Research progress of tumor metabolism in metastasis
2023-02-24MAOHuilanZHUHaitaoPEIWenhaoWANGWenruiCHENChangjieYANGQingling
MAO Hui-lan,ZHU Hai-tao,PEI Wen-hao,WANG Wen-rui,CHEN Chang-jie,YANG Qing-ling
[Abstract] An important biological characteristic of malignant tumors is metastasis,and it is often the primary reason why tumor treatments fail.The capacity of tumor cell proliferation and migration involves metabolic reprogramming that exists in almost all tumor cells and differs from other normal cells.This review summarizes the research progress of various metabolisms in tumor metastasis and the problems faced by metabolic therapy to inhibit metastasis.
[Key words] metabolism;metabolic reprogramming;tumor microenvironment;metastasis
In 1920,Otto Warburg pioneered the discovery that tumor cells produced energy by switching from mitochondrial respiration to glycolysis.Even under oxygen-rich conditions,cancer cells transfer pyruvate produced by glucose from aerobic oxidation to the pathway of lactic acid production,which is called aerobic glycolysis by Otto Warburg,also called the "Warburg effect"[1-2].As oncology research advances,tumor metabolism has become the focus of contemporary tumor research,and the tumor is also considered a metabolic disease.Metabolic reprogramming provides energy and material basis for the rapid proliferation and metastasis of tumors,and is one of the important markers of malignant tumors[3].Therefore,targeted metabolism has become one strategy for treating tumors.
Metabolic reprogramming is one of the characteristics of tumor cells and their adjacent cells in the tumor microenvironment (TME),which is regulated by carcinogenic mutation and the TME.There is an interactive relationship between tumor cells and TME at the metabolic level.Tumor cells can change the metabolites and conditions of TME in order to proliferate and develop in challenging environments.TME can also influence metabolic changes in tumor cells,which can change and maintain their occurrence and development[4-6].The two depend on each other and promote each other.In recent years,because of the progressive study of tumor-related cellular and molecular mechanisms,people have a deeper understanding of the role of tumors and their microenvironment,which is of great significance for understanding biological behaviors such as tumor occurrence,development and metastasis.
Metastasis is an important biological characteristic of malignant tumors and the principal cause of most tumor-related deaths.With the continuous development of cancer biological research and metastasis research,it has been found that tumor cells induce the process of metastasis through interaction with other cells and molecules in the TME[7-8].Metastasis is a complex and continuous multi-step process involving local invasion of the basement membrane,infiltration into the surrounding blood vessels or lymphatic system (intravascular),circulatory survival,and vascular extravasation and metastasis site colonization.To survive and develop in the metastasis cascade,tumor cells will choose the suitable growth metabolism according to the local environment of each stage of metastasis[9-10].Therefore,the metabolic limitation may provide a therapeutic approach for the prevention and cure of metastatic tumors.
The biological process of tumor metastasis is complex and challenging.Metastatic tumor cells adaptively regulate key metabolisms,such as glucose,amino acid and lactic acid metabolism,to respond to changes in the local microenvironment.Clarifying metabolic reprogramming during tumor metastasis is important to reveal the metabolic vulnerability of metastatic tumor cells and to determine new anti-tumor metastatic strategies.In latest years,the research on tumor metabolism in metastasis has been a new field,the literature is relatively less.This review summarizes the key metabolism affecting cancer metastasis and discusses the problems that need to be solved in metabolic therapy for metastasis inhibition in the future.
1 Glucose metabolism and metastasis
Glucose metabolism provides raw materials for biosynthesis,adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate,etc.,which is the principal energy source of organisms.Most tumor cells use aerobic glycolysis instead of oxidative phosphorylation (OXPHOS) as their main energy source[11].This metabolic change is disadvantageous to energy production,but it can achieve rapid production of energy and intermediate metabolites and promote tumor metastasis[11-12].Tumor cell metastasis is an energy-intensive process.For example,metastatic cells extend to invade pseudopodia when they locally invade the basement membrane or highly mechanically plastic matrix,which requires a lot of energy to support the actin cytoskeleton[13].With the increase in glucose level,the ratio of intracellular ATP:adenosine diphosphate (ADP),cell migration speed,and net migration distance of breast cancer cells cultured in the collagen matrix increased[14].This suggests that when the energy cost of metastasis increases significantly,tumor cells may ingest a large amount of glucose to enhance the supply of ATP,and then promote metastasis.
Glycolysis of tumor cells can respond quickly to the energy needs in the process of metastasis,which is related to cytoskeleton activity and faster migration.In prostate and breast cancer cells,mitochondrial-derived ATP is insufficient,and glycolysis is used to supply the energy needed for cell movement and cytoskeleton remodeling during metastasis[15].When tumor cells absorb glucose for glycolysis and energy supply,intracellular glucose-metabolizing enzymes and transporters will be up-regulated.As a result,researchers have gradually noticed a role in the expression of glucose-metabolizing enzymes and transporters in tumor proliferation and metastasis[16].
