抗-HBs联合抗-HBe对HBsAg/HBeAg/抗-HBc阳性患者HBeAg血清学转换的预测价值
2023-04-29谭亚峰孙凤兰夏巍欧阳耀灵李承彬陈珍霞吴松余运运江涛
谭亚峰 孙凤兰 夏巍 欧阳耀灵 李承彬 陈珍霞 吴松 余运运 江涛
摘要:目的建立HBsAg/HBeAg/抗-HBc阳性(以下简称“大三阳”)+抗-HBe阳性模式患者HBeAg血清学转换预测模型,并验证其预测价值。方法选取2018年4月1日—2022年8月1日于长江大学附属荆州医院行乙型肝炎血清标志物和HBV DNA定量检测的乙型肝炎患者6 055例,根据不同乙型肝炎血清学指标模式分为5组:HBsAg阴性组、HBsAg/抗-HBe/抗-HBc阳性组、大三阳+抗-HBe阳性组、大三阳+抗-HBs阳性组和大三阳组。分析不同乙型肝炎血清学指标模式的特点及不同组别中HBeAg水平的差异。非正态分布的多组连续性变量组间比较采用Kruskal-Wallis H检验。计数资料组间比较采用χ2检验。采用线性回归分析不同组别HBeAg水平差异。采用Logistic回归筛选独立影响因素,获得最优预测因子。通过受试者工作特征曲线(ROC曲线)验证预测效能。使用R Studio4.2.1建立预测模型并验证。结果与大三阳模式相比,大三阳+抗-HBe阳性、大三阳+抗-HBs阳性模式患者HBeAg水平均降低(P值均<0.01)。多因素Logistic回归分析显示,抗-HBe是HBeAg血清学转换的独立影响因素(P=0.014)。Lasso回归分析筛选结果显示,大三阳+抗-HBe阳性患者发生HBeAg血清学转换的最优预测因子为抗-HBe+抗-HBs。ROC曲线结果显示,抗-HBe+抗-HBs的AUC为0.733(95%CI: 0.588~0.878,P=0.004 8)。纳入抗-HBe+抗-HBs建立预测模型,其区分度(AUC=0.733)、准确度(C=0.733,B=0.20,P=0.946)、检测效能及稳定度(加强Bootstrap检验C=0.726)均表現良好。结论乙型肝炎患者体内抗-HBs或抗-HBe的出现均促进HBeAg水平下降,且抗-HBe促进HBeAg水平下降的能力强于抗-HBs。抗-HBe+抗-HBs可用于大三阳+抗-HBe阳性模式患者HBeAg血清学转换的预测。
关键词:乙型肝炎抗原; 血清转换; 预测
基金项目:荆州市科技计划项目(2022HC68)
Predictive value of anti-HBs+anti-HBe for seroconversion of HBeAg in patients with positive HBsAg/HBeAg/anti-HBc
TAN Yafeng SUN Fenglan XIA Wei OUYANG Yaoling LI Chengbin CHEN Zhenxia WU Song YU Yunyun JIANG Tao(a. Department of Clinical Laboratory, b. Department of Gynecology, Jingzhou Hospital Affiliated to Yangtze University, Jingzhou, Hubei 434000, China)
Corresponding author:JIANG Tao, jiangtao00358@126.com (ORCID:0000-0002-0852-9190)
Abstract:ObjectiveTo establish a predictive model for HBeAg seroconversion in patients with positive HBsAg/HBeAg/anti-HBc and anti-HBe, and to investigate the predictive value of this model. MethodsA total of 6 055 patients with hepatitis B who received the quantification of the serum markers for hepatitis B and HBV DNA in Jingzhou Hospital Affiliated to Yangtze University from April 1, 2018 to August 1, 2022 were enrolled, and according to the pattern of serological markers for hepatitis B, they were divided into negative HBsAg group, positive HBsAg/anti-HBe/anti-HBc group, positive HBsAg/HBeAg/anti-HBc+positive anti-HBe group, positive HBsAg/HBeAg/anti-HBc+positive anti-HBs group, and positive HBsAg/HBeAg/anti-HBc group. The characteristics of different patterns of serological markers were analyzed, and the level of HBeAg was compared between groups. The Kruskal-Wallis H test was used for comparison of non-normally distributed continuous variables between multiple groups; the chi-square test was used for comparison of categorical data between groups. The linear regression analysis was used to analyze the difference in HBeAg content between different groups; the logistic regression analysis was used to screen for independent influencing factors and obtain the optimal predictive factors. The receiver operating characteristic (ROC) curve analysis was used to validate the predictive performance of the model. R Studio4.2.1 was used to establish and validate the predictive model. ResultsCompared with the positive HBsAg/anti-HBe/anti-HBc group, the positive HBsAg/HBeAg/anti-HBc+positive anti-HBe group and the positive HBsAg/HBeAg/anti-HBc+positive anti-HBs group had a significant reduction in the level of HBeAg (both P<0.01). The multivariate logistic regression analysis showed that anti-HBe was an independent influencing factor for HBeAg seroconversion (P=0.014), and the Lasso regression analysis showed that anti-HBe+anti-HBs was the optimal predictive factor for HBeAg seroconversion in the patients with positive HBsAg/HBeAg/anti-HBc and positive anti-HBe. The ROC curve analysis showed that anti-HBe+anti-HBs had an area under the ROC curve (AUC) of 0.733 (95% confidence interval: 0.588-0.878, P=0.004 8). Anti-HBe+anti-HBs was included to establish a predictive model, which had good discriminatory ability (AUC=0.733), accuracy (C=0.733, B=0.20, P=0.946), predictive performance, and stability (C=0.726 based on the enhanced Bootstrap test). ConclusionThe presence of anti-HBs or anti-HBe in patients with hepatitis B promotes the reduction in HBeAg level, and anti-HBe had a stronger ability than anti-HBs in promoting such reduction. Anti-HBe+anti-HBs can be used to predict HBeAg seroconversion in patients with positive HBsAg/HBeAg/anti-HBc+positive anti-HBe.
