The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
2023-01-23ChenChenChenglongLiuKeZhangWentongXue
Chen Chen, Chenglong Liu, Ke Zhang, Wentong Xue*
College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
Keywords:Gut microbiota Composition Short-chain fatty acids Immune system Food allergy
A B S T R A C T Emerging evidence indicated that the increase in food allergy (FA) over the past few decades was associated with the abnormal compositional and metabolic changes of gut microbiota. Gut microbiota played a vital role in maintaining the homeostasis of the immune system and the dysbiosis of gut microbiota promoted the occurrence of FA. Recent research suggested that short-chain fatty acids (SCFAs), the main metabolites derived from gut microbiota, contributed to FA protection. Herein, we provided a comprehensive review on the relationship between gut microbiota and FA. The multifaceted mechanisms underlying benef icial effects of gut microbiota composition/metabolites on the regulation of diverse cellular pathways in intestinal epithelial cells,dendritic cells, innate lymphoid cells, T cells, B cells and mast cells in the immune system were discussed systematically. These f indings emphasized the positive function of gut microbiota in FA and provided novel ideas for the treatment or prevention of FA in the future.
1. Introduction
Food allergy ( FA) refers to the adverse immune response to allergens (usually proteins) after food entering human body [1].Physiological dysfunction or tissue damage caused by FA led to a series of clinical symptoms, such as urticaria of skin, asthma and cough of respiratory tract, nausea, vomiting and diarrhea of gastrointestinal tract, and shock or even death in severe cases [2,3].As a major public health and food safety issue of global concern, the incidence of FA is increasing by 10% every year, which shows a trend of diversif ication, complexity and wide area, affecting the health of vulnerable people seriously [4]. About 5% of adults and 8% of infants and young children are allergic to the allergen in foods [4], mainly peanuts, milk, wheat, soybeans, eggs, nuts, f ish and crustaceans [5].
In general, after allergens entering the body, mediators that trigger intestinal epithelial cells (IECs) secretion are involved in stimulating antigen-presenting cells, such as dendritic cells (DCs)which activate allergen-specif ic T cells differentiation and cytokine production, as well as mast cells, eosinophils, and other leukocyte infiltrations, further inducing subsequent immune intestinal tissue damage and mucosal disease [6]. The most typical FA is mediated by immunoglobulin E (IgE)-dependent pathways [7,8], which is also the focus of this review. Studies are accumulating that FA is not only related to the allergen structure in food itself, but also closely linked to the symbiotic microbiota in the gut [9-11].
Gut microbiota is a wide, complex and diverse ecosystem composed of a variety of microorganisms highly interacting with the host [12]. However, the gut microbiota of animals is not innate. The intestinal tract is originally sterile, and after breathing, microbiota begins to colonize the intestinal tract in contact with the external environment. With the intake of food, the species and diversity of intestinal microorganisms are constantly enriched, and eventually a stable structure of gut microbiota is formed [13,14]. There are about 10 trillion to 100 trillion kinds of microbiota in the intestinal tract of normal adults, most of them are bacteria, while a small number are archaea, viruses and single-cell eukaryotes [15-18]. The gut microbiota mainly includes Verrucomicrobia,Proteobacteria,Actinobacteria, Firmicutes andBacteroidetes, among which Firmicutes and Bacteroidetes usually account for more than 90% of the total gut microbiota [19]. As found by the growing body of research, the gut microbiota composition and its metabolites short-chain fatty acids(SCFAs) played a considerable role in the regulation and evolution of the immune system [20,21]. A network of interactions was formed between healthy gut microbiota and the host intestinal tract through direct contact or the secretion of metabolites, directly or indirectly participating in shaping the developmental and mature metabolic processes in the immune system, which is particularly pivotal for maintaining the balance of immune responses [22,23].
In this review, on the basis of expounding the relationship between gut microbiota and FA, molecular mechanisms of gut microbiota composition and the main metabolite SCFAs regulating the IECs, DCs, innate lymphoid cells (ILCs), T cells, B cells and mast cells in the immune system were discussed comprehensively, in order to provide a theoretical basis for the treatment or prevention of FA.
