Eff icient reduction of β-lactoglobulin allergenicity in milk using Clostridium tyrobutyricum Z816
2023-01-23QianruZhaoYuweiWangZhengmingZhuQuanyuZhaoLiyingZhuLingJiang
Qianru Zhao, Yuwei Wang, Zhengming Zhu, Quanyu Zhao, Liying Zhu*, Ling Jiang,*
a College of Biotechnology and Pharmaceutical Engineering, State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 210009, China
b College of Food Science and Light Industry, Nanjing Tech University, Nanjing 210009, China
c School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing 211816, China
d School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 210009, China
Keywords:Milk allergy β-Lactoglobulin Clostridium tyrobutyricum Permeabilized bacteria
A B S T R A C T Milk allergy is one of the most common food allergies, affecting 6% of young children, and β-lactoglobulin( β-LG) is the main milk allergen. Clostridium tyrobutyricum Z816 was selected for the degradation of β-LG,which was successfully reduced by about 90% using permeabilized bacteria under the optimized conditions.The hydrolyzed peptides were identif ied by liquid chromatography-tandem mass spectrometry (LC-MS/MS)and analyzed by molecular modeling, which indicated that C. tyrobutyricum Z816 could effectively degrade the antigenic epitopes of β-LG. Finally, the concentration and digestibility of β-LG in actual samples was quantified using enzyme-linked immunosorbent assay (ELISA) and gastrointestinal digestion simulation experiments. The results showed more than 92% of β-LG in actual samples was hydrolyzed, and the gastric and total digestibility of whey protein isolate (WPI) was improved by 85.96% and 64.51%, respectively.Therefore, C. tyrobutyricum Z816 offers an effective method to degrade β-LG and reduce the occurrence of milk allergies, which has great signif icance for the development of hypoallergenic dairy products.
1. Introduction
The increase in the prevalence of allergic diseases in recent years has become a serious public health issue around the world [1]. Food allergies are the most common cause of anaphylaxis, accounting for 81% of cases in children [2-4]. Some common foods, such as milk,eggs, f ish, peanuts, tree nuts, wheat and soy, are more likely to cause allergic reactions [5-7]. One of the most common food allergies in toddlers and young children is cow milk allergy (CMA) [8-11],which is characterized by immunoglobulin E (IgE)-mediated or cellmediated immune hyperactivation [12]. Milk allergy in infants is often exacerbated by diff iculty in digesting allergens in their intestinal system. Therefore, effective prevention and control of milk allergy has become the focus of food allergy research.
The World Health Organization and International Union of Immunological Societies (WHO/IUIS) office listedβ-lactoglobulin(β-LG),α-lactalbumin (α-LA) andα-casein (α-CN) as major milk allergens [13-17], among whichβ-LG is widely considered as the main milk allergen [18-21]. Because the tertiary structure ofβ-LG is extremely stable, it is resistant to digestion in the stomach. A variety of methods such as heat treatment and glycation [22-24] have been made to change structure ofβ-LG and reduce specific IgE againstβ-LG, thus reducing the occurrence of allergic reactions. However,these methods usually require stringent treatment conditions, which will destroy the f lavor and nutritional content of milk.
Microbial fermentation based on proteolytic system can be used as a mild alternative to decrease milk allergenicity. Milk and whey fermentation combined with probiotics has been widely used in the development of hypoallergenic milk in recent years [25,26]. Bioactive substances released during fermentation can have immunoregulatory effects additionally reducing milk protein allergenicity [27]. At the same time, probiotics have a beneficial influence on the balance of intestinal flora, which promotes the digestion process. Lactic acid bacteria (LAB) have been reported as an innovative model for the prevention and treatment of milk allergenicity using a non-proliferating bacteria system [28,29]. And the proteolytic system of LAB is thought to play a major role in the hydrolysis of allergens. Interestingly, Berni Canani et al. [30] found that infant formula containingLactobacillus rhamnosuscan cause the enrichment of butyric acid-producing bacteria in infants with food allergies. A number of studies have demonstrated that short chain fatty acid may positively modulate the immune response [31,32].Inspired by these studies, we aimed to degrade allergenic protein in milk usingClostridium tyrobutyricumthat is an organic heterotrophic obligate anaerobic bacterium with butyric acid production capacity,which is traditionally used as a probiotic to improve gastrointestinal function, since this bacterium can synthesize butyric acid, acetic acid and other short chain fatty acids [33]. However, there was no relevant research on the degradation of allergenic proteins byC. tyrobutyricum. Notably, our team carried out a series of studies using permeabilized bacteria as a whole-cell biocatalyst as an alternative to the traditional non-proliferating bacteria system [34,35].
