An antifouling polydopamine-based f luorescent aptasensor for determination of arginine kinase
2023-01-23YanboWangHuanLiJinruZhouFangtingWangYifanQianLinglinFu
Yanbo Wang, Huan Li, Jinru Zhou, Fangting Wang, Yifan Qian, Linglin Fu*
Food Safety Key Laboratory of Zhejiang Province, School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou 310018, China
Keywords:Food allergy Aptasensor Antifouling Polydopamine FRET effect
A B S T R A C T Simple yet eff icient detection methods for food allergens are in urgent need to help people avoid the risks imposed by allergenic food. In this work, a polydopamine (PDA)-based f luorescent aptasensor was developed to detect arginine kinase (AK), one of the major allergens in shellf ish. The aptamer towards AK was f irstly selected via systematic evolution of ligands by exponential enrichment method and labeled with f luorescein amidite (FAM) to build a fluorescence resonance energy transfer (FRET) system with PDA particles.Polyethylene glycol (PEG) was employed to construct an antifouling surface for the aptasensor to eliminate food matrix interferences. With the presence of AK, the PDA-based aptasensor exhibited elevated f luorescent signals as the FAM-labeled aptamer bound to AK and detached from the PDA particles. The aptasensor showed great stability and resistance to nonspecif ic interference of background proteins and had a limit of detection (LOD) of 0.298 μg/mL. The proposed aptasensor was further proved to be feasible for quantitative analysis of AK in nine species of shrimps and f ive commercial processed products, which indicated its high potential in tracing the presence of AK in complex aquatic products.
1. Introduction
Food allergy has become a global health issue due to the rising prevalence in the last decades [1]. Among the diverse daily foods,shellfish has been identified as one of the most common allergenic groups but sometimes could cause more severe manifestations than other food allergens [2,3]. Arginine kinase (AK), one of the leading allergens in shellf ish, is found to be responsible for 51% of the shellfish related allergy [4]. Due to the relatively high thermal stability, its allergenicity is difficult to be eliminated through food processing or cooking processes [5]. As the lack of achieved cure to food allergy [6], simple yet effective detection methods are in urgent need for tracing and labeling of AK in complex aquatic products to help allergic people avoid the intake of allergenic foodstuffs.However, the detection tools for AK are quite limited at this stage.
Conventional detection methods for food allergens such as enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR) method and mass spectrometry suffer from limitations such as long detection time, complicated operation, proneness to false results and expensive instruments [7,8]. In recent years, biosensor based on fluorescence resonance energy transfer (FRET) systems has been considered as one of the most potential candidates to meet the need for food allergen detection due to the simplicity, high sensitivity and cost-effectiveness [9,10]. In these cases, the presence of target would alter the distance between excited donor and energy acceptor to give out a variation in f luorescence signals so that the amount of target could be analyzed [9-11]. Polydopamine (PDA), a musselinspired catecholamine, has drawn great interest as an energy acceptor for constructing FRET-based biosensors [12-14]. For instance, Ma et al. [12]developed a FRET assay that employed Cy5-ssDNA and PDA nanoparticles as the energy donor-acceptor pair for reactive oxygen species (ROS) detection. The Cy5-ssDNAs adsorbed on the PDA nanoparticles could be cleaved by ROS to release the Cy5 molecules into solution, which led to a recovery in fluorescence signal for ROS quantification. Zhang’s group utilized the FRET effect between dyelabeled single-strand DNA (ssDNA) and PDA to successfully monitor the dynamic changes of the striatum antioxidants in rat cerebrospinal microdialysates during normal/ischemia/reperfusion process [13].Compared to other acceptor materials such as carbon nanotubes [15],graphene oxide [16], MoS2nanosheets [17] and metal-organic framework [18], PDA has the advantages of hydrophilicity, ease of preparation and abundant surface functional groups for further modification, which makes it a promising candidate for constructing FRET-based biosensors for AK.