2. The relationship between gut microbiota and FA
2.1 Gut microbiota composition and FA
The gut coevolved with the gut microbiota which is key to the efficacious development and maintenance of the intestinal barrier [24,25].Due to the absence of gut microbiota in germ-free animals, the integrity of intestinal epithelium, the development of intestinalassociated lymphoid tissue, innate and adaptive immunity systems were damaged in varying degrees [26]. The delicate balance between the pro-inflammatory and anti-inflammatory mechanisms necessary for intestinal immune homeostasis was influenced by the relative abundance and stability of gut microbiota composition, which affected the occurrence of allergic reactions [27-29]. Anomalous variations in the diversity and composition of the gut microbiota, known as gut microbiota dysbiosis, were important factors that increased susceptibility to diseases [30], such as FA [31], diabetes [32],cancer [33,34], asthma [35], malaria [36] and chronic urticaria [37].Specifically, gut microbiota dysbiosis refers to the breakdown of the dynamic balance between host and gut microbiota, and the imbalance of the proportion of aerobic bacteria and facultative anaerobes, which is one of the main causes of FA [38]. The results of human clinical studies showed the associations between altered gut microbiota composition and prevalence of FA [39,40]. Chen et al. [39]found that there were significant differences in gut microbiota composition between children with FA (n= 23) and healthy controls(n= 22). The children with FA possessed reduced diversity of the total gut microbiota with lower Bacteroidetes and higher Firmicutes.As revealed by Ling et al. [9], compared with the healthy controls(n= 45), the infants with IgE-mediated FA (n= 17) exhibited higher levels ofAnaerobacterandClostridium sensu strictoand lower levels ofClostridiumXVIIIandBacteroides. Azad et al. [10]explored relations of infant gut microbiota and FA by Illumina 16S rRNA sequencing. The results indicated that FA was attributed to the decreased gut microbiota richness with downregulated Ruminococcaceae and the elevated Enterobacteriaceae/Bacteroidaceae ratio in early infancy. Goldberg et al. [41] compared the gut microbiota of peanut (n= 71), milk (n= 66), tree nut (n= 58),and sesame (n= 38) FA patients with that of non-allergic healthy controls and found thatPrevotella copriwas the most abundant in healthy controls. Moreover, significant differences were captured in the characteristic gut microbiota among different IgE-mediated allergens in allergic reactions. For example, compared with peanut FA patients, there was a remarkable higher relative abundance in the milk and tree nuts FA patients. As for the children with cow’s milk allergy investigated by Canani et al. [42,43], the relative abundance ofBacteroidesandAllisteriaincreased with non-IgE mediated milk allergy while Bifidobacteriaceae were significantly upregulated in the IgE-mediated gut. As found by a survey of 226 milk FA children, the remission of FA reaction at 8 years was associated with enrichment of Clostridia and Firmicutes, which was depend on the gut microbiota composition at age 3 to 6 months [44]. Another exploration of the gut microbiota of 319 infants suggested that infants at risk of asthma showed transient gut microbiota disorders within 100 days after birth with the reduced relative abundance of the bacterial generaRothia,Lachnospira,Faecalibacterium,andVeillonella[45]. According to analysis results of the American Gut Project, the increase of Bacteroidales and the decrease of Clostridiales taxa resulted in nut and seasonal FA [46].

Fig. 1 Influence of gut microbiota composition on FA. (Under normal gut microbiota conditions, the intact intestinal barrier allows less allergens to enter the systemic circulation, and the naïve T cells tend to differentiate into Th1 and Treg cells, known as anti-inflammatory T cells, thereby reducing the release of inflammatory substances and maintaining immune tolerance. On the contrary, under gut microbiota dysbiosis, the disruption of the intestinal barrier increases the entry of allergens, and the subsets of pro-inflammatory T cells (Th2, Th17) secreting inflammatory cytokines will be activated, promoting the release of allergenspecific IgE by B cells and degranulation of mast cells, which results in the allergic reaction eventually. IECs, intestinal epithelial cells; M cell, microfold cell; DC,dendritic cell; IgE, immunoglobulin E.)
In the mouse model, Stefka et al. [47] demonstrated that the change of gut microbial composition enhanced food allergen sensitization of peanut allergy. As confirmed by Liu et al. [48], the alleviation of ovalbumin (OVA) allergy symptoms was due to the increase in the relative abundance of Bacteroidetes and the reduction of relative abundance of Firmicutes after Herbal Formula-3 treatment,which reversed the imbalance of gut microbiota. Evidence from another mouse model also suggested oral tolerance against OVA allergy was related to the distinct gut microbiota composition [49]. Therefore,FA is associated with gut microbiota dysbiosis to some extent (Fig. 1),and regulating the diversity and relative abundance of gut microbiota may be one of the effective strategies to relieve FA [50].
2.2 Gut microbiota metabolites and FA
There is a certain difference in microbial density between the small intestine and the colon in the human body. The microbial densities in human duodenum and ileum were about 1 × 103CFU/g and 1 × 108CFU/g, respectively, of which Firmicutes and Proteobacteria were the dominant phyla in the small intestine, while the microbial density of human colon was about 1 × 1011CFU/g, mainly Bacteroides and Firmicutes [51]. A recent study showed that the ratio of Firmicutes to Bacteroidetes varied with age, with the abundance of Firmicutes increasing and the number of Bacteroidetes decreasing.The variations in the density and physiological characteristics of the gut microbiota resulted in unique metabolites generation in each area of the intestine, which promoted the modulation of the host immune system [52,53]. Therefore, not only the gut microbiota composition,the metabolites derived from gut microbiota, such as SCFAs [54],bile acids [55], amino acids [13,56], and lactic acid [57] regulated intestinal homeostasis in their own unique ways.
SCFAs are the main products derived from gut microbiota through fermentation of non-digestible carbohydrates [58]. When the complex carbohydrates (such as dietary fibers) that cannot be digested in the small intestine reached the colon, they were converted into five- or six-carbon monosaccharides under the action of carbohydrate targeting enzyme (such as glucosidase polysaccharide lyase) released by anaerobic bacteria in the colon. Then through the pentose phosphate for five-carbon or Embden Meyerhof Parnas for six-carbon pathway, these monosaccharides were further catabolized to pyruvate,which could produce SCFAs via numerous biochemical pathways ultimately [59]. The total concentration of SCFAs in human intestinal cavity was about 130 mmol/kg intestinal contents [60], which was mainly determined by the gut microbiota composition, the time of intestinal transport, the intake of dietary fiber and the metabolic flux of host microorganisms [61,62].
As the effective regulators of the mucosal immune system,SCFAs are related to the induction of immune tolerance [63-65].The SCFAs concentration of FA patients was significantly lower than that of age-matched non-allergic control groups [41]. Current studies on SCFAs mainly focus on acetate, propionate and butyrate,which accounted for 90% of SCFAs produced by gut microbiota [66].Acetate was mainly produced byBifidobacterium[67], and propionate was mainly produced byBacteroidesand Firmicutes through the succinate pathway [68,69], as well as butyrate was mainly produced by the Clostridial clustersIVand XIVa through the action of butyrate kinase or butyryl CoA: acetate CoA transferase (Table 1) [70]. Human clinical investigation results indicated that the intake of acetate during pregnancy was linked to alleviated occurrence of FA in offspring [71].Roduit et al. [72] analyzed SCFAs levels in 301 fecal samples of 1-year-old children and found that propionate and butyrate levels were negatively correlated with atopic sensitization and future allergy. A study conducted by Cait et al. [73] noted that butyrate produced in early infancy was related to immune tolerance and the infants with allergic sensitization in late childhood lacked genes encoding key enzymes for butyrate production. In general, different SCFAs produced by gut microbiota were associated with the protection against FA.
3. Mechanisms of gut microbiota regulation of the immune system in FA
Under normal physiological conditions, the immune response was closely regulated by the immune system. Considered as one of the most important reasons for FA regulation, gut microbiota and its metabolites SCFAs affected the homeostasis of the immune system(Fig. 2) [74]. Although the underlying specific mechanism has notbeen stated explicitly, gut microbiota and its metabolites SCFAs are able to interact with innate and adaptive immune systems through different pathways, being advantageous to the development and maturation of the immune system [75].

Table 1 SCFAs and their biosynthetic pathways produced by different gut microbes.
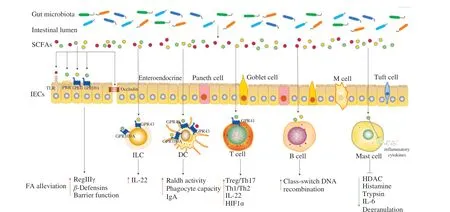
Fig. 2 Potential mechanisms of SCFAs regulating immune system in FA. (SCFAs fortify the intestinal barrier integrity by the receptors on the surface of IECs.Moreover, SCFAs induce the FA alleviation by acting on IECs, ILCs, DCs, T and B cells, or reduce inflammatory response by acting on mast cells, thereby relieving FA. SCFAs, short-chain fatty acids; IECs, intestinal epithelial cells; M cell, microfold cell; ILC, innate lymphoid cell; DC, dendritic cell; TLR, toll-like receptor, PRR, pattern recognition receptor; GPR, G protein-coupled receptor; RegIIIγ, regenerating islet-derived protein IIIγ; IL, interleukin; HIF1α, hypoxiainducible factor 1α; HDAC, histone deacetylase; FA, food allergy.)
3.1 Regulation of gut microbiota on IECs
IECs provided a physical and chemical barrier for the host immune system to resist the invasion of allergens. Professional immune cells will be activated when the barrier is broken. IECs included: enterocytes for absorbing nutrients and water, goblet cells for secreting mucin, enteroendocrine cells for secreting hormones,Paneth cells for releasing antimicrobial factors, tuft cells for detecting helminth and microfold (M) cells for antigen uptake [76].
As the key factor in maintaining intestinal tissue homeostasis, the integrity of the IECs barrier was influenced by the gut microbiota and the metabolites. Firstly, gut microbiota interacted with IECs directly to promote the production of defensins, thus strengthening the barrier function of IECs [77]. Moreover, the barrier function of IECs was also affected by the gut microbiota metabolites SCFAs. Acetate,propionate and butyrate alone or in combination markedly enhanced transepithelial electrical resistance and accelerated the formation of tight junction, accompanying obviously alleviated morphological destruction of occludin and zonula occludens-1, which suggested that SCFAs stimulated the formation of intestinal barrier [78,79]. Butyrate also promoted the barrier-protective effect of IECs by stimulating epithelial metabolism and depleting intracellular O2[80]. Furthermore,butyrate activated mTOR and STAT3 in IECs to induce antimicrobial peptide expression, such as RegIIIγ andβ-defensins, enhancing the intestinal barrier integrity [81]. In addition to the barrier function,IECs with many innate immune receptors were also the coordination hub of immune defense [82]. SCFAs were proven to work in direct contact with TLRs [83], PRRs [84], GPR43 and GPR109A [85] on IECs, promoting the interaction between gut microbiota and immune cells to maintain intestinal homeostasis.
3.2 Regulation of gut microbiota on DCs
DCs absorbed antigens and presented antigenic peptides to adaptive immune cells to accelerate the production of cytokines [86].Signals produced by gut microbiota could be recognized directly by DCs. CD103+DCs received microbial signals (such as flagellin)to produce interleukin (IL)-23, promoting the mucosal innate immune defense [87]. The indirect effect of gut microbiota on DCs was realized through metabolite-sensing receptors. Acetate acted on GPR43 of DCs to convert vitamin A into retinoic acid by the elevated expression of Aldh1a2, facilitating the production of IgA, which played an essential role in maintaining intestinal homeostasis and intestinal protection from inflammation [88]. The increased propionate content upregulated the phagocyte capacity of DCs, thereby alleviating allergic airway inflammation, which was dependent on GPR41 [89]. Acetate and butyrate produced by high fiber feeding prevented peanut allergy, which was due to the enhanced retinal dehydrogenase activity in CD103+DCs by GPR43 or GPR109A [90]. Butyrate improved the anti-inflammatory properties of CD103+DCs in colon by stimulating GPR109A [91].
3.3 Regulation of gut microbiota on ILCs
As one of the innate lymphocytes without antigen-specific receptors, ILCs which were predominantly localized in mucosal and barrier surfaces responded quickly to diverse soluble mediators released during cell stress and danger [92,93]. ILCs coordinated the pivotal functions of mucosal immunity in FA by mediating signals to regulate tissue repair, immunity, inflammation and homeostasis [94]. ILCs were divided into different subsets according to the transcription factors required for their development and the cytokines they produced [95].Non-cytotoxic subsets (ILC1s, ILC2s and ILC3s) and cytotoxic cells (natural killer cells) formed the ILCs family [84]. ILC1s secreted IFN-γ and produced the transcription factor T-bet, ILC2s produced proinflammatory cytokines IL-4, IL-5, IL-9, and IL-13 and expressed retinoic acid-related orphan receptor alpha (ROR-α)and GATA3, whereas ILC3s secreted IL-17A, IL-22, lymphotoxin,and Csf2 and produced retinoid-related orphan receptor γt [96-99].The realization of the normal function of natural killer cells mainly depended on T-bet expression and the cytokine IL-15 [100]. Although ILCs developed normally in the absence of microorganisms, the composition and metabolites of gut microbiota affected the maturation and specific function of ILCs [101-103].
Firstly, the direct patterns of the ILCs regulated by gut microbiota have been demonstrated. Bacterial components were sensed directly by human LTi-like ILCs through TLRs, promoting the expression of IL-2, IL-5, and IL-13 [104]. Chaushu et al. [105]reported that commensal bacteria were perceived by natural killer cells and ILCs directly through NKp44 and NKp46. Besides direct regulation, gut microbiota indirectly regulated ILCs through the interaction between metabolites and receptors. ILC receptors received the immune stimulation signals from SCFAs and produced cytokines that significantly changed intestinal homeostasis and inflammation [87]. SCFAs upregulated the IL-22 produced by ILC3s via activated AKT and STAT3 signaling, which was dependent on the metabolite-sensing receptor GPR43. Butyrate suppressed ILC3s frequency by GPR109A contributing to gut homeostasis [106].
3.4 Regulation of gut microbiota on T cells
T cells were located in the intestinal epithelium and lamina propria and performed various functions in the immune process,including effector function, coordinating adaptive immune response,activating B cells to differentiate into plasma cells and produce allergen specific IgE. Intestinal immune homeostasis was affected by the balance between anti-inflammatory T cells (Th1, Treg) and pro-inflammatory T cells (Th2, Th17) subsets [107]. As confirmed by Wang et al. [108], the imbalance of Th1/Th2 immune response was the main reason for gliadin-induced FA. A recent study reported that intestinal metabolites altered the balance of CD4+T cell subsets [109]. SCFAs inhibited histone deacetylase (HDAC) activity and stimulated phosphorylation rS6 and the acetylation of p70 S6 kinase to induce differentiation of naïve CD4+T cells into Th17, Th1 and IL-10+T cells by regulating the mTOR pathway [109]. Butyrate upregulated IL-22 produced by CD4+T cells via GPR41 to protect the gut from inflammation, which was dependent on the promoting aryl hydrocarbon receptor and hypoxia-inducible factor 1α (HIF1α) expression [110].
Furthermore, SCFAs were able to enhance the induction and function of Treg cells, which played a crucial role in the maintenance of immune homeostasis. The results of Ai et al. [111] indicated that the mice deficient in Treg cells were susceptible to FA. Butyrate has been shown to contribute to upregulate Tregs and maintain the integrity of the colonic mucosa to inhibit inflammation [64]. The offspring of high fiber diet-fed mice avoided FA with the higher frequencies of thymic Tregs and peripheral Tregs, which was because the butyrate promoted thymic Tregs differentiation through GPR41 [112]. The mice treated withClostridiumclustersIV and XIVashowed lower allergic symptom scores and OVA-specific IgE levels, which was due to theClostridium-mediated induction of Tregs accumulation [113].
3.5 Regulation of gut microbiota on B cells
Another target of the gut microbiota was B cells, which were derived from the differentiation of haematopoietic stem cells in the bone marrow. Mature B cells left the bone marrow and migrated into the surrounding lymphoid organs and lymphoid tissues. Stimulated by antigens, B cells performed the function of humoral immunity by differentiating into plasma cells and secreting antibodies [114,115].SCFAs supported host antibody response and promoted mucosal and systemic antibody response by regulating gene expression,accelerating cellular metabolism and differentiation of plasma B cells into B cells expressing different Ig subtypes (such as IgG1, IgG2a,IgG2b and IgG3) [116,117]. Recent studies indicated that the function of B cells in the small intestine and Peyer’s patches was influenced by butyrate [88,116]. An important B-cell-intrinsic mechanism regulated by SCFAs was demonstrated by Sanchez et al. [118]. In the mouse and human B cell models, propionate and butyrate inhibited classswitch DNA recombination to IgE by acting directly on B cells and dampened total and antigen (OVA)-specific IgE titers, which was because SCFAs decreased B cell Aicda and Prdm1 by increasing select miRNAs that target Aicda and Prdm1 mRNA-3’ UTRs through inhibition of HDAC of those miRNA host genes [118]. In addition,pentanoate and butyrate induced the secretion of immunomodulatory cytokine IL-10 in B cells, which promoted the differentiation of regulatory B cells [119,120].
3.6 Regulation of gut microbiota on mast cells
Classic FA reactions were mediated by the cross-linking of allergen-specific IgE bound to FcεRI receptors on mast cells and basophils [2,121]. Mast cells provided both immediate and delayed release of active inflammatory products when the sufficient FcεRI was aggregated for a certain time [122]. The generation and function of mast cells could be modulated by SCFAs, especially butyrate. Firstly,butyrate impeded the HDAC and transcription initiation to decrease the cytokine release of mast cells [123]. Moreover, butyrate also inhibited mast cells degranulation and reduced the release of inflammatory mediators (histamine, trypsin, TNF-α and IL-6) by downregulating JNK phosphorylation and specific trypsin expression [124].As indicated by Folkerts et al. [125], butyrate suppressed human or mouse mast cells degranulation and the release of allergen-induced histamine. Transcriptome analyses revealed butyrate reduced the Bruton’s tyrosine kinase, spleen tyrosine kinase, and linker for activation of T cells, which were the key factors of FcεRI-mediated signals for mast cell activation. Therefore, the direct inhibition of mast cell degranulation and inflammatory mediators released by SCFAs may be one of the effective strategies for the treatment of FA.
3.7 Overlapping effects of gut microbiota on the immune system
Considered as a complex process involving a series of immune cells in the immune system, FA was caused by the degranulation of mast cells through the binding of allergen-specific IgE to FcεRI receptor. The production of allergen-specific IgE was controlled by pro-inflammatory T cells (Th2 or Th17) subsets, which generated IL-4, IL-5, IL-13 or IL-17, the cytokines necessary for B cells class switching to the IgE isotype, while the anti-inflammatory T cells(Th1 or Treg) inhibited the release of inflammatory cytokines [28].The formation of pro- or anti-inflammatory T cells by naïve T cells induced by DCs under different circumstances. Gut microbiota and SCFAs regulated antigen presentation of DCs and naïve T cells differentiation, which influenced the degranulation of mast cells. For instance, the commensal A4 bacteria of the Lachnospiraceae family suppressed the development of Th2 cells and the production of IL-4 by inducing DCs to produce transforming growth factor-β (TGF-β),thereby reducing the risk of inflammatory reaction [126]. Other results of cell experimentsin vitroshowed that the immune tolerance was achieved because human DCs treated with butyrate induced naïve CD4+T cells to differentiate into IL-10+regulatory T cells, which was due to retinaldehyde dehydrogenase activity and GPR109A signaling [127]. In addition, SCFAs were conducive to promoting the preferential differentiation of naïve T cells into Treg cells directly [128]. Furthermore, the barrier of IECs strengthened by SCFAs was another pivotal way to prevent FA by reducing the entry of allergens.Synthetically, the innate immune cells (IECs, DCs, ILCs, mast cells) and adaptive immune cells (T and B cells) cooperated together to maintain the homeostasis of the immune system [129]. The impacts of gut microbiota and SCFAs on any of these FA related pathways may be overlapped,contributing to attenuate susceptibility to FA.
4. Conclusion
The development and maturation of the immune system were influenced by the gut microbiota and its metabolites. The dysbiosis of gut microbiota was one of the main causes of FA and gut microbiota composition in FA patients was significantly different from that in healthy controls. Therefore, targeted modulation of the gut microbiota composition may be one of the effective means to alleviate FA.Moreover, increasing evidence from human and mouse models also suggested that signals derived from gut microbiota metabolites SCFAs in the lumen of the intestine acted as key regulators of FA.The function of multiple types of immune cells was affected directly or indirectly by SCFAs through different mechanisms and pathways in intestinal immunity. Since the production of gut microbiota metabolites mainly depends on the dietary intake, in order to induce the production of specific metabolites, it is another possible way to protect against FA by changing diet components. Collectively, more efforts are needed to integrate the beneficial effects of gut microbiota and its metabolites to provide referable insights for the treatment or prevention of FA. Gaining a better cognition of the unique function of gut microbiota and its mechanism of action on the immune system in FA could be of great significance for the future.
Declaration of competing interest
All authors declare that there are no conflicts of interest regarding this article.
Acknowledgments
This study was financially supported by the National Key Research and Development Program of China (2019YFC1605000)and the National Natural Science Foundation (31872904).
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard
- An antifouling polydopamine-based f luorescent aptasensor for determination of arginine kinase