Key words:Hepatitis B Antigens; Seroconversion; Forecasting
Research funding:Jingzhou Science and Technology Plan Project (2022HC68)
成人感染HBV后,大多数患者可通过自身免疫清除病毒,仅5%~10%的患者发展为慢性乙型肝炎[1]。HBsAg、HBeAg阳性能够反映患者体内存在病毒复制,抗-HBs、抗-HBe及抗-HBc是患者免疫应答产生的抗体。HBeAg血清学转换通常是指HBeAg消失后抗-HBe出现,是临床抗病毒治疗的重要目标之一[2-3]。国内外相关研究显示,HBsAg/HBeAg/抗-HBc阳性(以下简称“大三阳”)乙型肝炎患者HBeAg血清学转换受病毒及宿主双方面的影响:(1)病毒方面,HBsAg、HBeAg、HBcrAg、HBV DNA及HBV RNA水平变化对HBeAg血清学转换有一定的预测价值[4-17];宿主方面,抗-HBc及其水平变化对HBeAg血清学转换也有一定预测价值[18-22]。随着检测技术的进步,越来越多特殊的乙型肝炎血清学指标模式被发现[23],包括大三阳+抗-HBe阳性模式和大三阳+抗-HBs阳性模式。目前,鲜有文献报道上述特殊模式中抗-HBs、抗-HBe对HBeAg水平的影响,也尚未见有研究探讨抗-HBs对乙型肝炎患者HBeAg血清学转换有无预测价值。本研究通过分析不同乙型肝炎血清学指标模式,探讨抗-HBs、抗-HBe对HBeAg水平的影响,建立预测模型,为此类乙型肝炎患者的疾病转归监测提供依据。
1资料与方法
1.1研究对象选取2018年4月1日—2022年8月1日于长江大学附属荆州医院行乙型肝炎血清标志物和HBV DNA定量检测的乙型肝炎患者。乙型肝炎诊断标准参照中华医学会《慢性乙型肝炎防治指南(2019年版)》[2]。排除标准:HAV、HCV感染,自身免疫性肝病以及药物性肝损伤、酒精性肝损伤等。根据不同的乙型肝炎血清学指标模式,将研究对象分为5组:(1)HBsAg阴性组;(2)HBsAg/抗-HBe/抗-HBc阳性(以下简称“小三阳”)组;(3)大三阳+抗-HBe阳性组;(4)大三阳+抗-HBs阳性组;(5)大三阳组。
1.2研究方法
1.2.1观察指标及检测仪器乙型肝炎血清标志物采用HISCL 5000全自动化学发光法免疫分析仪(日本希森美康公司)检测;血清HBV DNA定量采用ABI7500实时荧光定量聚合酶链式反应(PCR)仪(美国 ABI 公司)检测;AFP采用Cobas e602全自动免疫分析仪(瑞士罗氏公司,Elecsys Ⅱ 定量方法)检测。肝功能指标采用AU5800全自动生化分析仪(美国贝克曼库尔特公司)检测。所有仪器和试剂经过性能验证,均符合ISO15189医学实验室质量及能力认证要求。
1.2.2阳性判断标准(1)血清标志物阳性判断标准为:HBsAg>0.03 IU/mL、抗-HBs>5 mIU/mL、HBeAg>1 COI、抗-HBe>50 抑制率(Inh%)、抗-HBc>1 COI。所有乙型肝炎特殊模式样本均重复检测且通过ELISA方法复核确认;HBsAg及抗-HBs为定量检测,其单位分别为IU/mL及mIU/mL;乙型肝炎血清标志物半定量结果依据阴性质控品(Sysmex)(NC的发光强度)及阳性质控品(Sysmex)(PC的发光强度)计算获得,①HBeAg通过质控品的发光强度计算临界值(CO),即CO=(PC的发光强度-NC的发光强度)/50+NC的发光强度;然后计算检测样本的临界值指数(COI),即COI=(样本发光强度-NC的发光强度)/(CO-NC的发光强度),HBeAg>1 COI为阳性。②抗-HBe单位为Inh%,Inh%=(NC的发光强度-样本发光强度)/(NC的发光强度-PC的发光强度),抗-HBe>50 Inh%为阳性。