In this study, we developed a bioprocess for reducing the allergenicity ofβ-LG usingC. tyrobutyricumZ816 with an optimal concentration, pH and degradation time of bacteria. Furthermore, we investigated the ability to degradeβ-LG using permeabilized bacteria under the optimized conditions using a variety of detection methods.Importantly, the peptides released byβ-LG hydrolysis were identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS)and analyzed by molecular modeling. The relationship between the hydrolysis site ofβ-LG and antigen epitopes was investigated and compared with other methods. Finally, we used representative actual samples to evaluate the ability ofC. tyrobutyricumZ816 to hydrolyzeβ-LG and investigated the effect of the hydrolysates on digestion.
2. Materials and methods
2.1 Materials and microorganism
Theβ-LG standard (90% purity) and colistin sulphate used in this work were purchased from Sigma-Aldrich (St. Louis, MO, USA),trifluoroacetic acid (TFA) and acetonitrile were purchased from Sangon Biotech (Shanghai) Co., Ltd. (China). All other chemicals and reagents were from local commercial sources and of analytical grade.
TheC. tyrobutyricumZ816 strain used in this work was isolated from the feces of brown cattle. The bacterial suspension was diluted, spread on a plate containingβ-LG, and cultured at 37 °C.The strain that could produce transparent halos was selected for plate re-screening, and finally the target strain was obtained. It was routinely activated in reinforced clostridium medium (RCM) at 37 °C overnight. Cultures were preserved in 30% glycerol at -80 °C for long term storage.C. tyrobutyricumZ816 was deposited in Guangdong Culture Collection Center (GDMCC NO. 60753).
2.2 Hydrolysis of β-LG using non-proliferating and permeabilized bacteria
Cultures were incubated in RCM medium at 37 °C for 24 h. Fresh cells were collected at the exponential growth phase and centrifuged to remove the excess nutrients. Then, the cells were washed with deionized water 2 times and resuspended in 0.1 mol/L sodium phosphate buffer (pH 7.0) to a final optical density (OD600nm) of 5.The resulting suspension was mixed withβ-LG (2 mg/mL in sodium phosphate buffer) in an equal volume as hydrolytic substrate, and the mixture was shaken for 24 h at 37 °C. The mixtures were centrifuged at 4, 8, 12, 16, 20 and 24 h (4 000 ×g, 4 °C), and the supernatant was removed for analysis. The concentration, pH and degradation time of bacteria for the degradation ofβ-LG were optimized in single-factor experiments.
The permeabilization of bacteria can not only improve the catalytic efficiency, but also avoid the pollution of cell fragments and their intracellular substances. The bacteria were permeabilized with 0.2 g/L colistin sulphate for 30 min at 37 °C. The concentration of leaked protein was measured using an ultraviolet detector at 280 nm.The hydrolysis experiment using permeabilized bacteria was conducted the same way as described for non-proliferating bacteria.
2.3 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and reversed-phase-high performance liquid chromatography (RP-HPLC) analysis
Tricine SDS-PAGE was used to analyze the proteins present in the supernatant as described before [20]. Coomassie brilliant blue solution in 25% (V/V) ethanol and 8% (V/V) glacial acetic acid was added to dye the protein bands overnight, and the gels were decolorized with decolorization containing 25% (V/V) ethanol and 8%(V/V) glacial acetic acid. The QuickGel 6100 Gel imaging system was used to scan the gels and analyze the protein bands.
The content ofβ-LG in the supernatant were analyzed by RPHPLC (Dionex UltiMate 3000, USA). Samples (10 μL) were injected into C8column (4.6 mm × 150 mm, 5 µm) for detection, and gradient elution with a volume fraction of solvent A (water, 0.1% (V/V) TFA)and solvent B (acetonitrile, 0.1% (V/V) TFA) at a flow rate of 1 mL/min was used to analyze the peptides. Elution was performed by applying 30%-50% solvent A at 0-17.5 min, and 50%-70% solvent B at 17.5-20 min. The peptides were detected using a UV detector(UltiMate 3000, Thermo Fisher, USA) at 280 nm.