There have been many research results on the role of key enzymes and transporters in glucose metabolism (figure 1) in tumor metastasis.Such as glucose transporter (GLUT) and hexokinase (HK),the expression of its subtypes GLUT-1 and HK-Ⅱ in tumor cells is positively correlated with tumor glucose uptake[17-19]and affects tumor growth and metastasis by regulating glycolysis[20-22].In pancreatic cancer,the expression of GLUT1 and pyruvate kinase muscle isozyme 2 (PKM2) upregulated significantly except for lung metastasis.Key enzymes in metabolism can also contribute to tumor metastasis by regulating the expression of other proteins and signal pathways,such as PKM2,which can promote metastasis by phosphorylating p21 activated kinase 2 (PAK2) protein in pancreatic ductal adenocarcinoma[23].In breast cancer cells,methylated PKM2 can interact with inositol 1,4,5-trisphosphate receptors (IP3Rs) and inhibit the expression of IP3Rs to control the absorption of Ca2+by mitochondria,and changes the metabolic balance from OXPHOS to aerobic glycolysis,thus affecting migration and metastasis[24].PKM2 regulates the phosphatidylinositol 3-kinase/AKT (PI3K/AKT) pathway in gastric cancer cells,thereby inhibiting migration and autophagy[25].Regulating the expression of key enzymes and transporters in glucose metabolism can inhibit tumor metastasis,which shows that key enzymes and transporters are effective potential targets for tumor metastasis therapy.
2 Amino acid metabolism and metastasis
Amino acids are important alternative energy sources for tumor cells.The ability of cancer cells to use amino acids is related to more aggressive and migratory phenotypes.Amino acids are used for metabolite synthesis and are also involved in the defense of oxidative stress and cellular energy supply[26-27].It has been found that the metabolic changes of amino acids such as glutamine[28],serine[29],alanine[30],and asparagine[31]are strongly associated with growth and metastasis of tumor cells.An important change in tumor metabolism is glutamine addiction.Glycolysis of tumor cells transfers glucose-derived pyruvate to the production of lactic acid and away from mitochondria,which results in a marked increase in glutamine metabolism.Glutamine metabolism is related to tumor cell invasion and migration in vitro and tumor metastasis and colonization in vivo[32-33].At present,the treatment strategy for glutamine metabolism is mainly to reduce tumor metastasis by targeting glutamine transporter[34],glutaminase and glutamine synthetase[35-36].It is worth noting that dietary amino acid intake can also affect tumor cell metastasis[31].
For the past few years,it has been discovered that the amino acid metabolism of tumors is strongly associated with the TME and can affect the immune response related to tumorigenesis and metastasis,which in turn affect the metastasis of tumor cells[26,37].Glutamine metabolism can modulate the formation and recruitment of bone marrow-derived suppressor cells (MDSC) and the activation and differentiation of T cells.Targeted glutamine metabolism can affect the anti-tumor immunity of MDSC and T cells,thus influencing tumor growth and metastasis[38-39].The decomposition of glutamine to produce α-ketoglutaric acid is essential in the activation of M2 macrophages[40].
3 Lactic acid metabolism and metastasis
The major product of aerobic glycolysis of tumor cells is lactic acid.Its efflux will increase the acidity of the TME,cause extracellular acidification,promote the formation and maturation of invasive pseudopodia,and increase the invasive ability of metastatic cells through matrix metalloproteinases (MMPs) degradation of the surrounding matrix[41-42].Extracellular acidification also affects biological processes such as angiogenesis[43].When the supply of glucose is sufficient,tumor cells mainly carry out glycolysis,which causes an accumulation of intracellular lactic acid and an increase in extracellular acidification,but when lactic acidosis occurs,intracellular glycolysis slows down,and the rate of mitochondrial OXPHOS exceeds the rate of glycolysis[44].
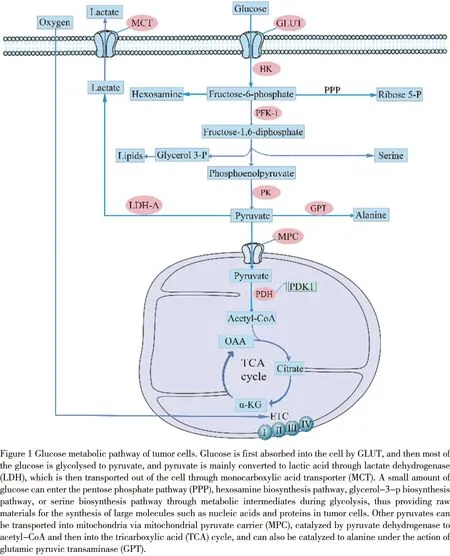
Lactate dehydrogenase A (LDH-A) mainly catalyzed the pyruvate produced by glycolysis in tumor cells to produce lactic acid,which is transported by the monocarboxylic acid transporter (MCT).Inhibition of LDH-A expression can suppress the invasion and migration of prostate cancer[45],pancreatic cancer[46],and renal cell carcinoma[47].Experimental studies of renal cell carcinoma in vitro and in vivo have suggested that LDH-A mediates tumor metastasis by promoting epithelial-mesenchymal transformation[48].MCT1 and MCT4 mainly transported lactic acid.Inhibition of MCT1 function will lead to the decrease of glutathione (GSH) caused by intracellular lactic acid accumulation,while the decrease of GSH will cause mitochondrial damage and eventually lead to cell death[49].High expression of MCT1 has been confirmed to be associated with tumor cell migration and invasion in bladder cancer[50],renal cell carcinoma[51],hepatocellular carcinoma[52],and osteosarcoma[53].MCT4 is suitable for the lactate output of glycolytic cells and is up-regulated because of hypoxia[54].MCT1 and MCT4 are prognostic biomarkers in patients with clear cell renal cell carcinoma.PAYENetal.found that MCT1 promotes cancer cell migration by activating transcription factor NF-κB.It has nothing to do with its transporter activity,suggesting that pharmacological inhibitors of MCT1-mediated lactic acid transport may not be effective in preventing tumour cell metastasis[54].Therefore,when targeting tumor metabolism by metabolic enzymes and transporters to inhibit metastasis and achieve prognostic treatment,we also need to consider the effectiveness of target inhibitors.
Recent studies have revealed that lactic acid can regulate the expression of programmed death-1 (PD-1) on T cells[55],or control immune escape through paracrine activation of G protein-coupled receptor 81 (GPR81) on stromal dendritic cells[56].This shows that lactic acid can affect the immune function of the TME,thus affecting the killing effect of immune cells on tumor metastatic cells.
4 Lipid metabolism and metastasis
Besides providing energy for tumor cells and taking part in biofilm synthesis,lipid metabolism also has an essential role in tumorigenesis,development,metastasis,and immune response in the TME[57-58].Changes in lipid metabolism have been found to impact the ability of many types of cancer to invade and metastasize,such as hepatocellular carcinoma (HCC)[59-60],breast cancer[61],and gastric cancer[62].The interaction between programmed ferroptosis and lipid metabolism in tumor cells can regulate tumorigenesis,development,and metastasis[63-64].Lipids can enter tumor cells through passive diffusion,fatty acid transporter (FATP) and fatty acid transporter CD36,or uptake of low-density lipoprotein and very-low-density lipoprotein[65-66].CD36 acts as a lipid sensor[67]and is related to tumor metastasis.Inhibition of CD36 can suppress the metastasis in oral cancer[68],esophageal squamous cell carcinoma[69],and gastric cancer[70].Fatty acids and cholesterol are essential lipids in lipid metabolism.Fatty acid oxidation can provide energy for tumor cells and affect the phenotypic transformation of melanoma[71].Cholesterol is involved in the formation of biofilm,lipid rafts,and other biomolecules.Too much cholesterol can reduce membrane fluidity,destroy lipid raft signals and cause cell death[57,72],which can promote metastasis in immune cells[73].
Lipid metabolism in tumor cells is associated with stromal cells (e.g.adipocytes,immune cells and tumor-associated fibroblasts) in the TME.Adipocytes activated by tumor cells drive tumor metastasis by lipolysis of stored triglycerides and secretion of fatty acids[74-75].The crosstalk of lipid metabolism between adipocytes and tumor cells benefits the invasion and metastasis of breast cancer[75-76],ovarian cancer[77],gastric cancer[78-79],and melanoma[80].Tumor-related fibroblasts are also participating in the lipid metabolism of cancer cells,affecting tumor proliferation,growth,and metastasis[81-84].It is worth noting that lipids play a contradictory role on immune cells,not only supporting the immune response but also inhibiting the immune response[57].
5 Mitochondrial metabolism and metastasis
Mitochondria are important productive organelles in cells.Tumor cells adapt to different microenvironments by regulating mitochondrial metabolism[85].In recent years,researchers have found that unlike "Warburg effect",mitochondrial OXPHOS expression is upregulated in some cancers,such as lymphoma,pancreatic ductal carcinoma and prostate cancer,and thus mitochondrial metabolism has begun to attract attention[86-90].In breast cancer cell line MDA-MB-468 and MCF-7,they have similar glycolysis flux under normoxic conditions,but the highly metastatic cell lines have higher levels of OXPHOS,while hypoxia can still promote glycolysis and inhibit OXPHOS[91].Therefore,in the process of relatively high oxygen concentration of tumor metastasis,tumor cells are likely to increase the level of OXPHOS to provide energy.