③抗-HBc通过质控品的发光强度计算CO,CO=(PC的发光强度-NC的发光强度)/5+NC的发光强度,然后计算检测样本的COI,COI=(样本发光强度-NC的发光强度)/(CO-NC的发光强度),HBcAg>1 COI为阳性。(2)HBV DNA临界值判断标准为:HBV DNA>2 log10 IU/mL为阳性。血清HBV DNA定量检测采用实时定量PCR法,HBV DNA扩增曲线呈“S”形,根据阳性参考品曲线自动换算出HBV DNA载量水平,HBV DNA>2 log10 IU/mL提示HBV DNA阳性,HBV DNA低于检测下限用0 log10IU/mL表示。(3)AFP临界值判定标准:AFP>7 ng/mL。(4)肝功能临界值判定标准,男性:DBil>9.3 μmol/L、IBil>23.5 μmol/L、TBil>26 μmol/L、Alb<55 g/L、A/G>2.4、Glo>40 g/L、TP>85 g/L、AST>40 U/L、ALT>50 U/L、GGT>60 U/L、ALP>150 U/L;女性:DBil>7.5 μmol/L、IBil>18.9 μmol/L、TBil>21 μmol/L、Alb<55 g/L、A/G>2.4、Glo>40 g/L、TP>85 g/L、AST>35 U/L、ALT>40 U/L、GGT>50 U/L、ALP>150 U/L。
1.3統计学方法采用SPSS 26.0软件进行统计学分析。计量资料以M(P25~P75)表示,组间比较采用Kruskal-Wallis H检验。计数资料组间比较采用χ2检验。采用Logistic回归筛选独立影响因素,增加Lasso(惩罚函数)消除共线性干扰获得最优预测因子,通过受试者工作特征曲线(ROC曲线)验证预测效能。使用R4.2.1建立预测模型并验证。P<0.05为差异有统计学意义。
2结果
2.1一般资料共纳入6 055例患者,HBsAg阴性组1 162例,小三阳组3 917例,大三阳+抗-HBe阳性组150例,大三阳+抗-HBs阳性组21例,大三阳组805例。
在大三阳+抗-HBe阳性中筛选出同期行乙型肝炎血清标志物、HBV DNA、AFP及肝功能检测的患者,最终观察到25例发生HBeAg 血清学转换,其中5例获得HBsAg阴转。本研究根据25例大三阳+抗-HBe阳性患者HBeAg血清学转换前后数据行单因素、多因素Logistic回归及Lasso回归分析,筛选出最优预测因子,应用最优预测因子建立大三阳+抗-HBe阳性患者HBeAg血清学转换的预测模型并验证。
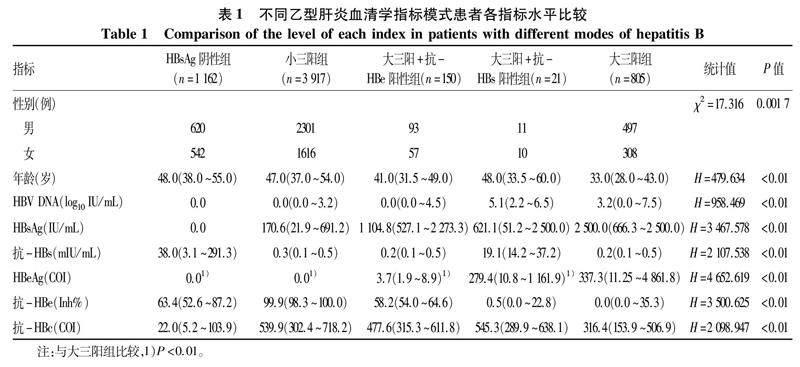
2.2不同乙型肝炎血清学指标模式患者各项指标水平比较不同乙型肝炎血清学指标模式患者各项指标水平差异均有统计学意义(P值均<0.01)(表1)。
2.3不同乙型肝炎血清学指标模式患者HBeAg水平比较分层分析显示:(1)大三阳+抗-HBs阳性患者由于抗-HBs的出现,其HBeAg含量较大三阳组低(P<0.01)。(2)大三阳+抗-HBe阳性患者由于抗-HBe的出现,其HBeAg含量较大三阳低(P<0.01)。(3)大三阳+抗-HBe阳性模式患者较大三阳+抗-HBs阳性模式患者HBeAg含量下降的更明显。(4)大三阳、大三阳+抗-HBs阳性、大三阳+抗-HBe阳性与小三阳4种模式HBeAg水平呈阶梯式降低趋势。
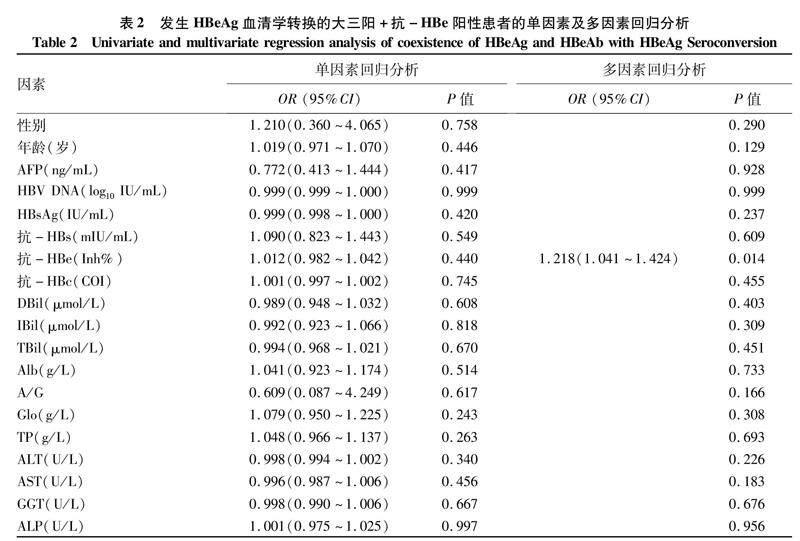