The interference of food matrix to detection process is one of the major obstacles for food allergen determination. Food matrix that contains diverse components could interact with detection probes nonspecifically to hamper the sensitivity [19]. For biosensors aimed to be used in complex samples, antifouling materials are often used to modify the surface of sensors to resist the interference from non-target species in the samples [20,21]. Moreover, antifouling coatings are found to protect the particulate systems from losing functionalities by improving the colloidal stability [21], which may benefit for improving the detection performance of the PDAbased sensors. Among the various category of antifouling materials,polyethylene glycol (PEG) is the most widely applicated one and has been recognized as the “gold-standard” [22]. Tran et al. [23] have proved that surface functionalization of the biosensor by PEG could effectively block the nonspecific interaction between the detection probe and the target allergen, Ara h1. Thus, the deviation from baseline caused by fouling of Ara h1 on the detection probe could be eliminated. However, if PEG could be used to inhibit the nonspecific interaction that arose from the complex food matrix rather than the single target allergen remains poorly understood.
In this work, we developed a fluorescent biosensor for AK based on the FRET effect between PDA particle and fluorescein amiditelabeled aptamer (FAM-APT). Aptamer (APT) has been considered as a promising recognition molecule for food allergens due to its high affinity and specificity, low production cost and feasibility for reporter molecule labeling [24]. Given these merits, a specific aptamer towards AK was selected by systematic evolution of ligands by exponential enrichment (SELEX) for the first time and further labeled with fluorescein amidite (FAM). The PDA-based biosensor was constructed by modifying the PDA particles with PEG and complementary sequence (CS), which was further hybridized with the fluorescence dye-labeled aptamer. It’s suggested that the fluorescence of the aptamer was quenched by the PDA particles due to FRET effect (Fig. 1a), whereas the presence of AK would allow the aptamer to detach from the CS and regain fluorescence signals (Fig. 1b). The antifouling ability of the PEG-modified PDA to nonspecific proteins as well as its influence on the detection properties were carefully studied to unveil the possibility of employing antifouling coatings to inhibit the interference caused by the nontarget food components.Moreover, the detection performance of the PDA-based aptasensor was carefully evaluated and quantitative analysis of AK in nine species of shrimps and five shrimp processed products was carried out to validate the feasibility of the proposed biosensor for practical application.
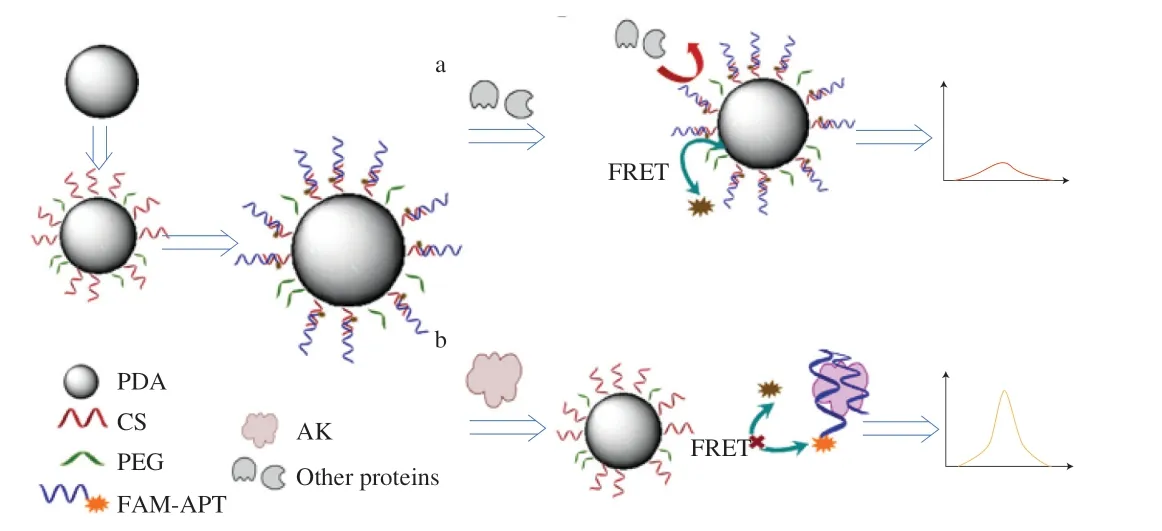
Fig. 1 Schematic diagram of the PDA-based aptasensor for AK detection.Fluorescence (a) turn off and (b) turn on in accordance with the presence of AK.