Mitochondrial metabolism is organ-specific in metastasis,and metastatic cancer shows different metabolic characteristics according to its metastatic site.Compared with bone or lung metastatic breast cancer cells,the transformation of pyruvate to lactic acid produced by glucose metabolism in liver metastatic breast cancer cells increased and mitochondrial metabolism decreased[92].Further analysis showed that liver metastatic breast cancer cells increased glycolysis by upregulating the expression of hypoxia-inducible factor 1α (HIF-1α) and then targeting pyruvate dehydrogenase kinase-1 (PDK1)[92].However,breast cancer cells with bone or lung metastasis increase oxidative stress by over-expressing peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α)[93].However,it is not clear whether the metabolic changes of the primary tumor determine the site of metastasis,or whether the metastatic site determines its metabolic reprogramming.
Targeting mitochondrial metabolism to inhibit tumor progression has gradually been gaining the attention of researchers.The transmembrane protein complex in the electron transport chain as an important regulator of mitochondrial metabolism has been studied,such as phenformin,petasin,and OXPHOS inhibitor IACS-010759 and other inhibitors can inhibit complex I and affect mitochondrial metabolism[94-96].Mitochondria are also participating in ferroptosis caused by cysteine deprivation.Targeting mitochondrial metabolism can achieve tumor inhibition by affecting ferroptosis[97-98].
6 Metabolism in the TME and metastasis
The TME is the direct niche around tumor cells,which is composed of blood vessels,stromal cells including immune cells and fibroblasts,extracellular matrix,and so on.It interacts with tumor cells in metabolism[99-100].Nutrients in the TME,characteristics of extracellular matrix,and interplay between tumor cells and stromal cells can affect metabolic reprogramming,and then affect tumor metastasis[101].Although blood vessels can supply nutrients and oxygen,except for tumor cells in the perivascular layer,most tumor cells are in an environment of hypoxia,nutrient deficiency,and acidity[102-104].This is not only the cause of metabolic reprogramming of tumor cells but also the result of metabolic regulation.
There is metabolic crosstalk among the cells in the TME,which affects the progression of tumor metastasis.Secretions metabolized by tumor cells can induce the differentiation of stromal cells,form a tumor-promoting environment,and then promote metastasis,and the metabolites of stromal cells will promote the epithelial-mesenchymal transformation of tumor cells[105-106].The metabolic changes of tumor cells in the TME can also impact the metabolic pathway of immune cells,such as T cells,macrophages,etc.,and then affect the immune effect and metastasis in the TME[107-109].
The TME is an important determinant of metabolic heterogeneity.Metabolic secretions between TME and tumor cells can affect the formation of a pre-metastatic niche in the pre-colonization stage.In the process of metastasis,tumor cells adjust metabolism to resist oxidative stress in the environment[110-114].Metastasis is a multi-stage cascade process,and different tumor types and different distant metastatic organs have different metabolic environments,so they have different metabolic regulations at different stages.Therefore,the metabolic characteristics of metastatic stages may offer a new treatment for tumor metastasis[7].Future work will need to study the local microenvironment and metabolic changes at various stages of metastasis,which is very important for the formulation of effective treatment strategies.
Discussion According to tissue specificity,different tumor cells have different metabolic characteristics,and the metabolic challenges they face in each stage of metastasis are not necessarily the same.Over the past few decades,statins,metformin,and SGLT2 inhibitors are a few examples of medications that target metabolism and have had substantial effects on human health and disease.However,there are still few metabolic therapeutic drugs successfully developed,the plasticity and fragility of metabolism will affect the effect of targeted metabolic therapy,and the interplay between tumor cells and stromal cells in the TME will also influence the therapeutic effect.Therefore,there is a need to further clarify the metabolic regulation related to tumor metastasis.In addition,due to the related toxicity of anti-metabolic drugs to host cells and other limitations,how to target key metabolic enzymes and key metabolic nodes without damaging normal tissue cells and ensuring immune cell function has become the key to targeted metabolic drug therapy.
At present,the research on metastasis is mostly focused on the early stage of metastasis,and there are few studies on the stages of circulation and colonization.We need more research to target the inhibition of metastasis in each stage according to the metabolic characteristics.More research is needed on the overall effect of tumor and its microenvironment metabolism and whether it can be combined with other treatments such as immunotherapy to inhibit metastasis.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions All authors listed have made a substantial,direct,and intellectual contribution to the work,and approved it for publication.