2.4 Analysis of peptides in the β-LG hydrolysates by LC-MS/MS
The peptides were purified and desalted using C18solid phase extraction (SPE) cartridges (standard density). The peptides were dissolved in 0.1% formic acid and 2% acetonitrile. The mixtures were centrifuged at 4 000 ×gand 4 °C for 20 min, and the supernatant was removed as sample for mass spectrometry. The specific LC parameters were as follows: (a) chromatographic column information:Acclaim PepMap RSLC C18column (300 µm × 5 mm, 5 µm);(b) solvent information: solvent A (0.1% formic acid) and solvent B(0.1% formic acid, 80% acetonitrile) at a flow rate of 300 nL/min;(c) solvent B rises from 5% to 90% within 65 min.
The separated peptides were directly injected into the mass spectrometer for online detection. The scan range was set between 400 and 2 000 Da in MS mode. The Mascot software was used to identify peptides by searching against the UniProt database (Mascot:http://www.matrixscience.com/) with the following parameters:enzyme: none; fixed modifications: carbamidomethyl (C); variable modification: oxidation (M); peptide mass tolerance: 20 ppm;fragment mass tolerance: 0.6 Da; mass values: monoisotopic.
2.5 Enzyme-linked immunosorbent assay (ELISA)
Allergen-specific IgE was measured using double antibody sandwich method. An ELISA kit was purchased from Jiangsu Meimian Industrial Co., Ltd. (Jiangsu, China). The samples were added to a 96-well plate with purified antibodies, and incubated with a HRP (horseradish catalase)-labeled anti-β-LG antibody for 1 h at 37 °C. Then, 100 μL of 3,3’,5,5’-tetramethylbenzidine (TMB)was added and incubated in the dark for 15 min at 37 °C as a color reagent to reveal the progress of the reaction. The absorbance at 450 nm was recorded using a SpectraMax M3 microplate reader(Molecular Devices, USA). 1 000 μg/mL of antigen was treated with permeabilized bacteria (inhibitors) at different optical densities,the concentration of bacterial concentration (inhibitor) required to inhibit 50% of the total binding of serum IgE (IC50) was calculated from the inhibition curves by relating absorbance intensity to inhibitor concentrations. Absorbance data were corrected by subtracting the absorbance of the control with no bacteria.
2.6 Hydrolysis of samples and digestibility of peptides in vitro
Protein solution (1% in 1 mol/L HCl, pH 3.0) was incubated at 37 °C for 10 min, after which pepsin was added to simulate the digestive process of gastric juice for 1.5 h. After that, pepsin was inactivated to terminate the reaction. To further simulate the digestive process of intestinal juice, the experimental steps were the same except replacing pepsin to trypsin. For the calculation of protein digestibility, the samples were combined with an equal volume of trichloroacetic acid (24%,V/V) solution to precipitate the protein.The supernatant was collected to determine the protein content by the Kjeldahl method [36] according to the following equation:
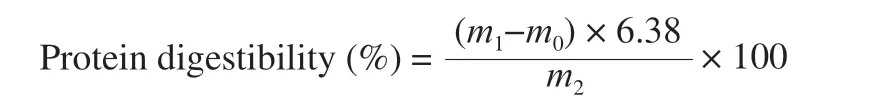
m1: nitrogen content in the sample supernatant;m0: nitrogen content in the blank supernatant;m2: protein content in the sample.
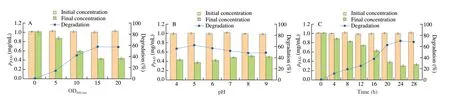
Fig. 1 Degradation of β-LG under different conditions. (A) Optical density (OD600 nm); (B) pH; (C) degradation time.
WPI, whey protein concentrate (WPC) and infant formula purchased in market with different content ofβ-LG were used as representative samples for the hydrolysis and digestibility experiments.
3. Results and discussion
3.1 Isolation and characterization of phenotypic of C. tyrobutyricum
The isolated strain can produce hydrolysis circle on the plate containingβ-LG (Fig. S1). The colony surface on the plate after proliferation culture is round, the middle is slightly protruding, and the edge is complete. The bacterial colony is gray, translucent and glossy on the surface. Its diameter is about 1-3 mm (Fig. S2). The 16S rDNA analysis results determined that it wasC. tyrobutyricum(supporting information), and the strain was named Z816. It shows 99% similarity withClostridium tyrobutyricumL319 (Accession No. SRR12149134) strain in our group [37]. We speculated that the genome information of Z816 is similar to that of strain L319,expecially the hypothetical proteins annotated as proteases in the gene information.