2.4大三阳+抗-HBe阳性患者发生HBeAg血清学转换的最优预测因子筛选及验证根据已出现HBeAg血清学转换的三阳+抗-HBe阳性患者数据进行探讨,如表2所示,单因素回归分析显示,所有观察指标均不是大三阳+抗-HBe阳性患者发生HBeAg 血清学转换的独立影响因素(P值均>0.05);多因素回归分析显示,抗-HBe是HBeAg血清学转换的独立影响因素(P=0.014)。提示抗-HBe在其他因素的协同作用下可促进HBeAg血清学转换。
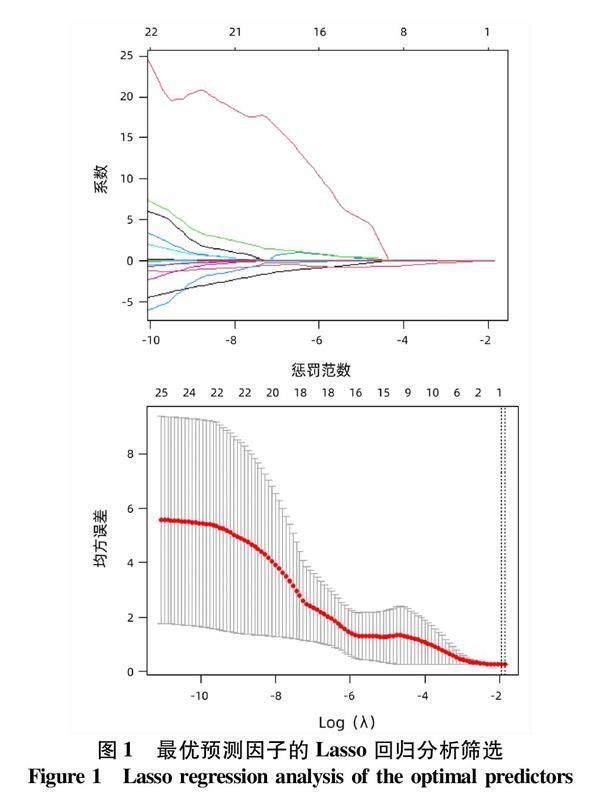
本研究进一步增加抗-HBe与抗-HBs、抗-HBc的不同协同模式及性别、年龄、AFP、HBV DNA、HBsAg、抗-HBs、抗-HBe、抗-HBc、DBil、IBil、TBil、TP、Alb、Glo、A/G、ALT、AST、GGT及ALP均纳入Lasso回归分析,应用惩罚函数消除共线性干扰,最终筛选出促进HBeAg血清学转换最优预测因子为抗-HBe+抗-HBs(图1)。ROC曲线分析显示,抗-HBe+抗-HBs预测大三阳+抗-HBe阳性患者发生HBeAg血清学转换的AUC=0.733(95%CI: 0.588~0.878,P=0.004 8)(图2)。
2.5大三阳+抗-HBe阳性患者发生HBeAg血清学转换预测模型的建立及验证列线图分析显示,抗-HBe+抗-HBs是HBeAg血清学转换的良好预测指标(图3)。该模型的区分度良好(AUC=0.733)(图4a)、准确度良好(C=0.733,B=0.20,P=0.946)(图4b)、检测效能良好(图4c)及稳定度良好(加强Bootstrap检验C=0.726,检验曲线与模型校准曲线贴合良好)(图4d)。
3讨论
抗-HBs、抗-HBe和抗-HBc是患者免疫应答过程中产生的抗体,抗-HBc出现最早,抗-HBe常出现于HBeAg消失后,抗-HBs可出现于健康人群疫苗接种后或感染HBV病毒完全康复后[24]。在临床实践中常见大三阳+抗-HBe阳性和大三阳+抗-HBs阳性模式的患者,其发生机制尚不清楚,此特殊模式出现对HBeAg水平的影响以往鲜有文献报道。本研究分层分析乙型肝炎不同模式对HBeAg水平的影响,证实了乙型肝炎患者体内抗-HBs或抗-HBe的出现均促进HBeAg水平的下降,且抗-HBe促进HBeAg水平的下降的效能强于抗-HBs。HBV 炎症与免疫反应相关研究[25-26]显示,抗-HBe为依赖T淋巴细胞介导的特异性抗体,对HBeAg具有较强的中和作用。故本研究认为大三阳+抗-HBe阳性患者HBeAg水平下降的原因可能是患者体内HBV病毒仍处于高复制状态,而体内产生的抗-HBe对HBeAg具有一定的清除作用,最终表现为HBeAg水平下降且处于HBeAg与抗-HBe动态平衡状态。另有国内外多项研究[27-30]显示,患者发生HBeAg血清学转换(HBeAg消失后出现抗-HBe)或HBsAg血清学转换(HBsAg消失后出现抗-HBs)过程中可观察到:细胞因子IL-21水平升高,NK细胞的活化性受体NKG2D、Toll样受体表达上调及PD-1表达降低,CD56bright NK细胞数量、病毒特异性CD8 T淋巴细胞数量增加及调节性T淋巴细胞数量减少等现象。本研究推测,大三阳+抗-HBs阳性患者HBeAg水平下降的原因可能是患者免疫力较以前增强,促进了NK细胞、特异性CD8 T淋巴细胞数量的升高及调节性T淋巴细胞数量减少等而间接增强了機体清除HBV病毒的能力,从而促使了HBeAg含量下降。但该阶段患者体内产生的抗-HBs为非HBeAg中和抗体,不能直接中和HBeAg,因此该模式促进HBeAg含量下降的效能较大三阳+抗-HBe阳性模式弱。