2. Material and methods
2.1 Materials and chemicals
Sodium chloride (NaCl), potassium chloride (KCl), sodium hydrogen carbonate (NaHCO3) and dipotassium hydrogen phosphate(K2HPO4) were purchased from Sigma-Aldrich (Shanghai, China).Ammonia and ethanol were purchased from Sinopharm Chemical Reagent (Beijing, China). Dopamine was purchased from Aladdin(Shanghai, China). Methoxy-PEG2000-thiol (HS-PEG) was purchased from Seebio (Shanghai, China). Bicinchoninic acid (BCA)protein assay and whole cell lysis assay kits were purchased from KeyGEN BioTECH (Nanjing, China). The sequences of the FAMAPT and the aptamer’s CS were 5’-FAM-AACGTTGACCTAGA AGCGGCGAACAGCAGCGCGATTCGGGTTGCGGATAGTG ACATA-3’ and 5’-GCTTCTAGGTCAACGTTTTTTTT-C6SH-3’respectively, which were synthesized by Sangon Biotech (Shanghai,China). Shrimp tropomyosin (TM) was prepared as the previously reported work [26] and briefly described in Supporting information.Bovine serum albumin (BSA), ovalbumin (OVA), pepsin and trypsin were purchased from Sangon Biotech (Shanghai, China).Penaeus chinensis,Solenocera crassicornis,Trachypenaeus curvirostris,Macrobrachium nipponense,Metapenaeus ensis,Pleoticus muelleri,Penaeus monodon,Oratosquilla oratoria,Litopenaeus vannamei,shrimp dumpling, shrimp taste dumpling, shrimp meatball, mashed shrimp wah, dried small shrimps, chicken soup and fish (Gadus)meat were purchased from a local supermarket (CenturyMart,Hangzhou, China).
2.2 Synthesis of the PDA particles
The PDA particles were fabricated in accordance with the work reported by Liu et al. [25]. Briefly, 3 mL ammonia (28%-30%),40 mL anhydrous ethanol and 90 mL deionized water were fully mixed under mild stirring at 30 °C for 30 min. Then 0.05 g/mL aqueous solution of dopamine was added to the above mixture and polymerized at 30 °C for 24 h. The obtained PDA was purified by 12 000 ×gcentrifugation at 25 °C for 3-4 times. The pellets of the PDA particles were re-dispersed in 50 mmol/L Tris-HCl buffer(pH 8.5). The PDA particles were characterized by transmission electron microscopy (TEM, JEM-1200EX, JEOL, Japan),dynamic light scattering (DLS, Zetasizer Nano-ZS, Malvern, UK)and ultraviolet-visible (UV-Vis) spectrophotometer (UV2600,Shimadzu, Japan).
2.3 Selection protocol for the aptamer towards AK
The aptamer selection protocol using magnetic bead-based SELEX method is described in Supporting information.
2.4 Construction of the PDA-based aptasensor
The PDA probes were prepared as follows. A mixture of 1 nmol CS and 1 nmol HS-PEG was added into 1 mL 2 mg/mL PDA solution and shaken overnight. Then it was centrifuged for 10 min at 8 000 ×gand 25 °C for 3-4 times to remove the unbound CS or HS-PEG so that the purified PDA-PEG/CS was obtained. Next, 1 nmol FAM-APT was added to the PDA-PEG/CS and incubated at 25 °C for 3 h. The obtained PDA-PEG/CS-APT was purified by 10 min centrifugation at 8 000 ×gand 25 °C for 3-4 times to remove the unbound aptamer. The PDA-CS-APT aptasensor was prepared in a similar procedure with 1 nmol CS and no HS-PEG added.