3.2 Changes of β-LG concentration during treatment with non-proliferating bacteria of strain Z816
We carried out the experiment of shaking flask degradation and foundC. tyrobutyricumZ816 was able to degradeβ-LG. The degradation ability ofC. tyrobutyricumZ816 was quantitatively analyzed by detecting the residual amount ofβ-LG in the supernatant.In previous studies, Pescuma et al. [18] investigated the degradation effect of the suspension ofLactobacillus delbrueckiisubsp.bulgaricusCRL 454 with different optical density with pH at 7.0. In a similar study, reaction mixtures containingβ-LG andLactococcus lactisBMC12C and BMC19H with an optical density at 600 nm of 25 were carried out [38], while a pH value of 4.6 was used forβ-LG hydrolysis usingLactobacillus caseiLcY under different reaction time conditions [39]. Accordingly, we also explored and optimized the degree of protein hydrolysis under different conditions of the degradation process, including the optical density (OD600nm), pH value and degradation time.
As shown in Fig. 1, the content of residualβ-LG decreased with the increase of bacterial optical density (OD600nm). When the optical density (OD600nm) of bacterial suspension was less than 5,the degradation efficiency ofβ-LG was only 10%. By contrast, the degradation efficiency reached 60% when the optical density (OD600nm)of the bacterial suspension was 15, while a further increase of bacterial optical density did not further improve the degradation efficiency. It is possible that excessively high concentration of bacteria affected the normal function of them. We speculated that the degradation process was related to the catalysis of enzymes in the organism, so we studied the effect of pH value on the degradation process. However, we did not find that the pH value of the environment had a significant effect on the degradation process. The degradation efficiency ofC. tyrobutyricumZ816 was about 40%-45% in the entire tested pH range of 4-9. The degradation efficiency at pH 5 was better in comparison, possibly becauseC. tyrobutyricumproduces butyric acid,which leads to the decrease of pH value in the fermentation process.At the same time, we optimized the degradation time and found that the degradation process tended to reach saturation after 24 h, which the degradation efficiency did not change significantly. Different from CRL 454, the degradation efficiency ofC. tyrobutyricumZ816 was highest after 12 h, while that of CRL 454 was highest in the first 4 h, and the final degradation efficiency reached 70% avter 18 h [18]. In conclusion,we proved that non-proliferating bacteria ofC. tyrobutyricumZ816 were able to degradeβ-LG (70%) under the optimized conditions of optical density (OD600nm) at 15 and pH at 5 after 24 h of incubation.
3.3 Hydrolysis of β-LG used permeabilized bacteria of strain Z816
Although we found that the bacteria could effectively degradeβ-LG, there were still about 30% of residualβ-LG that had not been degraded, which could still provoke allergic reactions. Biscola et al. [40]reported that proteases from LAB played a major role in the degradation of immunoreactive proteins in milk. In our previous studies, we found that permeabilization ofEscherichiacolicould significantly increase the production of trehalose due to better contact between the substrate and trehalase [34]. Our team also constructed permeabilized trehalase expressingBacillus subtilisbacteria to improve the catalytic activity of the enzyme. Therefore,we tried to further improve the degradation efficiency ofβ-LG used permeabilized bacteria. In this study, we used the biological permeabilization reagent colistin sulfate for bacteria to carry out permeability treatment. Compared with chemical reagents, it has the advantages of high safety and low toxicity, while also avoiding some shortcomings of physical methods such as cumbersome operation and cell damage.
As shown in Fig. 2, the leakage of intracellular proteins indicated the formation of large pores in the cell membrane. The extracellular protein of the non-proliferating bacteria without permeabilization treatment remained at about 0.15, but it could reach 0.6 after 10 min of permeabilization treatment, which was 4 times higher that of the non-proliferating bacteria. These results indicated that permeabilization treatment can promote the opening of the cell membrane and release intracellular enzymes. Therefore, we used the permeabilized bacteria to degradeβ-LG. The hydrolytic ability ofC. tyrobutyricumZ816 to degradeβ-LG was analyzed by SDS-PAGE and RP-HPLC, and the hydrolytic ability of non-proliferating and permeabilized bacteria toβ-LG was compared in Fig. 3 and Fig. 4. It was found that the band ofβ-LG became weaker with the extension of reaction time, and the band after permeabilization treatment was significantly weaker than that without treatment after 20 h, which confirmed that permeabilized bacteria ofC. tyrobutyricumZ816 had betterβ-LG degradation efficiency after incubation for 20 h. Further RP-HPLC indicated that the hydrolysis rate ofβ-LG was 70% with non-proliferating bacteria and 95% with permeabilized bacteria after 24 h of incubation (Fig. 4), which was consistent with the previous results. These results indicated that permeabilization treatment can significantly improve the whole-cell catalytic properties of bacteria,which is conducive toβ-LG degradation. The catalytic mass transfer of enzymes in the cell would be improved in permeabilized bacteria while the complete cell structure could provide good protection for the enzyme and prolong its life. We speculated that the degradation ofβ-LG was due to the production of protease byC. tyrobutyricum. Cell permeabilization improved the contact between the substrate protein and proteases to achieve better degradation efficiency.

Fig. 2 The leakage of proteins from C. tyrobutyricum Z816 after permeabilization, measured at 280 nm.
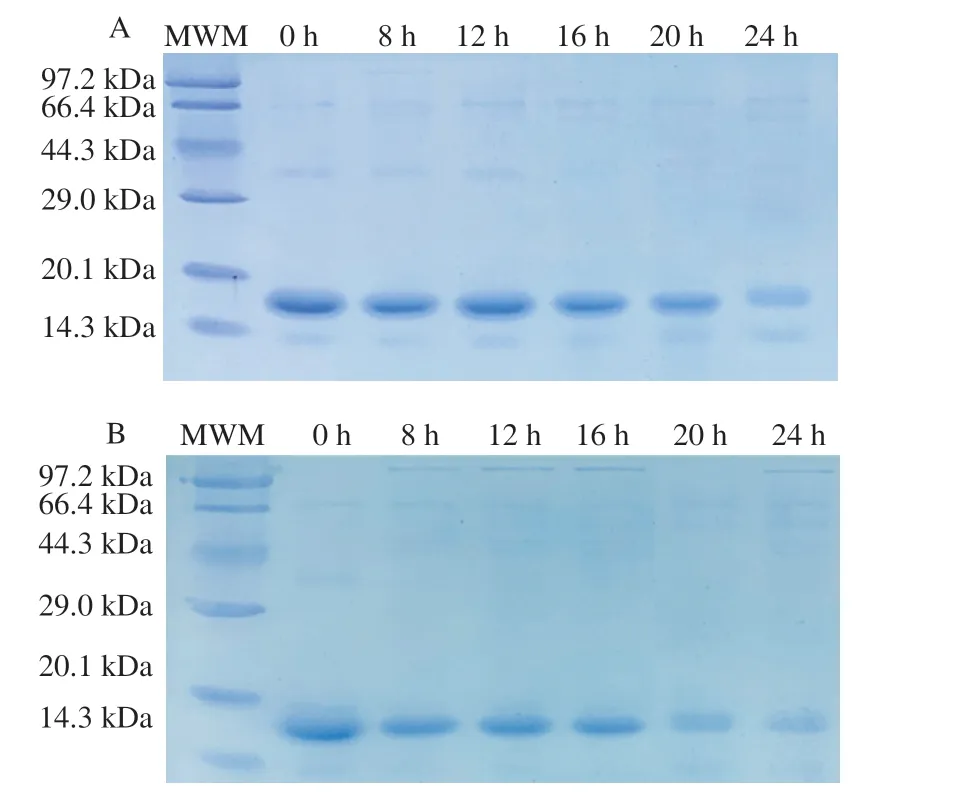
Fig. 3 SDS-PAGE showing the hydrolysis of β-LG by (A) non-proliferating bacteria and (B) permeabilized bacteria of C. tyrobutyricum Z816. A wide range (2-212 kDa) molecular weight marker (MWM) was used. The numbers indicate the incubation time in hours.