大三阳+抗-HBe阳性模式出现的原因已有学者探讨,有学者[31]认为是由基因变异引起,即C区G1896A突变、BCP区A1762T/G1764A双突变等,是HBV免疫逃逸的体现。另有学者[32]认为大三阳+抗-HBe阳性是感染了HBV不同亚型导致产生的抗-HBe无法中和体内的HBeAg所致。还有学者[33]指出HBeAg与抗-HBe是患者体内病毒复制和免疫清除动态平衡的体现,认为大三阳+抗-HBe阳性模式的出现是患者发生HBeAg血清转化的过渡阶段。本研究追溯到25例大三阳+抗-HBe阳性模式患者发生HBeAg血清学转换,且有5例获得HBsAg转阴,验证了大三阳+抗-HBe阳性模式可发生HBeAg血清学转换,支持大三阳+抗-HBe阳性模式的出现是患者发生HBeAg血清学转换的过渡阶段的观点。
《慢性乙型肝炎防治指南(2019年版)》[2]指出抗-HBe是大三阳患者HBeAg血清学转换的影响因素,即HBeAg消失后出现抗-HBe为传统的HBeAg血清学转换方式。但大三阳+抗-HBe阳性模式HBeAg未消失体内便已出现抗-HBe,其能否发生HBeAg血清学转换、转化方式是否具有特殊性、有无良好的预测因子等方面值得深究。本研究验证了大三阳+抗-HBe阳性模式可发生HBeAg血清学转换,回归分析结果表明,大三阳+抗-HBe阳性模式仅抗-HBe在其他因素的协同作用下可促进HBeAg血清学转换。本研究增加抗-HBe与其他抗体不同的协同模式后,将所有影响因素均纳入Lasso回归分析,筛选出最优预测因子为抗-HBe+抗-HBs。ROC曲线分析证实,相较于抗-HBe,抗-HBe+抗-HBs是大三阳+抗-HBe阳性模式发生HBeAg血清学转换更优的预测因子,基于抗-HBe+抗-HBs建立的预测模型,其区分度、准确度、检测效能及稳定度均表现良好,可用于此类乙型肝炎患者治疗过程的监测。
综上所述,本研究再次证实了乙型肝炎患者体内抗-HBs或抗-HBe的出现均会促进HBeAg水平下降,抗-HBe促进HBeAg水平的下降的效能强于抗-HBs,首次探寻出抗-HBe+抗-HBs是大三阳+抗-HBe乙型肝炎患者HBeAg血清学转换的最优预测因子。但本研究也有局限性:首先,药物对HBeAg血清学转换具有一定的影响,拉米夫定、恩替卡韦、阿德福韦酯、替比夫定治疗的1年累计HBeAg血清学转换率分别为17.24%、13.92%、15.95%和14.04%[8],但本研究未将药物对HBeAg血清学转换的影响纳入研究模型。其次,HBV基因型及突变对HBeAg血清学转换也有影响,B型HBV感染的HBeAg血清学转换率最高,为15.5%,其次为C型7.9%及E型7.4%,1762T/1764A和1862T突变有利于HBeAg血清学转换[34-36]。而本研究未行基础实验,未获得HBV基因型及基因突变等预测因子的支持。本研究还缺乏多中心研究数据支持,未行外部验证。后期笔者团队将增加基础实验并开展广泛合作,建立多中心数据库进行模型开发与验证。
伦理学声明:本研究方案于2022年6月30日经由长江大学附属荆州医院伦理委员会审批,批号:2022-06-30,所纳入患者均签署知情同意书。利益冲突声明:本文不存在任何利益冲突。作者贡献声明:谭亚峰、孙凤兰负责课题设计,资料分析,撰写论文;夏巍,欧阳耀灵负责收集数据和统计分析;李承彬、陈珍霞、吴松负责指导数据统计分析;江涛负责拟定写作思路,指导撰写文章并最后定稿。谭亚峰、孙凤兰对本文贡献等同,同为第一作者。
参考文献:
[1]DUAN MX, GU YN, YU MC, et al. Reasons and clinical significance of HBeAg and anti-HBe double positive in patients with chronic hepatitis B[J]. Chin J Nosocomiology, 2017, 27(19): 4332-4335.段夢夕, 谷娅楠, 于淼琛, 等. 慢性乙型肝炎患者HBeAg与抗-HBe双阳性的原因与临床意义[J]. 中华医院感染学杂志, 2017, 27(19): 4332-4335.
[2]Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019) [J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.
[3]GU ZY, WANG AH, HE WC, et al. Influencing factors for HBeAg seroconversion in patients with chronic hepatitis B[J]. J Clin Hepatol, 2022, 38(11): 2581-2585. DOI: 10.3969/j.issn.1001-5256.2022.11.029.顾子杨, 王安辉, 何文昌, 等. 慢性乙型肝炎患者HBeAg血清学转换影响因素的研究进展[J]. 临床肝胆病杂志, 2022, 38(11): 2581-2585. DOI: 10.3969/j.issn.1001-5256.2022.11.029.
[4]YAO CC, LEE CM, HUNG CH, et al. Combining age and HBsAg level predicts post-treatment durability of nucleos(t)ide analogue-induced HBeAg seroconversion[J]. J Gastroenterol Hepatol, 2015, 30(5): 918-924. DOI: 10.1111/jgh.12874.
[5]GISH RG, CHANG TT, LAI CL, et al. Quantitative hepatitis B surface antigen analysis in hepatitis B e antigen-positive nucleoside-naive patients treated with entecavir[J]. Antivir Ther, 2013, 18(5): 691-698. DOI: 10.3851/IMP2559.
[6]ZOULIM F, CAROSI G, GREENBLOOM S, et al. Quantification of HBsAg in nucleos(t)ide-nave patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study[J]. J Hepatol, 2015, 62(1): 56-63. DOI: 10.1016/j.jhep.2014.08.031.
[7]YANG SC, LU SN, LEE CM, et al. Combining the HBsAg decline and HBV DNA levels predicts clinical outcomes in patients with spontaneous HBeAg seroconversion[J]. Hepatol Int, 2013, 7(2): 489-499. DOI: 10.1007/s12072-012-9382-3.
[8]GENG MF, LI YX, GAO FY, et al. A scoring model predicts hepatitis B e antigen seroconversion in chronic hepatitis B patients treated with nucleos(t)ide analogs: Real-world clinical practice[J]. Int J Infect Dis, 2017, 62: 18-25. DOI: 10.1016/j.ijid.2017.06.016.
[9]WANG Y, LIAO H, DENG Z, et al. Serum HBV RNA predicts HBeAg clearance and seroconversion in patients with chronic hepatitis B treated with nucleos(t)ide analogues[J]. J Viral Hepat, 2022, 29(6): 420-431. DOI: 10.1111/jvh.13671.
[10]WANG B, CAREY I, BRUCE M, et al. HBsAg and HBcrAg as predictors of HBeAg seroconversion in HBeAg-positive patients treated with nucleos(t)ide analogues[J]. J Viral Hepat, 2018, 25(8): 886-893. DOI: 10.1111/jvh.12889.
[11]HUANG YJ, CHANG CS, PENG YC, et al. On-treatment HBV DNA level could predict HBeAg seroclearance in patients with HBeAg-positive chronic hepatitis B with entecavir therapy[J]. J Chin Med Assoc, 2017, 80(6): 341-346. DOI: 10.1016/j.jcma.2016.12.005.
[12]PENG CY, HSIEH TC, HSIEH TY, et al. HBV-DNA level at 6 months of entecavir treatment predicts HBeAg loss in HBeAg-positive chronic hepatitis B patients[J]. J Formos Med Assoc, 2015, 114(4): 308-313. DOI: 10.1016/j.jfma.2013.10.023.
[13]JI X, XIA MY, ZHOU B, et al. Serum hepatitis B virus RNA levels predict HBeAg seroconversion and virological response in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analog[J]. Infect Drug Resist, 2020, 13: 1881-1888. DOI: 10.2147/IDR.S252994.
[14]ZHANG M, LI GD, SHANG J, et al. Rapidly decreased HBV RNA predicts responses of pegylated interferons in HBeAg-positive patients: A longitudinal cohort study[J]. Hepatol Int, 2020, 14(2): 212-224. DOI: 10.1007/s12072-020-10015-3.
[15]YE F, ZHAO W, YANG X, et al. The decline of HBV RNA associated with HBeAg seroconversion and double-negative HBV DNA and RNA in chronic hepatitis B patients who received entecavir therapy: a 10-year retrospective cohort study[J]. Ann Transl Med, 2022, 10(16): 897. DOI: 10.21037/atm-22-3265.
[16]LUO H, ZHANG XX, CAO LH, et al. Serum hepatitis B virus RNA is a predictor of HBeAg seroconversion and virological response with entecavir treatment in chronic hepatitis B patients[J]. World J Gastroenterol, 2019, 25(6): 719-728. DOI: 10.3748/wjg.v25.i6.719.
[17]JIA W, ZHU MQ, QI X, et al. Serum hepatitis B virus RNA levels as a predictor of HBeAg seroconversion during treatment with peginterferon alfa-2a[J]. Virol J, 2019, 16(1): 61. DOI: 10.1186/s12985-019-1152-6.
[18]ZHAO XG, WANG J, LIU JC, et al. Baseline serum hepatitis B core antibody level predicts HBeAg seroconversion in patients with HBeAg-positive chronic hepatitis B after antiviral treatment[J]. Antiviral Res, 2021, 193: 105146. DOI: 10.1016/j.antiviral.2021.105146.
[19]WANG CT, ZHANG YF, SUN BH, et al. Models for predicting hepatitis B e antigen seroconversion in response to interferon-α in chronic hepatitis B patients[J]. World J Gastroenterol, 2015, 21(18): 5668-5676. DOI: 10.3748/wjg.v21.i18.5668.
[20]FAN R, SUN J, YUAN Q, et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues[J]. Gut, 2016, 65(2): 313-320. DOI: 10.1136/gutjnl-2014-308546.
[21]CAI SH, LI ZD, YU T, et al. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs[J]. Infect Drug Resist, 2018, 11: 469-477. DOI: 10.2147/IDR.S163038.
[22]FU XH, LOU HB, CHEN F, et al. Hepatitis B core antibody and liver stiffness measurements predict HBeAg seroconversion in HBeAg-positive chronic hepatitis B patients with minimally elevated alanine aminotransferase (ALT) levels[J]. Clin Exp Med, 2020, 20(2): 241-248. DOI: 10.1007/s10238-019-00603-5.
[23]XU WZ, TONG YQ, LI Y. Comparison of Roche Elecsys and Sysmex HISCL immunoassays for the screening of common blood-borne pathogens[J]. Ann Transl Med, 2019, 7(14): 300. DOI: 10.21037/atm.2019.05.83.
[24]WIJAYA RS, READ SA, TRUONG NR, et al. HBV vaccination and HBV infection induces HBV-specific natural killer cell memory[J]. Gut, 2021, 70(2): 357-369. DOI: 10.1136/gutjnl-2019-319252.
[25]TSUKUDA S, WATASHI K. Hepatitis B virus biology and life cycle[J]. Antiviral Res, 2020, 182: 104925. DOI: 10.1016/j.antiviral.2020.104925.
[26]KEENAN BP, FONG L, KELLEY RK. Immunotherapy in hepatocellular carcinoma: The complex interface between inflammation, fibrosis, and the immune response[J]. J Immunother Cancer, 2019, 7(1): 267. DOI: 10.1186/s40425-019-0749-z.
[27]CHEN T, ZHU L, SHI AC, et al. Functional restoration of CD56bright NK cells facilitates immune control via IL-15 and NKG2D in patients under antiviral treatment for chronic hepatitis B[J]. Hepatol Int, 2017, 11(5): 419-428. DOI: 10.1007/s12072-017-9803-4.
[28]HU X, MA S, HUANG X, et al. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease[J]. J Viral Hepat, 2011, 18(7): 458-467. DOI: 10.1111/j.1365-2893.2011.01475.x.
[29]YANG JZ, SHENG GP, XIAO DS, et al. The frequency and skewed T-cell receptor beta-chain variable patterns of peripheral CD4(+)CD25(+) regulatory T-cells are associated with hepatitis B e antigen seroconversion of chronic hepatitis B patients during antiviral treatment[J]. Cell Mol Immunol, 2016, 13(5): 678-687. DOI: 10.1038/cmi.2015.100.
[30]XIA J, HUANG R, CHEN YX, et al. Profiles of serum soluble programmed death-1 and programmed death-ligand 1 levels in chronic hepatitis B virus-infected patients with different disease phases and after anti-viral treatment[J]. Aliment Pharmacol Ther, 2020, 51(11): 1180-1187. DOI: 10.1111/apt.15732.
[31]AN X, LIU SH, XIA LN, et al. Efficacy and clinical outcomes of different antiviral regimens in HBeAg/HBeAb double-positive patients with chronic hepatitis B[J]. J Army Med Univ, 2022, 44(2): 162-167. DOI: 10.16016/j.2097-0927.202107114.安选, 刘书宏, 夏莉娜, 等. HBeAg/HBeAb双阳性慢性乙型肝炎患者不同抗病毒方案疗效及临床转归分析[J]. 陆军军医大学学报, 2022, 44(2): 162-167. DOI: 10.16016/j.2097-0927.202107114.
[32]LUO L, ZHANG TC, LIU SG. Clinical characteristics of chronic hepatitis B patients with both HBeAg and HBeAb positive[J]. J Mol Diagn Ther, 2019, 11(5): 387-390, 433. DOI: 10.3969/j.issn.1674-6929.2019.05.011.罗琳, 张廷超, 刘书刚. HBeAg与抗-HBe双阳性的慢性乙型肝炎患者临床特征分析[J]. 分子诊断与治疗杂志, 2019, 11(5): 387-390, 433. DOI: 10.3969/j.issn.1674-6929.2019.05.011.
[33]WANG H, WANG HP, QIAN LY, et al. Maternal breast feeding safety of hepatitis B virus carrying parturient women with hepatitis B surface antigen and hepatitis B e antigen double positive[J]. Chin J Infect Dis, 2020, 38(1): 44-48. DOI: 10.3760/cma.j.issn.1000-6680.2020.01.006.王虹, 王红萍, 钱琳妍, 等. 乙型肝炎表面抗原和乙型肝炎e抗原双阳性的乙型肝炎病毒携带产妇进行母乳喂养的安全性[J]. 中华传染病杂志, 2020, 38(1): 44-48. DOI: 10.3760/cma.j.issn.1000-6680.2020.01.006.
[34]KAO JH, CHEN PJ, LAI MY, et al. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers[J]. J Med Virol, 2004, 72(3): 363-369. DOI: 10.1002/jmv.10534.
[35]SHIMAKAWA Y, LEMOINE M, NJAI HF, et al. Natural history of chronic HBV infection in West Africa: A longitudinal population-based study from The Gambia[J]. Gut, 2016, 65(12): 2007-2016. DOI: 10.1136/gutjnl-2015-309892.
[36]KRAMVIS A, KOSTAKI EG, HATZAKIS A, et al. Immunomodulatory function of HBeAg related to short-sighted evolution, transmissibility, and clinical manifestation of hepatitis B virus[J]. Front Microbiol, 2018, 9: 2521. DOI: 10.3389/fmicb.2018.02521.
收稿日期:2022-12-02;錄用日期:2023-01-09
本文编辑:邢翔宇
引证本文:TAN YF, SUN FL, XIA W, et al. Predictive value of anti-HBs+anti-HBe for seroconversion of HBeAg in patients with positive HBsAg/HBeAg/anti-HBc[J]. J Clin Hepatol, 2023, 39(8): 1832-1840.