fracture of C-S bonds formed in the first stage[16,19,36]. The first-stage temperatures and char yields are significantly higher than those of the copolymers synthesized using canola oil,[19]myrcene, farnesol, or farnesene[13]. The char yield value at 800 ℃ for the pure TO-sulfur copolymer is 24%, which is higher than that of the mixed oil samples.When the proportion of SFO in the initial oils is higher than 65%, the char yields stabilize at around 18%. The copolymer has a higher initial decomposition temperature,indicating that the conjugated structure of TO makes the synthesized copolymer more highly crosslinked and more structurally stable. A high initial decomposition temperature is significant for improving the thermal stability of materials, and in this case it confirms the formation of polymer structures with high thermal stability.
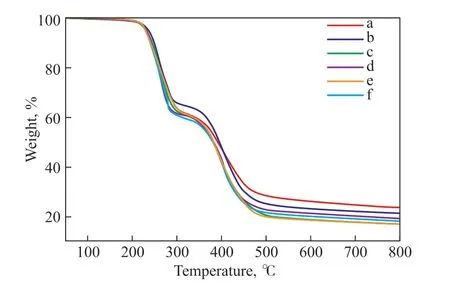
Figure 6 TGA of copolymers
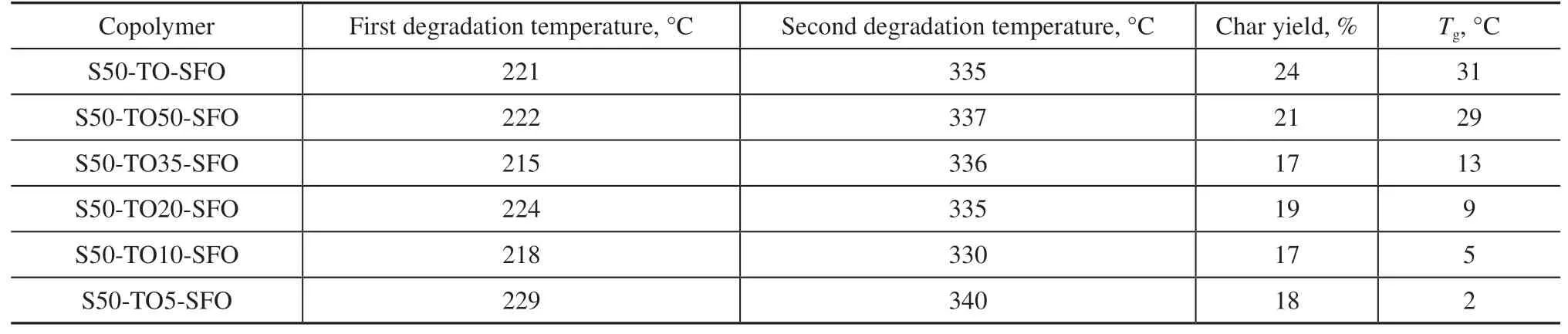
Table 4 Degradation temperatures, char yields, and glass transition temperatures Tg for sulfur-rich copolymers
The dependence of polymer mechanical properties on temperature reflects the role of molecular motion in determining these mechanical properties. Since the mechanical properties of a polymer are macroscopic manifestations of its various molecular motions, the influence of temperature on molecular motion is selfevident. Dynamic mechanical analysis (DMA) is a dynamic thermomechanical method used to study the relaxation behavior of substances subjected to alternating loads or vibrational forces under programmed heating conditions[37]. Although DSC can be used to determine the glass transition temperatureTgof a copolymer, the temperature range from glass to rubber shown on the DSC curve is not distinct. Therefore, DMA testing forTgis more accurate, which is why we chose to use it to determine theTgof the copolymers. The dynamic thermomechanical curve shown in Figure 7 was obtained by measuring the storage modulusE', loss modulusE'', and tanδvalues of the samples at each temperature under programmed temperature control conditions. It can be seen that the storage modulusE' of all the samples drops in a stepped pattern. At low temperatures, theE'of the sulfur-rich copolymers ranges between 1 GPa and 1.5 GPa. Since sulfur-rich copolymers are rubber-like materials at room temperature, the tanδvalue corresponds toTg. TheTgof the pure TO copolymer is 31 °C. TheTgof each material decreases as the TO content decreases.TheTgof the SFO-dominated S50-TO5-SFO copolymer is only 2 °C. A decrease inTgindicates that the relative mobility between molecules is increased, and the degree of crosslinking between them is reduced.
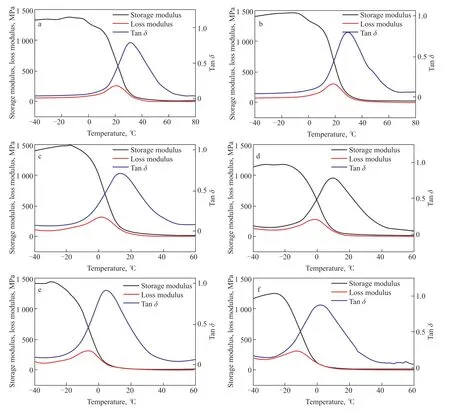
Figure 7 DMA of copolymers
The trend of decreasingTgand degree of crosslinking is related to the TO content of the raw material. Our previous work[24]and that of other researchers[21,38]haveshown that there are two reaction mechanisms involved in inverse vulcanization: a free radical mechanism and an anion mechanism. Conjugated trienes help form and stabilize sulfur chain radicals at lower temperatures,which contributes to the production of thiol radicals through hydrogen seizure reactions. These thiol radicals are then converted into anions for anionic mechanism reactions. Since sulfur chain radicals are biradicals, they can directly undergo addition reactions with conjugated trienes to form crosslinked products. The highTgand high degree of crosslinking of the pure TO copolymer indicate that the synthesis reaction is primarily based on the free radical mechanism. On the other hand, sulfur anions are more active and can react with non-conjugated double bonds. However, sulfur anions are prone to breaking into anions with a low number of sulfur atoms, which further enhances their activity[4,39,40], but this is not conducive to crosslinking. Therefore, the anionic mechanism is the prevailing reaction mechanism for the vulcanization of mixed oils with low TO content.
3.5 Mercury capture
To test the mercury capture capabilities of the copolymers, 100 mg of copolymer and 10 mL of HgCl2solution were added to a vial and mixed for 3 h at room temperature. The amounts of Hg2+in the original and treated solutions were measured using an inductively coupled plasma atomic emission spectrometer, with the results shown in Figure 8. For the 3.87 mg/L solution,the copolymer was able to adsorb at least 97% of Hg2+,and the adsorption capacity of the individual copolymer samples was very similar. To further test the adsorption capacity, a 1000 mg/L solution of Hg2+was used, and it was found that the addition of 0.1% copolymer removed between 15% and 33% of mercury ions. This indicates that the copolymer has an adsorption capacity of up to 33 mg Hg2+per gram of adsorbent, demonstrating the feasibility of sulfur-rich copolymers as mercury removal adsorbents. However, it is important to note that the adsorption performance of existing materials needs improvement, as the material is crushed into small particles with a diameter of only 0.5 mm and has a limited surface area. To address this issue, our next step is to foam the sulfur-rich copolymers into porous materials to increase their adsorption capacity.
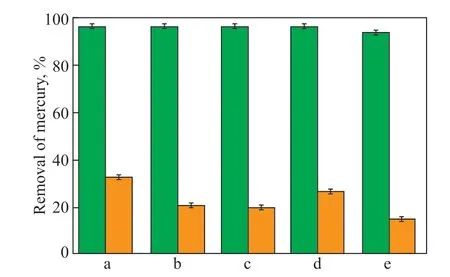
Figure 8 Removal of mercury
4 Conclusions
In this study, we synthesized sulfur-rich copolymers using tung oil and vegetable oil containing non-conjugated double bonds at 130 °C. The structures of the products were analyzed by1H-NMR and FTIR spectroscopy, the results of which indicated that most of the double bonds in the raw materials were saturated and that carbon–sulfur bonds were generated. Conjugated trienes in tung oil can produce highly active free radicals and activate nonconjugated double bonds. The copolymers exhibited good thermal stability, with high glass transition temperatures and degrees of crosslinking. We found that increasing the amount of tung oil in the raw material mixture decreased the gel time, but increased the degradation temperature,indicating that the synthesis reaction was based on both free radical and anion mechanisms. Additionally, we demonstrated the feasibility of sulfur-rich copolymers as mercury removal adsorbents, with an adsorption capacity of up to 33 mg Hg2+per gram of adsorbent. Our next step will be to foam the sulfur-rich copolymers into porous materials to increase their adsorption capacity.Overall, our study provides insights into the synthesis and applications of sulfur-rich copolymers, which could have potential applications in environmental remediation and other fields. The results of this study also highlight the importance of understanding the reaction mechanisms involved in copolymer synthesis,which can guide the optimization of reaction conditions and improvements in the performance of the resulting materials.
杂志排行
中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics