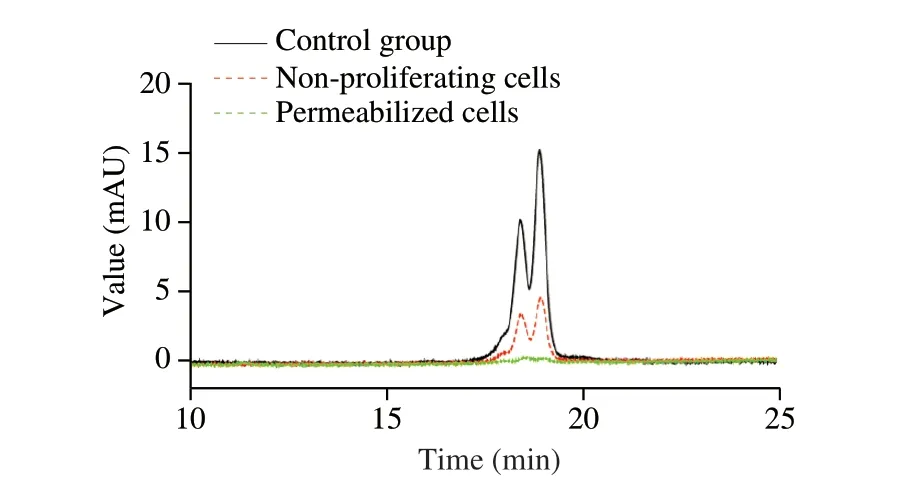
Fig. 4 RP-HPLC profiles of β-LG before and after hydrolysis using nonproliferating bacteria and permeabilized bacteria of C. tyrobutyricum Z816 after incubation for 24 h.
3.4 Identification of polypeptides in the β-LG hydrolysate by LC-MS/MS
LC-MS/MS is commonly used to identify the amino acid sequence of peptides to deduce the target sites of proteases. In order to further explore the hydrolysis sites ofβ-LG, we examined the peptide fragments before and afterβ-LG hydrolysis by LC-MS/MS. The comparison database was the proteome ofC. tyrobutyricumZ816, and the target protein database was based on the amino acid sequence ofβ-LG. A total of 23 peptides with molecular weights between 376.20 and 972.90 Da were detected in the hydrolysate (Table 1). The amino acid sequences of the identified peptides were deduced as shown in Fig. 5 with KPTPEGDLEI as an example. The results showed thatβ-LG was hydrolyzed into multiple polypeptide fragments withC. tyrobutyricumZ816, and it was worth exploring if the sequence corresponding to the truncated part of the main allergenic sequence(V41-K60, Y102-R124) ofβ-LG was found in the hydrolysates. The V41-K60 sequence was the most strongly truncated epitope [19]while 14 peptides were detected in this study. We found that the hydrolysis site of the proteases fromC. tyrobutyricumZ816 was different from those ofLactobacillus[18]. According to the research inLactobacillus, 44% of the peptides contained glutamine or glutamic acid residues at the amino-terminus, while 5 peptides contained alanine at the carboxy-terminus. By contrast, 48% of the peptides identified in this study contained leucine at the carboxy-terminus,three of which contained valine and three contained glutamic acid at the amino-terminus. Different resulting polypeptide sequences indicated that there were differences in the proteases that played a role inLactobacillusandC. tyrobutyricumZ816.
Based on the molecular simulation structure diagram shown in Fig. 6, it could be seen thatβ-LG was composed ofα-helices,β-sheets and random coils. The peptide fragments before and after hydrolysis was analyzed by molecular modeling to observe the hydrolysis site ofβ-LG more intuitively. We found that most of the antigenic epitopes and hydrolysis sites were located in theβ-sheets, which showed that Z816 has a good ability to hydrolyze allergen.
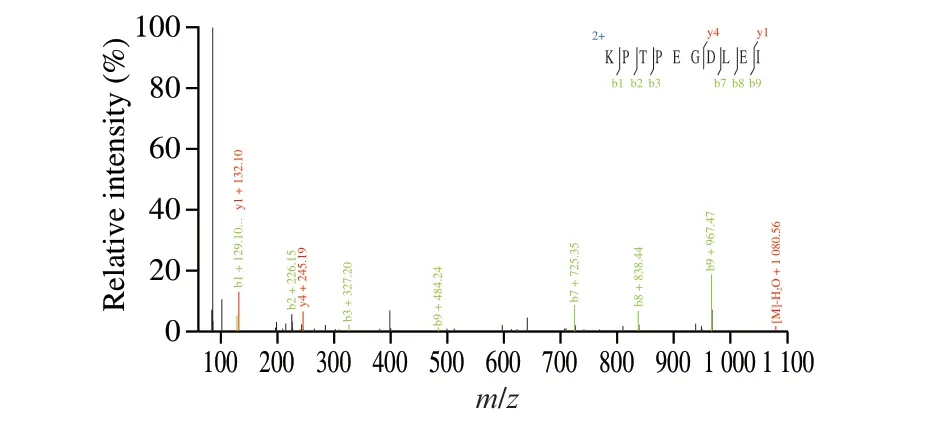
Fig. 5 Mass spectrogram of peptide fragments (KPTPEGDLEI).
