The Role of Endoplasmic Reticulum Stress in Melanoma
2023-10-20HaoZeShiJianFangSunHaoChen
Hao-Ze Shi, Jian-Fang Sun*, Hao Chen*
Department of Pathology, Hospital for Skin Diseases (Institute of Dermatology), Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, Jiangsu 210042, China.
Abstract
Keywords: biological process, endoplasmic reticulum stress (ER stress), melanoma
Introduction
Endoplasmic reticulum (ER) stress is a critical process in skin physiology and pathology.Physiologically, ER stress can affect the fate of melanocytes and regulate lysosome biogenesis and keratinocyte differentiation to maintain epidermal homeostasis.1Pathologically, melanoma may be associated with ER stress.Moderate ER stress generally promotes cell proliferation and survival, whereas prolonged or severe ER stress often leads to irreversible cell apoptosis.2ER stress plays a vital role in the pathogenesis of melanoma, cell survival and death, metastasis,drug resistance, and other processes.We performed the literature searching from January 1, 2005, to September 30, 2020, and keywords related with (“ER stress”)OR(“tumor” and “ER stress”)OR (“melanoma” and “ER stress”) OR (“melanoma”and “ER stress” and “survival”)OR (“melanoma” and “ER stress” and “metastasis”)OR(“melanoma” and “ER stress” and “cell death”)OR(“melanoma” and “ER stress” and “drug resistance”)OR (“melanoma” and “ER stress” and “therapy”), and summarized current progress of ER stress and its specific role in melanoma.
Brief Network of ER Stress
The development of ER stress may be either intrinsic or extrinsic.Tumors are often characterized by a high metabolic rate and rapid growth, causing increased protein generation and resultant ER stress.Extrinsic stressors often include hypoxia, disturbances in temperature, acidosis, reactive oxygen species (ROS) production, and disturbances in calcium homeostasis.2All of these intrinsic and extrinsic conditions lead to an ER stress response characterized by misfolded proteins in the ER, and an unfolded protein response (UPR) can subsequently develop.Three sensors regulate the development of a UPR: protein kinase RNA-activated-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6(ATF6).These sensors can trigger various pathways.The responses of ER stress are generally categorized as adaptation, alarm, and apoptosis, and activation of different sensors can affect various processes and destinations.
PERK/elF2α Pathway
Two molecules interact with PERK at its two ends to maintain its stability: heat shock protein 90 and 78-kDa glucose-regulated protein (GRP78).Exacerbation of the ER stress condition can lead to the dissociation of GRP78, causing oligomerization, autophosphorylation, and activation of PERK.The activation of PERK can consequently lead to phosphorylation of eukaryotic translation initiation factor 2α (eIF2α),which is responsible for decreased protein translation.Activation of eIF2α can also activate ATF4 and ATF5.CCAAT/enhancer-binding protein homologous protein(CHOP; also known as DDIT3 and GADD153) and growth arrest and DNA damage-inducible protein 34(GADD34 or PPP1R15A) are well-known targets of ATF4.
CHOP has various roles in cell biology, especially in cell survival.Prolonged ER stress can cause the activation of CHOP, which generally interacts with Bcl-2 family members such as Bcl-2, Bax, and Bim to regulate mitochondrial apoptosis.CHOP can also interact with the p53 upregulated modulator of apoptosis, which can also determine cell fate.Furthermore, CHOP influences autophagy in multiple ways.In contrast, GADD34 benefits survival by dephosphorylating eIF2α.3
IRE1α/XBP1 Pathway
The two paralogs of IRE1αre IRE1α and IRE1β.IRE1α,which is encoded by ERN1, is more prevalent than IRE1β in cellular functions.Like PERK, the two ends of IRE1α are also connected to heat shock protein 90 and GRP78,which are used to maintain the inactivated status of IRE1α.Once activated by ER stress, IRE1α can serve as an endoribonuclease that converts the unspliced form of X-box binding protein 1 (uXBP1) into the spliced form(sXBP1) by targeting the pre-mRNA of X-box binding protein 1 (XBP1).sXBP1 is often involved in restoration of ER function.IRE1α can also affect various pathways;for instance, beclin 1-dominated autophagy can be influenced by c-Jun N-terminal kinase (JNK), which is the downstream molecule of IRE1α.4
Bcl-2 family members also have an impact on IRE1α.For instance, Bax and Bak can activate IRE1α, and Bcl-2 and Bcl-XL can attenuate this process.Combined with tumor necrosis factor receptor-associated factor 2 and apoptosis signal-regulating kinase 1 (ASK1), the phosphorylation of JNK can also influence the expression of some Bcl-2 family members.3
ATF6 Pathway
In the absence of ER stress, ATF6 is often combined with GRP78; however, this complex can also dissociate once confronted with ER stress.The ATF6 then translocates into the Golgi apparatus for processing.3,5The processed ATF6 can further move into the nucleus and serve as a transcriptional factor.The genes influenced by ATF6 are often related to the restoration of ER function.Furthermore, ATF6 can interact with PERK and IRE1α.For example, PERK and IRE1α have a positive effect on synthesis of ATF6, and ATF6 can facilitate the formation of sXBP1 by interacting with IRE1α.However, the specific function of the ATF6 pathway is more elusive than that of the PERK/eIF2α and IRE1α/XBP1 pathways, and some research has suggested that the function of ATF6 is not indispensable for the process of ER stress.6
ER Stress in Melanoma
ER stress plays a dual role in determining cell fate.Adaptation or alarm caused by moderate ER stress generally leads to tumor progression, whereas severe ER stress often induces an inhibitory effect on the tumor.
Cell Survival
In general, the pro-survival role of ER stress is often caused by the intrinsic condition of the tumor, and these intrinsic protective methods often emerge when the tumor is exposed to unfavorable conditions.
Because of the rapid progression of melanoma formation and development, the UPR is up-regulated, and ER stress subsequently develops.In the presence of ER stress, pro-survival phenomena that are beneficial to melanoma progression occur (part of this process is shown in Figure 1).Compared with normal tissues, the IRE1α-XBP1 branch of ER stress in melanoma is highly up-regulated, which can elevate the expression of interleukin 6,and the elevation of interleukin 6 can boost the proliferation of melanoma cellsviathe JAK/STAT3 pathway.7Other ER-related proteins such as GRP78 can also promote tumor progression and maintain homeostasis of melanoma.8Therefore, clinically, a high level of GRP78 generally indicates a poor prognosis in patients with melanoma.Moreover, an ER transmembrane protein called selenoprotein K, which is involved in ER stress and calcium pathways, can also facilitate the progression of melanoma.9Selenoprotein K can interact with DHHC6, an enzyme located on the ER membrane, forming a complex that can palmitoylate IPR3, which can, in turn, affect the level of calcium.9Autophagy activated by ER stress can also have a positive effect on melanoma progression.10-16Increases in autophagy and ER stress-related markers in BRAF-mutated melanoma cells indicate that melanoma cells may have become adapted to their microenvironment, enhancing their survival.For example, receptor(TNFRSF)-interacting protein kinase 1 (RIPK1)-induced autophagy can relieve severe ER stress induced by tunicamycin or thapsigargin and thus facilitate cell surviv-al.11The activation of the IRE1α-XBP1 axis caused by ER stress can influence heat shock factor 1.As a downstream molecule of heat shock factor 1, RIPK1 can modulate mitogen-activated protein kinase 8/9, which can dissociate the complex of beclin 1 and Bcl-2, leading to the process of autophagy.11Autophagy can inhibit the initial ER stress, forming a self-protective mechanism.
Cell Metastasis and Migration
Besides survival, adaptation to ER stress can also enhance tumor metastasis.For instance, two of three branches of the UPR (ATF6 and PERK) may increase the expression of fibroblast growth factors 1 and 2 and enhance cell migration17(Fig.1).This is further demonstrated by the association of ATF6 and PERK with poor survival of patients with melanoma.Moreover, Krüppel-like factor 4 can regulate the ER stress adaptation and promote melanoma cell metastasis, and this may be achieved by enhanced expression of the downstream molecule nucleobindin 218(Fig.1).In BRAFV600Emelanoma cell lines, phosphorylated eIF2α is generally localized in the nucleus, which can promote the ER stress response and melanoma metastasisviathe lysosomal and Wnt/ β-catenin pathways.19
Molecules such as mechano growth factor E peptide can not only inhibit melanoma cell invasion by affecting the expression of lysyl oxidase family members and matrix metalloproteinases, but such molecules can also induce ER stress and then increase the expression of CHOP, suppressing melanoma survival.20
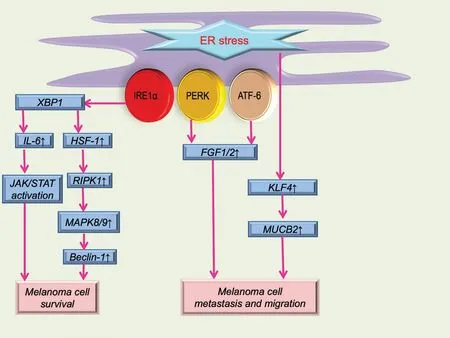
Figure 1.Schematic illustration of ER stress in melanoma cell survival, metastasis, and migration.ER stress can serve as a self-protective mechanism for melanoma.The IL-6/JAK/STAT pathway and autophagy-related pathway are the main methods of maintaining melanoma cell survival.Molecules such as FGF1 and 2, KLF4, and NUCB2 are mainly involved in melanoma cell metastasis and migration.ATF-6: activating transcription factor 6; ER: endoplasmic reticulum; FGF: fibroblast growth factor; HSF-1: heat shock factor 1; IRE1α: inositol-requiring enzyme 1α; IL-6: interleukin 6; KLF4: Krüppel-like factor 4; NUCB2: nucleobindin 2; MAPK: mitogen-activated protein kinase; PERK: protein kinase RNA-activated-like ER kinase; RIPK1: receptor (TNFRSF)-interacting protein kinase 1; XBP1: X-box binding protein 1.
Cell Death
Drugs and compounds associated with cell deathviaER stress are generally caused by chronic or severe ER stress and subsequent molecule activation.Multiple pathways related to cell death are triggered by ER stress and its subsequent molecule activation, including the IRE1α pathway, transcription factors such as CHOP, mitochon-drial Bcl-2 family members, proteases such as caspase family members, and other unexplored mechanisms.However,distinguishing these processes separately is difficult because of the diverse and intricate connections among these elements, as mentioned above.For instance, the IRE1α pathway can serve as the upstream of CHOP,mitochondrial Bcl-2 family members, and caspase family members, while the Bcl-2 family member Bax can also play a role in regulating IRE1α.21The downstream molecules of IRE1α, including JNK and p38 mitogen-activated protein kinase, can also affect the expression of the Bcl-2 family and CHOP.21Some members of the caspase family can be affected by mitochondrial dysfunction, which is correlated with Bcl-2 family caspase.Furthermore,overproduction of ROS can aggravate ER stress.22The production of ROS is generally influenced by calcium disturbances and mitochondrial function.23
CHOP often plays a vital or main role in melanoma inhibition caused by ER stress; the anti-melanoma effect of some drugs and chemicals generally leads to CHOP activation and its interaction with other ER stress pathways (Fig.2).Honokiol, vitamin E δ-tocotrienol (δ-TT),and 25-epi ritterostatin GN1N are all related to melanoma cell death caused by activation of CHOP induced by ER stress.24-26Honokiol can enhance ER stress and activate CHOP expression, then suppress melanoma growth by down-regulating melanocyte-inducing transcription factor (MITF) and β-catenin pathways.Additionally, MITF expression is correlated with CDK2 expression, thus determining the development of melanoma.MITF also regulates molecules (BCL2,I BIRC7)participating in processes such as cell survival and the cell cycle.25Inhibition of MITF often leads to the arrest of G0/G1 in melanoma cells and causes senescence.δ-TT is involved in melanoma inhibition, mainlyviathe process of ER stress.26δ-TT can elevate the levels of PERK and IRE1α, which are upstream of CHOP activation.26In addition, ER stress can facilitate calcium release from the ER, and elevation of calcium can activate calpain,which can cleave capase-4; pro-capase-4 can also be processed by the IRE1α branch.26Both activation of CHOP and capase-4 can mediate melanoma cell apoptosis.Furthermore, this inhibition process can be attenuated by ER stress inhibitors such as salubrinal.26The mechanism of 25-epi ritterostatin GN1N in inhibiting melanoma might involve CHOP elevation and GRP78 down-regulation, and the decrease of GRP78 can promote the increase of caspase 7.24Besides its ability to directly regulate cell death, CHOP can also increase isocitrate dehydrogenase 1 expression transcriptionally,which can decrease the expression of hypoxia-inducible factor 1α and indirectly facilitate hypoxia-induced apoptosis of melanoma cells.27
Several drugs and chemicals can also play a role in influencing the network among CHOP and other essential molecules associated with cell death (Fig.2).Pemetrexed or cisplatin can induce ER stress of choroidal melanoma cells with higher expression of IRE1α and CHOP,which can increase the expression of NOXA and result in mitochondrial apoptosis.28Panduratin A is involved in the network of mitochondrial apoptosis and ER stress.The CHOP elevation induced by the PERK/eIF2α/ATF4/CHOP pathway can decrease expression of Bcl-2 and increase the release of cytochrome c from the mitochondria, leading to activation of caspase 3, a mediator of cell apoptosis.29-30Xanthohumol can cause cell apoptosis by increasing the expression of CHOP, which is essential for ER stress-induced apoptosis.31The patient's physical condition can also have an impact on melanoma apoptosisviaCHOP expression (Fig.2).Hyperthermia can achieve a therapeutic effect on melanomaviaER stress.32For instance, low hyperthermia (43 °C) can activate the IRE1α and ATF6 branches, whereas high hyperthermia(45 °C) can lead to the activation of PERK.32
Other cell death-related molecules or pathways of ER stress can also contribute to the fate of melanoma.Pinocembrin can induce cell apoptosis by increasing caspases 4 and 12, which are downstream molecules of ER stress triggered by the IRE1α-XBP1 branch.16
ER stress, ROS generation, calcium disturbances, and mitochondrial dysfunction can also form an intricate network that affects the fate of melanoma23(Fig.2).Shikonin can elevate the ROS level, which can cause ER stress; the subsequent expression of phosphorylated eIF2α, CHOP, and cleaved caspase 3 can then inhibit the proliferation of melanoma.10Both resveratrol and phy-tochemicals are capable of inhibiting melanoma cells.33-34They can activate the ROS/p38/p53 pathway and ER stress pathways, which can lead to apoptosis by up-regulating Bax expression and down-regulating Bcl-2 expression, respectively.33-34Imiquimod can also form an intricate network including ER stress, the JNK and p38 pathways, and intracellular calcium and ROS disturbances.35Imiquimod can activate the PERK and IRE1α branches and the ASK1/JNK pathway.35These processes can increase the expression of NOXA, which can facilitate ER stress and cell apoptosis.35Imiquimod can also bind to Toll-like receptor 7/9 located on the ER membrane, causing intracellular calcium elevation and subsequent ROS production.35The increase in ROS can activate the ASK1/JNK/p38 pathway and NF-κB pathway, and it may also aggregate ER stress.35These processes can have an impact on the mitochondrial membrane potential, and subsequent cytochrome c release, cleavage of caspases 9 and 3,and poly (ADP-ribose) polymerase can all contribute to melanoma cell apoptosis.35Luteolin also affects various aspects of ER stress associated with cell apoptosis.36It can increase the expression of ATF6, CHOP, and cleaved caspase 12.36Additionally, mito-chondrial calcium overloading and elevated ROS are also observed after melanoma cells have been treated with luteolin, which can further affect ER stress and promote cell apoptosis.36Besides the above-mentioned drugs or chemicals, several extracts can also give rise to ER stress-induced apoptosisviaelevation of ROS.The green tea polyphenol analog JP8 can increase the level of ROS in melanoma B16-F10 cells, and ROS accumulation often serves as the initiator of ER stress-mediated apoptosis.37Dictyopteris undulata ethanol extract leads to cell apoptosis by disturbing the generation of ROS, inducing mitochondrial calcium overload, and causing ER stress.38
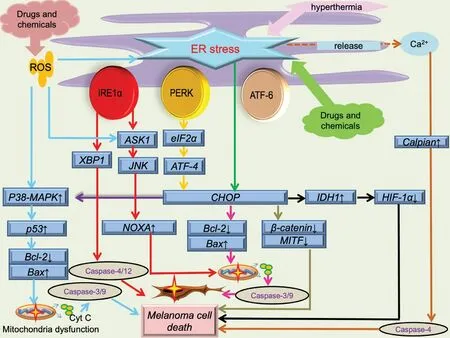
Figure 2.Schematic illustration of ER stress in melanoma cell death.ER stress-induced by drugs, chemicals, and physical condition.Besides ER stress, process including ROS overproduction, mitochondrial dysfunction (membrane potential depolarization and cytochrome c release), calcium disturbance are all involved in melanoma cell death.ASK1: apoptosis signal-regulating kinase 1; ATF-4: activating transcription factor 4; ATF-6: activating transcription factor 6; CHOP: CCAAT/enhancer binding protein-homologous protein; ER: endoplasmic reticulum; HIF-1a: hypoxia inducible factor 1 subunit alpha; IDH1: isocitrate dehydrogenase 1; IRE1α: inositol-requiring enzyme 1α; JNK:c-jun N-terminal kinases; MITF: microphthalmia-associated transcription factor; PERK: protein kinase RNA-activated-like ER kinase; ROS:reactive oxygen species; XBP1: X-box binding protein 1.
Many compounds can inhibit melanoma cellsviainteractions between ER stress and mitochondrial dysfunction (Fig.2).Kalantuboside B can promote cytochrome c release and activation of caspase family members such as caspases 3, 9, and 4.39Besides mitochondrial dysfunction,the venom of the crown-of-thorns starfish (Acanthaster planci) can cause ER stressviaelevation of calcium, and the subsequent CHOP activation is the effector of melanoma cell apoptosis.40Bornyl cis-4-hydroxycinnamate can induce apoptosisviamitochondri-al dysfunction and CHOP activationviathe PERK branch of ER stress.41The inhibitory effect of rottlerin on melanoma cells has various causes.42Rottlerin can down-regulate the expression of Bcl-2 and the membrane potential of mitochondria and cause apoptosisviacaspases 3 and 9.The decrease in Bcl-2 can induce ER stress, and activation of the PERK/eIF2α/ATF4/CHOP pathway can further inhibit Bcl-2 expression and increase expression of death receptor 5.Death receptor 5 can then facilitate cell apoptosisviacaspases 3 and 8.42Nuclear receptor subfamily 4 group A member 1 can not only regulate the activity of the mitochondrial membrane but also affect the ER outer membrane by interacting with Bcl-2 or translocon-associated protein subunit g, which is upstream of ER stress-induced apoptosis.43Camphene can induce cell apoptosisviadisturbance of calcium, mito-chondrial dysfunction, and ER stress.44Inhibition of ubiquitin-specific peptidase 14 can also trigger cell death in melanoma caused by ER stress,mitochondrial dysfunction, and ROS generation.45
Drug Resistance and Synergic Therapy
Even though various drugs such as chemotherapy and immunotherapy agents have been widely used in melanoma, the therapeutic effects are not promising.BRAF inhibitor (BRAFi) resistance is a major problem in melanoma treatment; thus, a thorough understanding of the underlying mechanisms is urgently needed.Research has demonstrated that ER stress and its related processes,such as autophagy, can also be effectors of drug resistance and promising targets of melanoma treatment.
Autophagy induced by ER stress is an important mechanism underlying the resistance of melanoma cells to certain drugs, especially BRAFi.BRAFi can induce the presence of ER stress in melanoma, laying the foundation for BRAFi resistance through increased autophagy.12,46Increasing evidence has shown that resistance to BRAFi drugs such as vemurafenib and dabrafenib in melanoma cells can be explained by ER stress-induced autophagy.12-13Some molecules can promote BRAFi resistance in association with ER stress.Signal sequence receptor 2 can promote BRAFi resistance of melanoma cellsviaprotein translocation.47Furthermore, XBP1s can increase the expression of signal sequence receptor 2.47Galectin-3, a β-galactoside-binding lectin, is often absent in melanoma,which can facilitate vemurafenib resistance.48The resistance may be attributed to ER stress because the absence of galectin-3 might generate a microenvironment leading to ER stress, as demonstrated by the increased expression of GRP78.48An elevated autophagy status induced by fenretinide and bortezomib is also associated with chronic ER stress and subsequent increase of beclin 1.49
Under such circumstances, the establishment of synergic methods to evade drug resistance is urgently needed.Two main methods have been proposed.The first method is to inhibit the pro-survival mechanisms caused by ER stress.49For instance, ER stress-induced autophagy is associated with temozolomide resistance in the treatment of melanoma.Inhibition of GRP78 can decrease the level of autophagy and cause cell cycle arrest, making the melanoma cells sensitive to temozolomide treatment.14Moreover, after exposure to certain drugs, melanoma can also become resistant to vinca alkaloids, and some researchers have confirmed that the resistance is caused by ER stress.50The second method is to further elevate pro-apoptosis elements in ER stress.For example, histone deacetylase 6 (HDAC6) overexpression can also contribute to vemurafenib resistance, which can be relieved by the HDAC6 inhibitor ACY-1215.51ACY-1215 can cause accumulation of polyubiquitinated protein, which is essential for ER stress, and the subsequent activation of the PERK/eIF2α/CHOP pathway can counteract the vemurafenib resistance caused by HDAC6.51The resistance to BRAFi can also be overcome by HA15-induced ER stress.HA15 is a thiazole benzenesulfonamide, the function of which mostly relies on CHOP.52
Notably, a more robust inhibitory effect can be achieved by combination with mitochondrial disturbance.For instance, a combination of sorafenib and α-mangostin can achieve a more promising result.The ER stress caused by sorafenib can be enhanced by α-mangostin,and the inevitable autophagy induced by ER stress can be inhibited by α-mangostin.53Another example is the combination of BRAFi and mitochondriotropic drugs.54BRAFi can cause calcium to be released from the ER and lead to ER stress.The calcium can be absorbed by mitochondria, causing mitochondrial calcium overload and consequent cell apoptosis.54However, a BRAFi such as vemurafenib can reprogram the mitochondrial structure and increase the ability of mitochondria to absorb calcium, which can delay the mitochondrial calcium overload and disturb the process of ER stress-induced apoptosis.54Therefore, the combination of a BRAFi and modulator of a mitochon-drial calcium transporter such as mitochondrial calcium uniporter might achieve a stronger inhibitory effect by influencing calcium uptake by mitochondria.54
Furthermore, as we mentioned above, the IRE1α-XBP1 branch can serve as a cytoprotective method for cell survival.17-Aminogeldanamycin can induce ER stress while concomitantly inhibiting the IRE1α/XBP1 s pathway and suppressing extracellular signal-regulated kinase 1/2 (ERK1/2) activity.55Therefore, a more robust inhibitory effect on melanoma can be achieved by combination of17-aminogeldanamycin with an inhibitor of MAP kinase-ERK kinase 1/2(MEK1/2) or BRAF.55
Conclusion
There are several limitations of our review: (1) We mainly focus on the pathological role of ER stress in melanoma and have less discussion on its role in malignant transformation of melanocyte due to the limited literatures.(2)More literatures may be needed to understand the role of ER stress in melanoma or even other skin tumors.
ER stress plays a dual role in cell biology, especially in tumor pathogenesis.Through different sensors including the PERK-, IRE1α-, and ATF6-dominated pathways,mild to moderate ER stress can facilitate the tumor to overcome harmful or lethal conditions; additionally, prolonged ER stress, especially that caused by external substances, can inhibit the progression of various tumors.ER stress participates in the survival, apoptosis, metastasis,migration, and drug resistance of melanoma.Each branch of ER stress is involved in these processes.Most inhibitory effects of multiple drugs or chemicals are associated with overactivation of ER stress and other processes, whereas some of them inhibit melanoma, perhaps by attenuating ER stress to disturb the adaptation of melanoma to the unfavorable microenvironment.Therefore, finding a more specific target or suitable functional model associated with ER stress in treating melanoma and exploring synergic therapies may be more promising.
Source of funding
This work was supported by the Nanjing Incubation Program for National Clinical Research Center (No.2019060001), PUMC Youth Fund (No.3332017168),and Six Major Talent Summit in Jiangsu Province (No.WSN-030).
杂志排行
国际皮肤性病学杂志的其它文章
- Transcription and Secretion of lnterleukin-1β and HMGB1 in Keratinocytes Exposed to Stimulations Mimicking Common lnflammatory Damages
- Dermoscopic Assessment of Pityriasis Versicolor: A Cross-Sectional Observational Study
- Evaluation of Serum Vitamin D Concentration and Blood Eosinophil and Basophil Counts in Patients With Vitiligo: A Cross-sectional Study From Rafsanjan and Zarand, Iran
- Teledermatology During the COVID-19 Pandemic in a Developing Country: Could This Be the Answer to Improving the Reach of Dermatology Care?
- Basal Cell Carcinoma Excision Guided by Dermoscopy: A Retrospective Study in Macau
- The Relationship Between Ultraviolet B and DNA Methylation in Skin Cancers