2.5 Analysis of the antifouling ability of the PDA probes
Nonspecific protein adsorption on the probe surface was employed as the key indicator to study the antifouling ability of the PDA probes since shrimp is abundant with proteins in a matrix. In addition, protein has been considered as one of the major biomolecules that could nonspecifically adsorb onto a sensing interface [21]. Briefly, the PDA probes were incubated with the solution of BSA or whole shrimp protein extract for 30 min. The hydrodynamic sizes of the PDA probes before and after incubation were collected to analyze if there was any nonspecific protein adsorption on the probes’ surface. In addition, the amount of adsorbed nonspecific proteins was evaluated by comparing the protein amounts in the supernatant (obtained by 10 min centrifugation at 8 000 ×gunder 25 °C and measured by BCA assay) before and after incubation with the PDA probes.
2.6 Detection of AK by the PDA-based aptasensor
The standard or commercial samples were mixed with the PDA-based aptasensor and shaken for 2 h at 25 °C. Then it was centrifugated for 10 min at 8 000 ×gunder 25 °C and the fluorescence intensity of the supernatant was measured at 485 nm excitation and 525 nm emission with a microplate reader (Spectra Max i3x; Molecular Devices, USA). ELISA was used for verification(detailed procedures in Supporting information).
For the specificity test, the PDA-based aptasensor was mixed with AK, TM, BSA, OVA, pepsin and trypsin respectively, with the final concentration of the proteins being 2 μg/mL. After 2 h incubation at 25 °C, the supernatant was collected by 10 min 8 000 ×gcentrifugation at 25 °C and measured at 485 nm excitation and 525 nm emission with the microplate reader. For the recovery test,the commercial chicken soup (diluted to 10 times) and fish (Gadus)meat powder were chosen as the representatives for non-seafood and seafood food matrix and spiked with the standard AK. The final AK concentration in the spiked chicken soup was 0, 1 and 2 μg/mL, which was measured by the aptasensor directly. The final AK concentration in the spiked fish meat powder was 0, 10 and 20 μg/g. The whole protein of the AK spiked fish meat powder was further extracted by a commercial extraction kit (Sangon Biotech, Shanghai, China) with 100 mg fish meat in 1 mL lysis buffer. Then the AK concentration was measured by the aptasensor.
2.7 Preparation of the standard AK samples
AK was extracted following the previously reported method with slight modification [4]. Briefly, shrimp muscle ofLitopenaeus vannameiwas deveined and homogenized in the extraction buffer(50 mmol/L NaCl, 2 mmol/L NaHCO3, 10 mmol/L EDTA-2Na,pH 8.0). The mixture after 1 h extraction was subjected to centrifugation, and the supernatant was treated with 70% and 90%saturated ammonium sulfate for 6 h successively. The extraction,centrifugation (8 000 ×g, 20 min) and ammonium sulfate treatment were all conducted at 4 °C. After centrifugation, the precipitate was dialyzed in buffer (20 mmol/L Tris, 1 mmol/L NaCl, pH 8.0 by 1 mol/L HCl) for 16 h to obtain the whole protein extract, which was eluted at 1 mL/min in elution buffer (0.5 mol/L NaCl, 0.1 mol/L Tris-HCl, pH 8.0) through AKTApure 25 (General Electric Company,Boston, USA) with DEAE-Sepharose Fast Flow ion-exchange column to collect the pure AK. The eluates without AK were also collected and referred to the whole shrimp proteins without AK.The protein concentration was determined by BCA assay. The pure AK was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using AlphaView SA 3.4.0 software(Fig. S1), which showed high purity.
2.8 Preparation of the commercial shrimp and processed product samples
The commercial shrimp and processed product samples were prepared according to the previously reported work with slight modification [26]. For the shrimp samples, 0.1 g shrimp muscle frozen by liquid nitrogen was grounded into powder, and the whole proteins were extracted by a commercial kit (KeyGEN, Nanjing, China).The extraction was conducted at 4 °C for 2 h, and the whole protein extract in the supernatant was obtained after 5 min of 8 000 ×gcentrifugation at 4 °C. The processed product samples were prepared by cutting the products into 0.5 cm pieces and homogenized by MicroBlender2TM400 (Seroat, Escondido, USA). The whole protein extract was similarly collected as above by the commercial kit with 6 h extraction at 4 °C. The concentration of the whole protein extracts was determined by BCA assay. Both the commercial shrimp and processed product samples were diluted by 10 mmol/L Tris-HCl buffer (pH 8.0) to allow the AK concentration to fall into the linear quantification range.