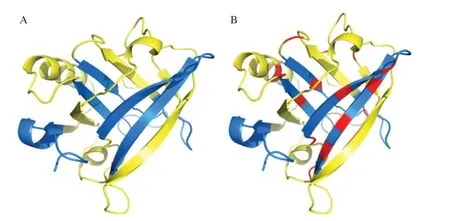
Fig. 6 The crystal structure of β-LG PDB ID: 5IO6 was analyzed using PyMol and different regions were labelled with different colors. (A) The initial crystal structure of β-LG. (B) The crystal structure of β-LG after hydrolysis.The red regions are hydrolytic fragment, the yellow regions were nonantigenic epitopes, and the blue regions are the main allergenic epitopes.
3.5 Detection of antigenicity by ELISA
After analyzing the peptide fragments produced by the hydrolysis ofβ-LG, we used ELISA to determine whetherβ-LG hydrolysates(treated with non-proliferative and permeabilized bacteria for 24 h)were still reactive as IgG or IgE binding epitopes. The results in non-proliferative and permeabilized bacteria were compared under different concentrations of antigen. As shown in Fig. 7, when the antigen concentration was in the range of 1-1 000 μg/mL, the binding ability of antigens treated byC. tyrobutyricumZ816 to IgG or IgE was also greatly reduced. The degradation efficiency of non-proliferative bacteria varied from 24% to 72%, while that of permeabilized bacteria varied from 60% to 92% after calculation. Our results indicated that the degradation efficiency was much higher than that ofL. delbrueckiisubsp.bulgaricusCRL 656 (92% vs 35%) [21]. In the presence of 1 000 μg/mL antigen, the inhibition rate was calculated to be 74.4% under the optimal hydrolysis conditions. The optical density(OD600nm) of the bacterial suspension (inhibitor) required to IC50was 10 according to inhibition curves (Fig. S3). ELISA results suggested that the release of intracellular proteases fromC. tyrobutyricumled to more effective cleavage of antigenic epitopes, which reduced the immunoreactivity of the antigen.

Table 1 Peptides identified by LC-MS/MS in the β-LG hydrolysate produced by C. tyrobutyricum.

Fig. 7 The changes of antigen concentration following treatment with C. tyrobutyricum Z816 under different conditions. The black squares represent the untreated antigen, the red circles represent the antigen treated with nonproliferative bacteria (bacterial suspension), and the blue triangles represented the antigen treated with permeabilized bacteria.
3.6 Hydrolysis of actual samples and digestibility of peptides in vitro
As showed in Table 2, we selected WPI, WPC and infant formula as representative substrates for this experiment. The hydrolysis rate ofβ-LG in whey powder concentrate reached 56.70% after 12 h treatment byL. delbrueckii, while the hydrolysis effect ofC. tyrobutyricumwas higher than that ofL. delbrueckii(93.25%vs56.70%) [21]. The permeabilized bacteria more effectively reduced the concentration of allergenicβ-LG in dairy products. Milk allergy in infants is often exacerbated by the difficulty of digesting allergens in their intestinal system. In addition, the ability of intestinal digestion may depend on the degree of hydrolysis of the allergen and the released peptide sequence [41], so we simulated the digestion ofβ-LGin vitrousing pepsin and trypsin, which is of more practical significance. As shown in Fig. 8, the gastric digestibility and total digestibility of the treated samples was significantly improved.The gastric digestibility and total digestibility of WPI were improved by 85.96% and 64.51%, respectively, after treatmentwithC. tyrobutyricumZ816. Similarly, the digestibility of WPC improved by 36.37% and 52.94%, while that of infant formula improved by 51.48% and 13.84%, respectively.C. tyrobutyricumZ816 could improve the digestion of proteins in dairy products, and its effect was greatest with WPI. Previous studies also found that the digestibility of proteins increased to a certain extent when pasteurized milk was fermented (45% within 2 h) and the immunochemical reactivity of all fermented samples was lower than that of non-fermented samples [42],which was also consistent with the results of this experiment. These results also indicated that the burden of gastrointestinal digestion could be reduced to a certain extent when the macromolecular whey protein was hydrolyzed, and the hydrolysates could be well absorbed and utilized. In a word, permeabilized bacteria of strain Z816 can achieve high hydrolysis efficiency of allergen, and the hydrolyzed sample is more easily absorbed by the intestine and stomach.
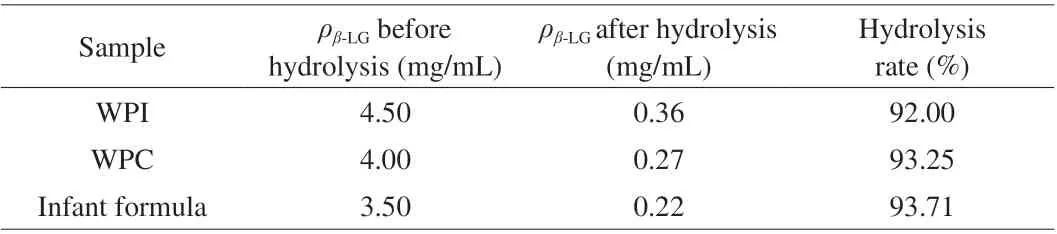
Table 2 Hydrolysis of real-world samples.
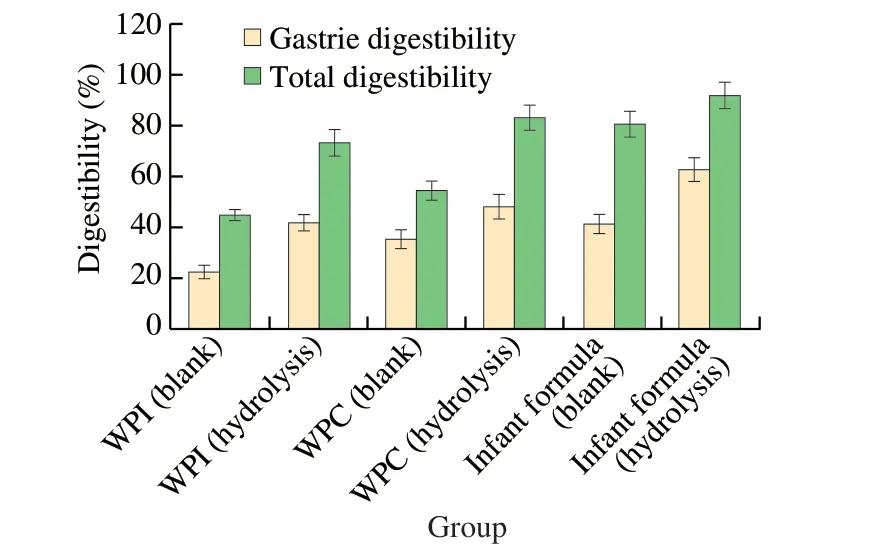
Fig. 8 In vitro simulated digestion of real-world samples before and after treatment with C. tyrobutyricum Z816.
To verify the potential application value ofC. tyrobutyricumZ816 in degrading milk allergens, it is necessary to consider the safety of strains when adding microorganisms to foods products.Recent genomic studies found thatC. tyrobutyricumhas great potential as a probiotic that produces short-chain fatty acids in the intestines. It may be a promising functional probiotic and butyric acid producing bacterium, while butyric acid itself also has beneficial effects on human health [43]. For example, it was reported that butyrate produced byC. tyrobutyricumhas a positive effect on ulcerative colitis in mice [44]. However, potential applications in food production and human health, require a thorough genetic safety assessment. It was reportedC. tyrobutyricumhas no transferable resistance genes, invasive defensive pathogenic factors or harmful enzymes. The genomic analysis ofC. tyrobutyricumindicates that it has good safety [37]. Through the above analysis,C. tyrobutyricumhad a potential application value in the field of food.
4. Conclusions
In this work, the strainC. tyrobutyricumZ816 showed excellent degradation ability for the milk allergenβ-LG, and the degradation efficiency reached 70% after optimizing the parameters.The degradation efficiency was further improved to 95% by permeabilization of the bacteria. We also identified the polypeptides inβ-LG hydrolysate by LC-MS/MS and found that the main allergenic sequences ofβ-LG were truncated. Further detection of the binding ability of specific IgE toβ-LG hydrolysate from actual samples showed that the level of immunoreactivity ofβ-LG was greatly decreased. In addition, the hydrolysates were more accessible to simulated gastrointestinal digestion. It stands to reason that the intracellular protease released from permeabilized bacteria ofC. tyrobutyricumcould degrade multiple antigenic epitopes ofβ-LG.Therefore, this study provides a workable strategy to reduce the occurrence of milk allergies caused byβ-LG.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFC1600404), the National Natural Science Foundation of China (31922070, 22008114), and the Natural Science Foundation of Jiangsu Province (BK20180038, BK20200684).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.09.017.
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard