25-羟维生素D、铁蛋白与代谢相关脂肪性肝病及FIB-4指数的相关性分析
2023-04-29李文豪刘志平赵致维张金华
李文豪 刘志平 赵致维 张金华
摘要:目的探討血清25-羟维生素D[25(OH)D]和铁蛋白(SF)水平与代谢相关脂肪性肝病(MAFLD)及FIB-4指数的相关性。方法回顾性分析2020年8月—2021年12月在武汉市汉口医院消化内科住院的595例患者临床资料。比较MAFLD患者(242例)与非MAFLD患者(353例)临床特征的差异。比较不同25(OH)D水平组MAFLD患病率及SF水平的差异。非正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。采用Spearman相关性分析不同人群中血清25(OH)D与SF的相关性。采用二元Logistic回归分析25(OH)D和SF与MAFLD患病风险及FIB-4指数的关系。通过受试者工作特征曲线(ROC曲线)评估25(OH)D和SF预测MAFLD合并肝纤维化的诊断价值。结果与非MAFLD患者相比,MAFLD患者血清25(OH)D水平[15.35(11.26~20.02) vs 21.71(15.39~27.84)]明显降低,而SF水平[365.50(251.75~525.00) vs 205.00(112.50~275.00)]明显升高(Z值分别为-9.761、-13.317,P值均<0.05)。随着血清25(OH)D水平的降低,MAFLD患病率呈升高趋势, SF水平亦呈升高趋势(Z=75.512,P<0.05)。MAFLD患者中血清25(OH)D水平与SF存在显著负相关(r=-0.460,P<0.05)。Logistic回归分析结果显示,血清25(OH)D降低(OR=0.934,95%CI:0.879~0.992,P=0.028)和SF升高(OR=1.009,95%CI:1.006~1.013,P<0.001)是MAFLD的独立危险因素,而且25(OH)D降低还是MAFLD患者FIB-4指数升高(>2.67)的独立危险因素(OR=0.852,95%CI:0.752~0.965,P=0.012)。ROC曲线分析显示,血清25(OH)D、SF及二者联合预测MAFLD患者FIB-4指数升高(>2.67)的曲线下面积分别为0.793、0.829和0.851(P值均<0.05)。结论血清25(OH)D与SF存在负相关,血清25(OH)D降低和SF升高与MAFLD患病风险及FIB-4指数升高相关,血清25(OH)D和SF水平对预测MAFLD患者合并肝纤维化具有一定的临床价值。关键词:代谢相关脂肪性肝病; 25-羟维生素D; 铁蛋白质类; 肝纤维化基金项目:武汉市卫健委医学科研重点项目(WX20A08)
Association of 25-hydro xyvitamin D and ferritin with metabolic associated fatty liver disease and fibrosis-4 index
LI Wenhao, LIU Zhiping, ZHAO Zhiwei, ZHANG Jinhua. (Department of Gastroenterology, Wuhan Hankou Hospital, Wuhan 430012, China)
Corresponding author:LIU Zhiping, xiao6599@sina.com (ORCID:0000-0002-3447-0356)
Abstract:ObjectiveTo investigate the association of serum 25-hydroxyvitamin D [25(OH)D] and serum ferritin (SF) with metabolic associated fatty liver disease (MAFLD) and fibrosis-4 (FIB-4) index. MethodsA retrospective analysis was performed for the clinical data of 595 patients who were hospitalized in Department of Gastroenterology, Wuhan Hankou Hospital, from August 2020 to December 2021. Clinical features were compared between 242 patients with MAFLD and 353 patients without MAFLD, and the prevalence rate of MAFLD and SF level were compared between the groups with different 25(OH)D levels. The non-normally distributed continuous data were expressed as M(P25-P75), and the Mann-Whitney U test was used for comparison between two groups; the chi-square test was used for comparison of categorical data between groups. A Spearman correlation analysis was used to investigate the correlation between serum 25(OH)D and SF in different populations; a binary logistic regression analysis was used to investigate the association of 25(OH)D and SF with the risk of MAFLD and FIB-4 index; the receiver operating characteristic (ROC) curves were used to assess the value of 25(OH)D and SF in the diagnosis of liver fibrosis in patients with MAFLD. ResultsCompared with the non-MAFLD patients, the MAFLD patients had a significant reduction in serum 25(OH)D level [15.35(11.26-20.02) vs 21.71(15.39-27.84), Z=-9.761, P<0.05] and a significant increase in SF level [365.50(251.75-525.00) vs 205.00(112.50-275.00) , Z=-13.317, P<0.05]. The prevalence rate of MAFLD and SF level tended to increase with the reduction in serum 25(OH)D level (Z=75.512, P<0.05). Serum 25(OH)D level was significantly negatively correlated with SF in MAFLD patients (r=-0.460, P<0.05). The logistic regression analysis showed that the reduction in serum 25(OH)D level (odds ratio [OR]=0.934, 95% confidence interval [CI]: 0.879-0.992, P=0.028) and the increase in SF level (OR=1.009, 95%CI: 1.006-1.013, P<0.001) were independent risk factors for MAFLD, and the reduction in serum 25(OH)D level (OR=0.852, 95%CI: 0.752-0.965, P=0.012) was also an independent risk factor for elevated FIB-4 index (>2.67) in MAFLD patients. The ROC curve analysis showed that serum 25(OH)D, SF, and their combination had an area under the ROC curve of 0.793, 0.829, and 0.851, respectively, in predicting elevated FIB-4 index (>2.67) in MAFLD patients (all P<0.05). ConclusionSerum 25(OH)D is negatively correlated with SF, and the reduction in serum 25(OH)D and the increase in SF are associated with the risk of MAFLD and elevated FIB-4 index. Serum 25(OH)D and SF levels have a certain value in predicting liver fibrosis in patients with MAFLD.
Key words:Metabolic-Associated Fatty Liver Disease; 25-Hydroxyvitamin D; Ferritin; Hepatic Fibrosis
Research funding:Key Project of Wuhan Municipal Health Commission (WX20A08)
非酒精性脂肪性肝病(NAFLD)作为一种潜在的严重肝病,其患病率约占全球成年人口的四分之一,造成严重的健康不良负担,具有广泛的社会和经济影响[1]。近来相关研究[2-3]发现,维生素D缺乏、血清铁蛋白(serum ferritin, SF)水平升高可能与NAFLD和/或晚期肝纤维化密切相关,但相关研究结果的一致性尚有待进一步验证。随着对NAFLD发病机制研究的不断深入,发现代谢紊乱在肝脏脂肪变性中发挥着关键作用,国际专家小组于2020年提出将NAFLD更改为新的命名:代谢相关脂肪性肝病(metabolism-associated fatty liver disease,MAFLD)[4]。与NAFLD相比,MAFLD患者的平均年龄、BMI水平、胰岛素抵抗水平、脂质代谢紊乱、肝酶水平等均显著升高[5]。但另外一项横断面研究[6]则发现MAFLD和NAFLD在流行病学上似乎没有显著差异。目前关于中国人群中血清维生素D和SF水平与MAFLD之间的相关性尚不十分清楚。本研究旨在研究25-羟维生素D[25-hydroxyvitamin D, 25(OH)D]、SF水平与MAFLD及无创肝纤维化评估指标FIB-4指数(Fibrosis-4 index, FIB-4)的相关性,探讨血清25(OH)D和SF在MAFLD中的临床意义和诊断价值,为MAFLD的临床诊治和预防提供帮助。
1资料与方法
1.1研究对象回顾性收集2020年8月—2021年12月在武汉市汉口医院消化内科住院的1 029例接受过腹部超声检查的患者临床资料。排除标准:(1)年龄<18岁;(2)合并活动性病毒性肝炎者;(3)合并自身免疫性肝病、Wilson病、药物性肝损伤或慢性血吸虫感染者;(4)合并恶性肿瘤者;(5)各种原因导致的贫血者;(6)合并急性或慢性感染者;(7)合并严重心肺、肾脏及脑血管疾病者;(8)合并甲状旁腺疾患或服用含有维生素D药物者。按照上述排除标准进行筛选后,最终纳入595例患者。结合腹部超声检查及 MAFLD的诊疗共识[4]中相关诊断标准(基于肝脏脂肪积聚的证据,且同时合并以下3项条件之一:超重/肥胖、2型糖尿病、代谢功能障碍),其中242例患者诊断为MAFLD(MAFLD组),另外353例患者为非MAFLD(非MAFLD组)。
1.2资料收集收集所有研究对象的性別、年龄、既往病史、身高和体质量等信息。通过医院管理信息系统收集相关实验室指标,包括:全血细胞计数、ALT、AST、GGT、BUN、血肌酐(SCr)、血尿酸(SUA)、空腹血糖(FPG)、TC、TG、HDL-C、LDL-C、25(OH)D、SF等指标。肝纤维化采用无创性指标FIB-4指数进行评估,以FIB-4>2.67提示合并肝纤维化[7]。
1.3统计学方法采用SPSS 22.0和MedCalc 20.100软件进行统计学分析,非正态性分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。血清25(OH)D与SF水平的相关性检验采用Spearman相关性分析。采用二元Logistic回归分析评估25(OH)D及SF与MAFLD患病风险及FIB-4指数的相关性。采用受试者工作特征曲线(ROC曲线)评估25(OH)D和SF预测MAFLD合并FIB-4指数升高(>2.67)的诊断价值。P<0.05为差异有统计学意义。2结果
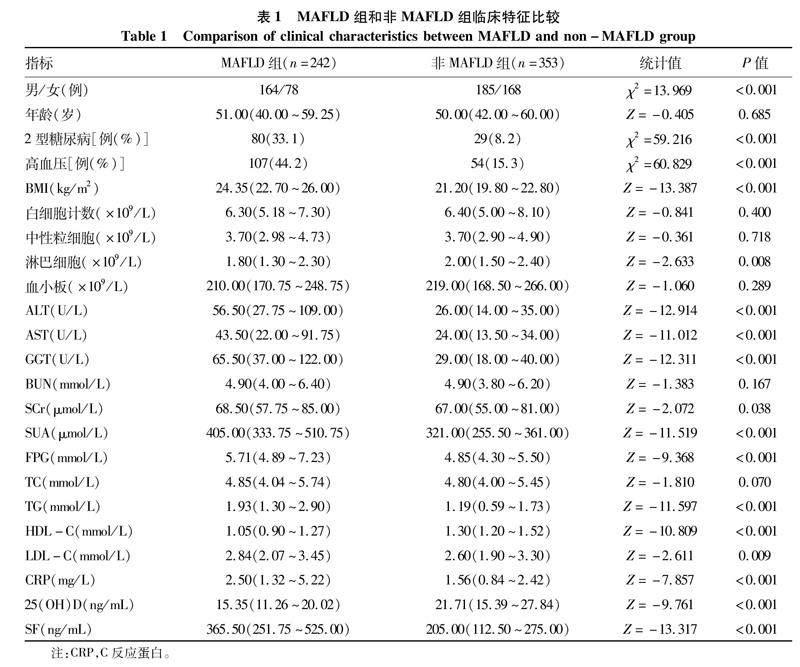
2.1一般资料与非MAFLD组相比,MAFLD组男性患者更多,2型糖尿病和高血压患病率更高,BMI、ALT、AST、GGT、SCr、SUA、FPG、TG、LDL-C、CRP及SF水平明显升高,而淋巴细胞、HDL-C及25(OH)D水平则降低,差异均有统计学意义(P值均<0.05)(表1)。
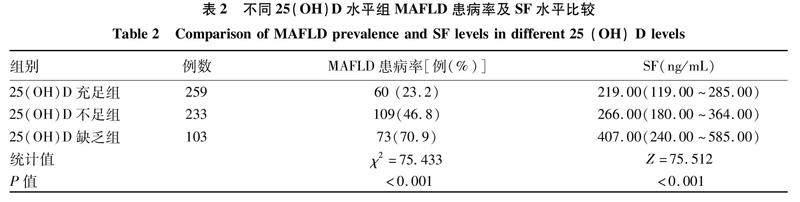
2.2不同25(OH)D水平组MAFLD患病率及SF水平比较根据相关指南[8]将受试者按血清25(OH)D水平分为维生素D充足(≥20 ng/mL)、不足(<20 ng/mL,≥12 ng/mL)和缺乏(<12 ng/mL)三组,比较MAFLD患病率及SF水平的差异。随着血清25(OH)D水平的降低,MAFLD患病率呈升高趋势,SF水平亦呈升高趋势(P值均<0.05)(表2)。
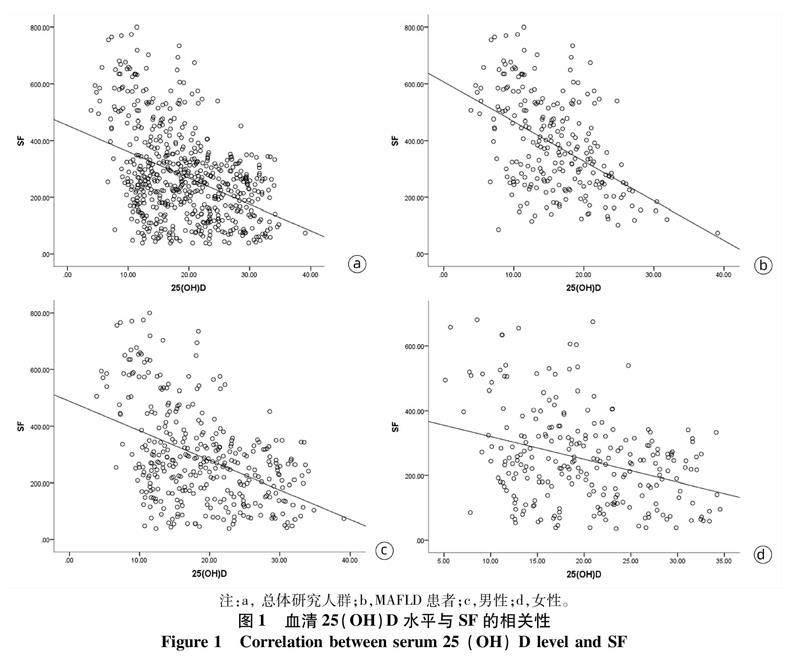
2.3血清25(OH)D水平与SF的相关性Spearman相关性分析显示,血清25(OH)D水平与SF在总体研究人群(r=-0.358,P<0.001)和MAFLD患者(r=-0.460,P<0.001)中均存在显著的负相关。按性别分层后发现,男性和女性受试者中25(OH)D水平与SF之间亦存在显著负相关(r值分别为-0.404、-0.275,P值均<0.001)(图1)。
2.425(OH)D和SF与MAFLD患病风险的Logistic回归分析以25(OH)D和SF为自变量、发生MAFLD为因变量进行Logistic回归分析发现,校正潜在的混杂因素后,25(OH)D水平降低、SF水平升高是MAFLD患病风险的独立危险因素(P值均<0.05)(表3)。
2.525(OH)D和SF与MAFLD合并FIB-4升高的Logistic回归分析以25(OH)D和SF为自变量、MAFLD合并FIB-4升高(>2.67)为因变量进行Logistic回归分析显示,未校正混杂因素时,25(OH)D水平降低、SF水平升高是 MAFLD合并FIB-4升高的危险因素(P值均<0.05);但是校正相关混杂因素后发现,仅25(OH)D降低是MAFLD合并FIB-4升高的独立危险因素(P=0.012)(表4)。
2.625(OH)D和SF对MAFLD合并FIB-4升高的预测价值以ROC曲线评估25(OH)D和SF及二者联合对MAFLD患者合并FIB-4升高的诊断效能。25(OH)D诊断MAFLD患者合并FIB-4升高的ROC曲线下面积为0.793(95%CI:0.737~0.842,P<0.05),敏感度80.4%,特异度74.3%,临界值为13.33 ng/mL;而SF的曲线下面积为0.829(95%CI:0.775~0.874,P<0.05),敏感度74.5%,特异度82.7%,临界值为494 ng/mL;而25(OH)D联合SF的曲線下面积则达到0.851(95%CI:0.799~0.893),敏感度80.4%,特异度84.3%;25(OH)D联合SF的诊断价值高于25(OH)D(Z=2.228,P=0.026),而与SF比较差异无统计学意义(Z=1.243,P=0.214)(图2)。
3讨论周荃等[9]研究发现,在非酒精性脂肪性肝炎患者中,25(OH)D水平与病情严重程度及FIB-4指数呈负相关。孙彩娟等[10]认为SF是临床上评估 NAFLD 患者肝损伤严重程度的实用指标,动态监测SF变化有助于监控NAFLD患者病情的变化。本研究通过比较MAFLD和非MAFLD受试者的临床特征后亦发现相似的结果。MAFLD患者血清25(OH)D水平明显低于非MAFLD受试者,而SF水平则明显升高。相关性分析[11-12]显示, 25(OH)D水平与SF存在显著负相关,在进行性别分层分析后亦得到了同样的结果。25(OH)D水平与SF之间的这种相关性可能与25(OH)D调控铁调素的表达有关。进一步Logistic回归分析显示,25(OH)D水平降低和SF水平升高是MAFLD患病风险的独立危险因素。
FIB-4是一种广泛用于评估晚期肝纤维化的无创指标,与诊断纤维化的金标准肝活检具有较高的一致性[13-14]。本研究显示,25(OH)D水平降低是MAFLD患者FIB-4升高(>2.67)的独立危险因素,提示25(OH)D水平降低增加了MAFLD患者肝纤维化风险。Yang等[15]研究发现,NAFLD患者血清25(OH)D水平与肝纤维化程度呈负相关,而在伴有晚期纤维化的NAFLD患者中,血清25(OH)D水平与转化生长因子-β1呈正相关。25(OH)D缺乏在慢性肝病相关肝纤维化患者中非常普遍,25(OH)D通过25(OH)D受体介导的特异性信号转导途径抑制促纤维化基因的表达,从而对肝星状细胞发挥抗纤维化作用[16]。另外,SF水平升高(>1.5倍正常值上限)与NAFLD患者肝脏铁沉积、非酒精性脂肪性肝炎诊断和组织学活性恶化有关,是NAFLD患者晚期肝纤维化的独立预测因子[17]。在468例活检证实为NAFLD的患者中,F3期与F0~F1期相比,SF水平明显增加,但在F4期则降低[18]。然而,Yoneda等[19]发现,虽然NAFLD患者中性别差异、脂肪变性分级和纤维化分期与SF水平独立相关,但SF对肝纤维化的诊断准确性较低。本研究则发现,MAFLD患者中SF水平升高与FIB-4指数升高相关,且ROC曲线下面积达到了0.829,而与25(OH)D联合诊断的ROC曲线下面积高达0.851(95%CI:0.799~0.893),提示联合检测血清25(OH)D和SF水平对评估MAFLD患者肝纤维化风险具有一定的临床价值。
本研究尚存在以下局限性:首先,本研究为回顾性横断面研究,不能说明25(OH)D和SF与MAFLD之间的因果关系,尚需进一步前瞻性、随机对照研究来证实;其次,本研究中受试者无肝活检资料,以FIB-4指数评估肝纤维化,可能存在漏诊和误诊,需要在大样本人群中开发出一种准确度更高的无创诊断MAFLD肝纤维化的临床指标,以减少肝活检组织学检查的需求[20]。
综上所述, MAFLD患者存在25(OH)D水平降低和SF水平升高,25(OH)D水平降低和SF水平升高不仅与MAFLD发病风险独立相关,而且与MAFLD患者FIB-4指数升高存在相关性。进一步深入研究25(OH)D和SF在MAFLD及肝纤维化中的作用及相关机制有助于丰富MAFLD的临床防治手段。
伦理学声明:本研究方案于2021年3月5日经由武汉市汉口医院伦理委员会审批,批号:2020-HKYY017。利益冲突声明:本文不存在任何利益冲突。作者贡献声明:刘志平负责研究的构思与设计,撰写论文;李文豪、赵致维负责数据收集与整理,统计学分析;张金华指导论文的撰写及内容的修改。
参考文献:
[1]LAZARUS JV, MARK HE, ANSTEE QM, et al. Advancing the global public health agenda for NAFLD: A consensus statement[J]. Nat Rev Gastroenterol Hepatol, 2022, 19(1): 60-78. DOI: 10.1038/s41575-021-00523-4.
[2]DU SX, LU LL, GENG N, et al. Association of serum ferritin with non-alcoholic fatty liver disease: A meta-analysis[J]. Lipids Health Dis, 2017, 16(1): 228. DOI: 10.1186/s12944-017-0613-4.
[3]ALI SANGOUNI A, GHAVAMZADEH S, JAMALZEHI A. A narrative review on effects of vitamin D on main risk factors and severity of non-alcoholic fatty liver disease[J]. Diabetes MetabSyndr, 2019, 13(3): 2260-2265. DOI: 10.1016/j.dsx.2019.05.013.
[4]ESLAM M, NEWSOME PN, SARIN SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement[J]. J Hepatol, 2020, 73(1): 202-209. DOI: 10.1016/j.jhep.2020.03.039.
[5]LIN S, HUANG JF, WANG MF, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world[J]. Liver Int, 2020, 40(9): 2082-2089. DOI: 10.1111/liv.14548.
[6]CIARDULLO S, PERSEGHIN G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population[J]. Liver Int, 2021, 41(6): 1290-1293. DOI: 10.1111/liv.14828.
[7]SHAH AG, LYDECKER A, MURRAY K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2009, 7(10): 1104-1112. DOI: 10.1016/j.cgh.2009.05.033.
[8]LIAO XP, ZHANG ZL, ZHANG HH, et al. Application guideline for vitamin D and bone health in adult Chinese(2014 standard edition) vitamin D working group of osteoporosis committee of China gerontological society[J]. Chin J Osteoporos, 2014, 20(9): 1011-1030. DOI: 10.3969/j.issn.1006-7108.2014.09.002.廖祥鵬, 张增利, 张红红, 等. 维生素D与成年人骨骼健康应用指南(2014年标准版)[J]. 中国骨质疏松杂志, 2014, 20(9): 1011-1030. DOI: 10.3969/j.issn.1006-7108.2014.09.002.
[9]ZHOU Q, LI JQ, LI XW. Influence of vitamin D deficiency on fibrosis-4 index and disease severity in patients with nonalcoholic steatohepatitis[J]. J Clin Hepatol, 2022, 38(6): 1293-1298. DOI: 10.3969/j.issn.1001-5256.2022.06.015.周荃, 李金强, 黎晓武. 维生素D缺乏对非酒精性脂肪性肝炎患者FIB-4指数及病情严重程度的影响[J]. 临床肝胆病杂志, 2022, 38(6): 1293-1298. DOI: 10.3969/j.issn.1001-5256.2022.06.015.
[10]SUN CJ, ZUO XQ, YAO N, et al. Study on the correlation between serum ferritin and non-alcoholic fatty liver disease[J]. Zhejiang J Integr Tradit Chin West Med, 2019, 29(5): 371-375. DOI: 10.3969/j.issn.1005-4561.2019.05.008.孙彩娟, 左昔清, 姚娜, 等. 血清铁蛋白与非酒精性脂肪性肝病相关性研究[J]. 浙江中西医结合杂志, 2019, 29(5): 371-375. DOI: 10.3969/j.issn.1005-4561.2019.05.008.
[11]BACCHETTA J, ZARITSKY JJ, SEA JL, et al. Suppression of iron-regulatory hepcidin by vitamin D[J]. J Am Soc Nephrol, 2014, 25(3): 564-572. DOI: 10.1681/ASN.2013040355.
[12]LIU ZP, ZHANG JH, WANG XN, et al. Effect of vitamin D on serum markers of iron metabolism in patients with non-alcoholic fatty liver disease[J]. Tianjin Med J, 2018, 46(12): 1316-1318. DOI: 10.11958/20180847.刘志平, 张金华, 王湘宁, 等. 维生素D对非酒精性脂肪性肝病患者铁代谢的影响[J]. 天津医药, 2018, 46(12): 1316-1318. DOI: 10.11958/20180847.
[13]PATEL YA, GIFFORD EJ, GLASS LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the veterans health administration[J]. Dig Dis Sci, 2018, 63(9): 2259-2266. DOI: 10.1007/s10620-018-5123-3.
[14]MA XH, ZHANG X, YOU Y, et al. Diagnostic value of APRI combined with FIB-4 for significant liver fibrosis in patients with chronic hepatitis B[J]. Chin J Gastroenterol, 2017, 22(9): 544-547. DOI: 10.3969/j.issn.1008-7125.2017.09.007.马晓辉, 张新, 游云, 等. APRI、FIB-4联合对慢性乙型肝炎患者显著肝纤维化的诊断价值[J]. 胃肠病学, 2017, 22(9): 544-547. DOI: 10.3969/j.issn.1008-7125.2017.09.007.
[15]YANG BB, CHEN YH, ZHANG C, et al. Low vitamin D status is associated with advanced liver fibrosis in patients with nonalcoholic fatty liver disease[J]. Endocrine, 2017, 55(2): 582-590. DOI: 10.1007/s12020-016-1152-x.
[16]UDOMSINPRASERT W, JITTIKOON J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies[J]. Biomed Pharmacother, 2019, 109: 1351-1360. DOI: 10.1016/j.biopha.2018.10.140.
[17]KOWDLEY KV, BELT P, WILSON LA, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease[J]. Hepatology, 2012, 55(1): 77-85. DOI: 10.1002/hep.24706.
[18]BUZZETTI E, PETTA S, MANUGUERRA R, et al. Evaluating the association of serum ferritin and hepatic iron with disease severity in non-alcoholic fatty liver disease[J]. Liver Int, 2019, 39(7): 1325-1334. DOI: 10.1111/liv.14096.
[19]YONEDA M, THOMAS E, SUMIDA Y, et al. Clinical usage of serum ferritin to assess liver fibrosis in patients with non-alcoholic fatty liver disease: Proceed with caution[J]. Hepatol Res, 2014, 44(14): E499-E502. DOI: 10.1111/hepr.12327.
[20]ZENG J, FAN JG. Clinical significance of renaming nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36(6): 1205-1207. DOI: 10.3969/j.issn.1001-5256.2020.06.002.曾靜, 范建高. 非酒精性脂肪性肝病更名的临床意义[J]. 临床肝胆病杂志, 2020, 36(6): 1205-1207. DOI: 10.3969/j.issn.1001-5256.2020.06.002.
收稿日期:2022-11-12;录用日期:2022-12-12
本文编辑:王莹
引证本文:LI WH, LIU ZP, ZHAO ZW, et al. Association of 25-hydroxyvitamin D and ferritin with metabolic associated fatty liver disease and fibrosis-4 index[J]. J Clin Hepatol, 2023, 39(8): 1867-1873.