3. Results and discussion
3.1 Selection of the AK-specific aptamer
Magnetic bead (MB)-based SELEX (Fig. S2) was employed to rapidly screen specific aptamer towards AK as it’s the simplest and most widely used method for aptamer selection [27]. The selection procedure involved three steps: (a) positive selection was conducted by incubating the MB-AK with a random oligonucleotide library and the MB-AK-ssDNAs were separated from the unbound DNA.The selected ssDNAs were detached from the MB-AK and amplified by PCR; (b) negative selection was conducted by mixing the MB with the oligonucleotide library. ssDNAs that showed high affinity to MB were excluded during this process; (c) counter selection was conducted by mixing the oligonucleotide library with the conjugates of the MB and whole shrimp proteins without AK. ssDNAs that showed high affinity to the non-AK proteins modified MB were excluded during this process.
MB-AK for positive selection was firstly prepared. The SEM images (Fig. S3a and S3b) showed that the MB and AK-modified MB (MB-AK) were all round-shaped. The stretching vibration of the N-H at 3 300 cm-1and C=O at 1 650 cm-1of the amido linkage in the subtraction FT-IR spectrum indicated that AK was successfully coupled onto the MBs’ surface (Fig. S3c). The optimal feed ratio between MB and AK was determined to be 50:4 (Fig. S4). After 15 round selection (detailed selection mode showed in Table S1), the recovery reached about 44% (Fig. S5). 8 representative sequences(Table S2) were chosen as candidates and subjected to highthroughput sequencing. The predicted secondary structures showed that the candidate sequences all contained stem ring and hairpin structures (Fig. S6). The 8 sequences all showed encouraging affinity to AK (Fig. 2a) as theKdvalues were all lower than μmol/L range [28].Among them, the candidate sequence I, II, VII showed the most encouraging affinity as theKdvalues were the lowest. Therefore,these three sequences were subjected to a specificity test to select the best sequence. According to Fig. 2b, the sequence II and VII both had great specificity to AK but the sequence II had a better affinity to AK (Fig. 2a). Therefore, sequence II (5’-GGCGAACAGCAG CGCGATTCGGGTTGCGGATAGTGACATA-3’) was chosen as the optimal aptamer to construct the PDA-based aptasensor.Considering the highly conserved sequence of AK among shrimp,oyster and squid [29], the aptamer may work as a recognition element for tracing AK of shellfish in food.
3.2 Preparation of the PDA-based aptasensor
As Fig. S7a shows, the PDA particles were round-shaped and well dispersed. The UV-Vis spectrum of the PDA particles in Fig. S7b indicated that there was a typical broad absorption band [25] of the PDA particles in the range of 300-900 nm. Therefore, the PDA particles were successfully prepared and suitable for further construction of PDA-based aptasensor.
The PDA-based aptasensor, PDA-PEG/CS-APT, was prepared by immobilization of FAM-APT onto the HS-PEG2000 and HS-CS co-modified PDA particles through hybridization between APT and CS. It could be found in Fig. 3a that the PDA-PEG/CS-APT particles were round-shaped and Fig. 3b indicated that the particles had a broad absorption band in 300-900 nm. From Fig. 3c, it could be seen that the hydrodynamic size gradually increased from (141.8 ± 1.5) nm for the bare PDA particles to (190.1 ± 3.7) nm for the HS-PEG2000 and HS-CS co-modified PDA particles. After complementation with FAM-APT, the hydrodynamic size of the particles further increased to (220.2 ± 5.2) nm. The increment in hydrodynamic size indicated that PEG, CS and FAM-APT were successfully immobilized onto the PDA particles’ surface. To optimize the ratio between PEG and CS, the PDA-PEG/CS-APT with different molar ratios of PEG/CS(1:1, 1:2, 1:4) were studied for AK detection. From Fig. S8, it could be seen that the fluorescence signal increased with the concentration of AK. This was due to the competition of AK for aptamer, which would result in detachment of FAM-APT from CS and recovery of fluorescence signals as Fig. 1 shows. The aptasensor with PEG/CS ratio being 1:2 exhibited the best sensitivity for AK detection, as the fluorescence signal was the largest among the three different aptasensor under the same AK concentration. Therefore, the molar ratio of PEG/CS was chosen to be 1:2 for further experiments.
The PDA-CS-APT without surface modification of the HS-PEG2000 was prepared as the control group. The increase in hydrodynamic size during stepwise surface immobilization indicated that the PDA-CS-APT was successfully prepared (Fig. S9).Fig. 3d showed the stability of PDA-PEG/CS-APT and PDA-CS-APT in 50 mmol/L Tris-HCl (pH 8.0). No agglomeration or increment in hydrodynamic size could be observed for the PDA-PEG/CS-APT,whereas the hydrodynamic size dramatically increased along with time and eventually sediments could be found in the bottom of the bottle for the PDA-CS-APT. The improved stability of the PDA-PEG/CS-APT over the PDA-CS-APT was suggested to arise from the great hydrophilicity of PEG [22].
3.3 Antifouling ability of the PDA-based aptasensor

Fig. 2 (a) Saturation curves along with Kd values for candidate sequence I-VIII from eight families. (b) Selectivity of sequence I, II, VII to AK over whole shrimp proteins without AK, TM, OVA, and BSA.

Fig. 3 (a) TEM image and (b) UV-Vis spectrum of PDA-PEG/CS-APT. (c) The hydrodynamic size distribution of PDA,PDA-PEG/CS and PDA-PEG/CS-APT. (d) Stability of PDA-PEG/CS-APT and PDA-CS-APT in 50 mmol/L Tris-HCl (pH 8.0).
The antifouling ability of the PDA-based aptasensors was carefully studied. Firstly, the hydrodynamic size of the PDA-based aptasensors before and after incubation with the protein solution was carefully studied. For the PDA-CS/PEG, there was no significant increase in the hydrodynamic size after 30 min incubation with BSA(Fig. 4a) or whole shrimp protein extract (Fig. 4b). For the PDA-CS,the hydrodynamic size dramatically increased from (190.1 ± 3.7) nm to (255.4 ± 3.7) nm and (342.2 ± 0.8) nm after incubation with BSA and the whole shrimp protein extract, respectively. This indicated that the PDA-CS/PEG had better ability than PDA-CS to resist the nonspecific adsorption of proteins, which was due to the antifouling ability of PEG. PEG is a highly flexible polymer that could generate a “conformational cloud” that works as a steric barrier to prevent interaction with biocomponents such as proteins [22,30]. Moreover,the hydrogen bond induced hydration layer of PEG further reinforced its antifouling ability [22]. The amounts of the nonspecifically adsorbed proteins on the PDA particles’ surface were further measured by BCA method. From Fig. 4c and 4d, it could be found that the amounts of proteins adsorbed on PDA-CS/PEG were greatly reduced than PDA-CS, which further confirmed the antifouling ability of the PDA-CS/PEG.
Next, the effect of the antifouling ability of PEG on the detection efficiency of the PDA-based aptasensors was carefully examined. As mentioned before, the PDA-CS-APT had relatively poor colloidal stability, which could lead to continuous agglomeration of the particles(Fig. 3d). In fact, when employing PDA-CS-APT for AK detection,no linearity could be achieved (Fig. S10) due to the instability of PDA particles. In order to eliminate the influence of colloidal stability, both PDA-CS-APT and PDA-PEG/CS-APT aptasensors were subjected to ultrasonic treatment before applicated for AK detection. As could be seen from the square of determination coefficient (Fig. S11), PDAPEG/CS-APT (R2= 0.992 5) showed better linearity in AK detection when compared to PDA-CS-APT (R2= 0.963 5). Moreover, PDAPEG/CS-APT exhibited stronger fluorescence signal intensity than PDA-CS-APT under the same concentration of AK. The improved detection performance of PDA-PEG/CS-APT was suggested to benefit from the antifouling ability of PEG. On the one hand, the PEG coating could reduce the nonspecific interaction between AK and the PDA particles, which could dramatically enhance the stability of the detection systems and lead to better linearity. On the other hand,the antifouling ability of the PEGylated PDA aptasensor allowed it to prevent the nonspecific adsorption of AK on the particles, which would bring no contribution to fluorescence signals.
3.4 Performance of the proposed aptasensor for AK detection
As the result showed in Fig. 5a, when using the PDA-PEG/CS-APT to detect AK there was a linear relationship between the concentration of AK and fluorescence intensity in the range of 0-2.5 μg/mL.The standard curve was found to bey= 1 897x+ 82.66, with the square of the determination coefficient (R²) being 0.990 5. Here,ywas the fluorescence intensity andxwas the concentration of AK.The limit of detection (LOD) and limit of quantification (LOQ) were further calculated to be 0.298 μg/mL (RS/N= 3) and 0.995 μg/mL(RS/N= 10), respectively.
TM, BSA, OVA, pepsin and trypsin were employed to explore the specificity of the PDA-based aptasensor for AK detection. The fluorescence signals observed in Fig. 5b for the nonspecific proteins were significantly lower than that of AK, which demonstrated the excellent specificity of the PDA-based aptasensor towards AK. To further study the specificity of the PDA-based method, the detection process was conducted in AK and whole shrimp proteins without AK. It could be seen from Fig. S12, the fluorescence signal in AK samples was dramatically larger than that in whole shrimp proteins without AK. This further confirmed the excellent specificity of the proposed method.
Finally, the recovery test was performed in chicken soup and fish meat that spiked with AK. Table 1 showed the recovery ranged from 88.84%-104.01%, indicating that the method had good accuracy(between 85%-110% [31]). Therefore, the PDA-based method is highly promising for AK detection with great linear response,sensitivity, specificity and accuracy.

Fig. 4 The change in hydrodynamic size for (1) PDA-CS/PEG and (2) PDA-CS incubated in (a) BSA solution and (b) whole protein solution of Litopenaeus vannamei. The amounts of nonspecifically adsorbed proteins on PDA-CS/PEG and PDA-CS in (c) BSA solution and (d) whole protein solution of Litopenaeus vannamei measured by BCA method. **P < 0.01 (t test), n = 3.
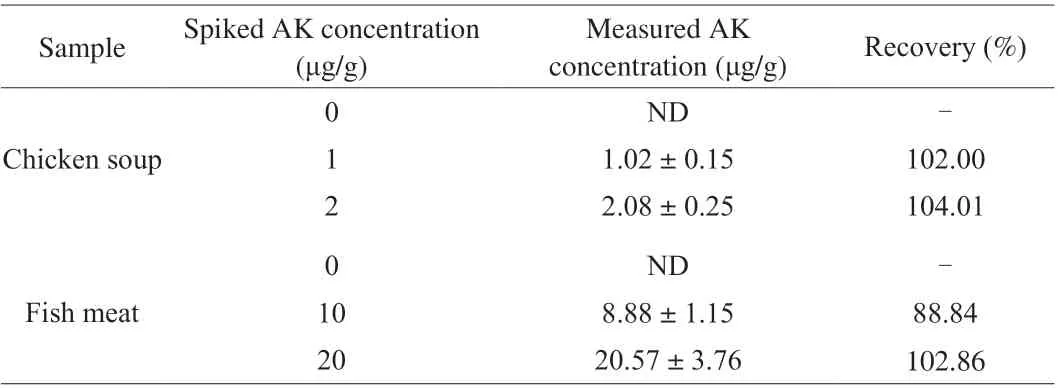
Table 1 Recovery ratios of the spiked AK in real samples (n = 3).
3.5 AK detection in shrimps and processed shrimp products
Arginine kinase is a relatively conserved protein among different shellfish [29], which allowed the PDA-based aptasensor to be used for AK detection in different species of shrimp or processed shrimp products. Therefore, the capability of the PDA-based aptasensor in detecting AK in different shrimp species (SDS-PAGE image in Fig. S13) and processed shrimp products (SDS-PAGE image in Fig. S14)was carefully studied to evaluate its potential in practical applications.For different shrimp species, the AK contents were determined to be(1.16 ± 0.37), (1.13 ± 0.19), (0.98 ± 0.11), (0.37 ± 0.05), (1.92 ± 0.12),(0.98 ± 0.11), (0.55 ± 0.03), (1.89 ± 0.18) and (1.63 ± 0.20) mg/g forPenaeus chinensis,Solenocera crassicornis,Trachypenaeus curvirostris,Macrobrachium nipponense,Metapenaeus ensis,Pleoticus muelleri,Penaeus monodon,Oratosquilla oratoriaandLitopenaeus vannamei, respectively (Table 2). For different shrimp products purchased from a local market, the AK contents were analyzed to be (0.70 ± 0.02), (0.17 ± 0.04), (2.56 ± 0.25), (0.89 ± 0.07) and(0.45 ± 0.05) mg/g for shrimp dumpling, shrimp taste dumpling,shrimp meatball, mashed shrimp Wah and dried small shrimp,respectively (Table 3). The AK contents in shrimp and processed products samples measured by the PDA-based aptasensor were found to be comparable to that by ELISA (Table S3 and Table S4). As the general threshold of food allergen is 10 μg/g (allergen in food taken by individuals) according to the Japanese Food Allergen Labeling Regulation [32], the above shrimp and processed products all held potential risk to allergic people. In addition, though a notable decrease in signals was observed for AK with thermal treatment (Fig. S15), it’s found that AK contents in thermally treated samples (shrimp meatball and dried small shrimp) measured by the proposed aptasensor were in consistent with those measured by the ELISA method. Therefore,the aptasensor could be potential for indicating the allergenic risk of thermally processed food products, at least with a similar extraction ratio as the ELISA method. Moreover, as the aptasensor didn’t need the process of overnight immobilization, successive addition of reagents and laborious washing operations, AK detection could be completed within simplified operations and shortened time(< 2.5 h) when compared to ELISA. More importantly, the aptasensor employed an aptamer as a recognition element, which is cost-effective and easy to synthesize in comparison with the antibodies used in ELISA [24]. Thus, the PDA-based aptasensor is highly promising for AK determination in shrimp samples at a relatively low cost.Given the high sequence conservation of AK among phylogenetically different species [29], the proposed aptasensor could be extended as an effective tool for tracing AK in various species of shrimp or recognizing adulteration in shrimp products using AK as a marker.

Table 2 Detection of AK in shrimp samples (n = 3).

Table 3 Detection of AK in shrimp product samples (n = 3).

Fig. 5 (a) The standard curves of the PDA-PEG/CS-APT for AK detection.(b) Specificity of the PDA-PEG/CS-APT towards AK over TM, BSA, OVA,pepsin and trypsin. **P < 0.01 (t test), n = 3.
4. Conclusions
In this work, we developed a PDA-based aptasensor that relied on the FRET effect between the PDA particles and FAM-APT. The surface modification by antifouling PEG increased the stability and resistance of the PDA-based aptasensor to nonspecific interference of background proteins. When applied to AK detection, the aptasensor showed a linear quantification range of 0.995-2.5 μg/mL(R2= 0.990 5). The LOD of the method was as low as 0.298 μg/mL.Moreover, the method showed both good specificity and accuracy.Finally, the PDA-based aptasensor was subjected to quantitative analysis of AK in nine shrimp samples and five shrimp processed products, which proved that the aptasensor was highly potent for determination of food allergens in practical samples.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
This study was financially supported by the National Key R&D Program of China (2019YFC1605002), the National Natural Science Foundation of China (31871735) and Xinmiao Talent Project of Zhejiang Province (2019R408063).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.09.007.
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard