碳量子点阳离子表面活性剂的多功能性
2023-01-13杨健雷辰刘祥张建孙玉蝶张铖叶明富张奎
杨健,雷辰,刘祥,*,张建,孙玉蝶,张铖,叶明富,2,张奎,*
1安徽工业大学化学与化工学院,安徽 马鞍山 243032
2内蒙古工业大学,风能太阳能利用技术省部共建教育部重点实验室,呼和浩特 010051
1 Introduction
As a new photoluminescent material, carbon quantum dots(CQDs) or carbon nanodots1,2had been discovered for decades.Numerous researchers published their works about the preparation with various raw materials and the fluorescent performances3-11.The cheap raw materials and convenient preparing approaches evoked extensive interests on CQDs12-16.Most CQDs were producedviapyrolysis on organic molecules at higher temperature4,12that resulted in carbonization of the starting materials accompanied by various reactions such as oxidation, condensation, dehydration, esterification,etc.In addition, some unpredicted reactions might take place simultaneously under such violent conditions.Hence, it was a tough problem for learning the precise structure of the CQDs even though there were modern detecting equipments.The accurate information about chemical structure of CQDs was not very clear until today17.However, functional groups of CQDs could be speculated according to the chemical structures of raw materials and the reaction types among them.For example,CQDs made from hemicelluloses should be rich in hydroxyl and ether groups.Such groups were sensitive to Ag+which should be due to the intensive interaction between the oxygen-contained groups and Ag+.And these caused fluorescence quenching18.CQDs derived from citric acid, urea and oxalic acid should own rich carboxyl groups on account of the multiple carboxyl groups of citric acid, which provided coordination sites for Hg+19.he authors attributed this as a determinative cause of the fluorescent quenching.The destruction of fluorescence was an efficient way of detecting several metallic cations20or organic molecules21.Therefore, the utility of CQDs was determined predominantly by the surface functional groups according to analyses on these examples.
In fact, some of functional groups of the raw materials were intact during the production of CQDs.The CQDs prepared from citric acid owned carboxyl groups even though some of them were consumed during the carbonization.The carboxyl groups could be activated byN-hydroxysuccinimide for realizing the bondage of primary amines of biomacromolecules and the fluorescent CQDs22.Since polyethyleneimine owned plenty of amino groups CQDs made from the mixture of glycerol and polyethyleneimine through one-step hydrothermal treatment were therefore positive-charged with a capability of serving as DNA condensation.With progress of the carbonization, amino groups of the raw materials were introduced into the CQDs that determined the chemical properties and the utility23.Therefore the chemical structure and the utilities of CQDs depended mainly on right choice of the raw materials24.Actually, the surface functional groups could be modified chemically that might open a door of exploiting new applications of CQDs even though the accurate structure of CQDs was difficult to be understood at present25.CQDs prepared by hydrothermal approach are soluble readily in aqueous medium depending on hydrophilicity of the surface functional groups.If a hydrophobic group is attached to one of these surface functional groups it endows hydrophobicity to the modified CQDs.Thus such modified CQDs become amphiphilic and possesses surface activity in declining surface tension of water which is similar to a conventional surfactant.
Herein, an approach of manufacturing a novel CQDs cationic surfactant will be depicted in detail in this work.Ethylenediamine tetraacetic acid (EDTA) and ethylenediamine(EDA) are chosen as raw materials of preparing the CQDs.Nitrogen doping also favored for enhancement of fluorescent quantum yield26-30.The introduced tertiary amine may be involved in the quaternization with halogenated hydrocarbon,which described in Scheme 1.This provides an economic route of synthesizing cationic surfactant with versatility such as surface activity, fluorescence and antibacterial effect that is incomparable by conventional surfactants.

Scheme 1 Schematic description of preparing the CQDs and the successive quaternization with n-C12H25Cl.
2 Experimental sections
2.1 Chemical reagents
Ethylenediamine tetraacetic acid (EDTA, 99.5%), 1-chlorododecane (n-C12H25Cl, 98%), 1-chlorotetradecane (n-C14H29Cl, 98%), 1-chlorohexadecane (n-C16H33Cl, 97%) and quinine sulfate fluorescence standard substance (98.6%) were purchased from Aladdin Biochemical Technology Co.Ltd.(Shanghai, China).Ethylenediamine (EDA, ≥ 99.0%) and hydrogen peroxide solution (H2O2, 30.0% (w) in water) were bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai,China).Double distilled water was used throughout the work.
2.2 Preparation of the carbon quantum dots and the successive quaternization
Firstly, weighed 3.0 g EDTA (0.010 mol) and dissolved in 6 mL water.The solution was mixed with dropwise EDA (3.5 mL,their molar ratio = 1 : 5) with a stirring.Then hydrogen peroxide of 1 mL was input into the mixture slowly with an agitation.Such a mixture was transferred into a Teflon vessel (25 mL) which placed in a stainless steel autoclave.The pyrolysis was carried out at 180 °C for 60 min.The solution in Teflon vessel was transferred completely into a round-bottom flask when the autoclave was cooled to ambient temperature.The carbon quantum dots were obtained by removing the solvent and excess EDAviareduced pressure distillation at 90 °C.Similar approach was carried out for synthesizing carbon quantum dots without hydrogen peroxide.Such two carbon dots were represented with OX-CQDs and CQDs respectively.The amount of synthesized OX-CQDs was recorded for calculating the yield.
As-prepared OX-CQDs were dissolved entirely in 15 mL methanol and mixed with 3 mLn-C12H25Cl (0.010 mol).The mixture was heated with a reflux at 65 °C for 5 h.Finally the solvent methanol was removed completely by reduced pressure distillation.The remaining was mixed with 25 mL water and transferred into a separating funnel for removing the supernatant(n-C12H25Cl).Such operation was repeated twice for removing the excessn-C12H25Cl.The suspension was placed in an oven with ventilation at 70 °C until the water was removed entirely.Amount of the product (represented with OX-CQDs-C12H25)was noted down for calculating the yield.In order to explore the effect of length of hydrocarbon chain on the surface activity quaternization of OX-CQDs withn-C14H29Cl andn-C16H33Cl were also carried out.The products were represented with OXCQDs-C14H29and OX-CQDs-C16H33respectively.
Similarly, quaternization of CQDs withn-C12H25Cl was executed (the product was represented with CQDs-C12H25) for comparing the performance of declining surface tension of water with that of OX-CQDs-C12H25.
2.3 Measurements of fluorescence quantum yields and surface tensions of the solutions of samples
The fluorescence emission areas of CQDs, OX-CQDs and OX-CQDs-C12H25solutions and the corresponding UV-visible absorbance (< 0.05) were recorded for linear regressions for calculating the slopes of the lines.Fluorescence quantum yields of these samples were obtained by comparing the slopes with that of standard substance of quinine sulfate according to
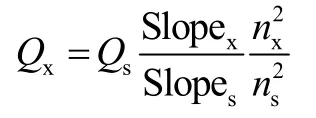
whereQsandQxrepresents quantum yields of fluorescent standard substance (55.00% in 0.05 mol·L-1sulphuric acid solution) and sample; SlopexandSlopesrefer to slopes of the linear regressions of sample and standard substance respectively;nxandnsrepresent to refractive index of sample and standard substance respectively (they are equal approximately in this case).
Surface tensions of the aqueous solutions of OX-CQDs-C12H25, CQDs-C12H25, OX-CQDs-C14H29and OX-CQDs-C16H33were measured by virtue of maximum bubble pressure method.Concentrations of the measured solutions were increased gradually until surface tension of the solution did not decrease almost.
2.4 Measurement of minimal inhibitory concentrations of OX-CQDs-C12H25 and OX-CQDs
Firstly, took eight EP tubes and prepared varied concentrations of OX-CQDs-C12H25and OX-CQDs (3.30, 1.65, 0.83, 0.41,0.21, 0.10, 0.052 and 0.026 mg·mL-1for the samples respectively) with test bacteria solution (Escherichia coli).Three times of 100 µL of each sample was added to three wells of a 96-well plate for a parallel comparison (including a sample of positive control).Then the plate was placed in an incubator at constant temperature of 37 °C for 18-24 h.The OD (optical density) values of such mixtures were measured with an enzymatic marker.Thus the antibacterial percentages were obtained by calculations on these OD values.
2.5 Characteristic techniques
Morphologies of CQDs, OX-CQDs and OX-CQDs-C12H25were illustrated by images of transmission electron microscopy(Tecnai G2 F20 S-TWIN).Fluorescence spectra (LS 45,PerkinElmer) of OX-CQDs, CQDs and OX-CQDs- C12H25were measured for calculating the fluorescence quantum yields.Structural information of OX-CQDs and OX-CQDs-C12H25were revealed by infrared (IR),1H nuclear magnetic resonance(1H-NMR) and X-ray photoelectron energy (XP) spectra respectively (corresponding to equipments of Nicolet 380,BRUKER 400 and KRATOS AMICUS respectively).Percentages of elemental C, N and O of OX-CQDs and OXCQDs-C12H25were received by analysis on XP spectra of a wide scanning.Narrow scanning on C 1sand N 1swas also carried out for demonstrating the quaternization of OX-CQDs.
3 Results and discussion
Purposes of choosing EDTA as a raw material of preparing OX-CQDs are based on the following two considerations.(1)The four terminal carboxyl groups ensure the reactivity with EDA and possibility of carbonizationviapyrolysis12.The combination with EDA may increases hydrophilicity of OXCQDs.Additionally the doped nitrogen may lead to an enhancement of fluorescence quantum yield of CQDs31,32.(2)The tertiary amines of EDTA can involve in successive quaternization with chlorohydrocarbon.If the hydrocarbon chains are introduced onto OX-CQDs a cationic OX-CQDs surfactant with bright fluorescence may be synthesized.Hydrogen peroxide, as intensive oxidant, may benefit for fluorescence quantum yields, which is demonstrated in our previous work33,34.It does not lead to excessive operation since the final product is water.During the synthesis, EDA is excess intentionally which ensures the complete conversion of EDTA.The excess EDA can be removed completely by reduced pressure distillation.This favors the purity of synthesized OXCQDs.The experimental result shows that the yield of OXCQDs and OX-CQDs-C12H25are 82.6% and 65.4% respectively(the respective amounts of OX-CQDs and OX-CQDs-C12H25are 5.49 and 6.03 g).The carbonization of the raw materials is proved by images of Fig.1a and Fig.S1 (Supporting Information).The sizes of OX-CQDs are distributed mainly at 1.8-2.0 nm with lattice distance of 0.3 nm35,36.
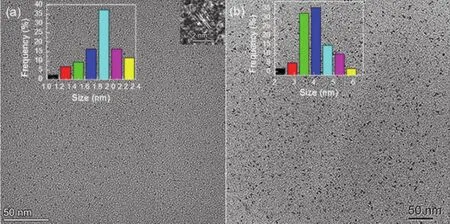
Fig.1 Transmission electron microscopy images and particle distributions of OX-CQDs and OX-CQDs-C12H25 corresponding to (a) and (b) respectively.
3.1 Analyses on OX-CQDs and OX-CQDs-C12H25 with IR, 1H-NMR and XP spectra
IR spectrum of OX-CQDs shown in Fig.2a reveals the intensive absorption between 3000 and 3500 cm-1.This should be attributed to overlapping of O-H and N-H stretching vibration of -OH and -NH2respectively.And the deep absorption near 3000 cm-1is due to stretching vibration of CH of alkyl.Peaks of 1634 and 1588 cm-1should be arisen from modes of amide I and II respectively, which proves the amidation between EDTA and EDA.Also, Peaks of 1396 and 1317 cm-1are due to the coupling of stretching vibration of C-N and scissoring vibration of N-H.The spectrum demonstrates the combination between EDTA and EDA.
Fig.2a also tells us that there is no obvious variation between OX-CQDs and OX-CQDs-C12H25.The spectra can not provide clear evidences to prove the quaternization of OX-CQDs.This may be due to the weak signals of the introduced hydrocarbon chains.However1H-NMR spectra of Fig.2b demonstrate the quaternization.The dense signals centered at 3.0 of both OXCQDs and OX-CQDs-C12H25are arisen from -CH2N- and-CH2C=O, which causes the1H signal atαposition of+NCH2CH2- in OX-CQDs-C12H25is obscure.Nevertheless,OX-CQDs-C12H25provides two signals at chemical shifts of 0.75 and 1.20, which belong to -CH3and -CH2-respectively37,38.That is due to the introduced hydrocarbon chains39,40.The inset of Fig.2b displays the enlarged range from 10.0 to 7.0.It exhibits the weak signals at 7.9 and 8.4 marked with asterisks, which should be due to1H of amide -NHCO-.This is consistent with the IR analysis.
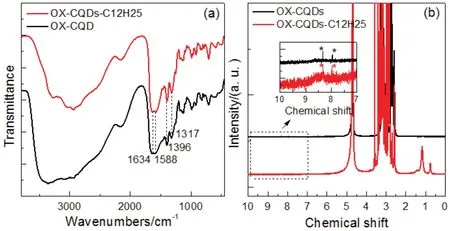
Fig.2 IR and 1H-NMR spectra of OX-CQDs and OX-CQDs-C12H25 corresponding to (a) and(b) respectively (chemical shift at 4.74 is due to solvent D2O).
XP spectra of Fig.3 also supply the structural information of OX-CQDs and prove the quaternization of OX-CQDs.Peaks of 286.9 eV of OX-CQDs and 287.2 eV of OX-CQDs-C12H25in C 1sspectra are arisen from -ONH-.These agree with IR analyses.Signals of 285.0 eV of OX-CQDs and 285.1 eV of OXCQDs-C12H25are attributed to -NHH2-.Peaks of 283.8 eV of OX-CQDs and 284.0 eV of OX-CQDs-C12H25are due to -H2-.Beside, N 1ssignal of OX-CQDs-C12H25shifts to a higher binding energy compared with that of OX-CQDs marked with a dash line, which indicates some of nitrogen elements are quaternized partly41.Table S1 (Supporting Information) also reveals the successful attachment of the hydrocarbon chain to OX-CQDs.After the quaternization the atomic percentage of carbon reaches 73.73%, which is more than 70.05% of OXCQDs.Such evidences prove the quaternization of OX-CQDs.Summarily, both1H-NMR and XPS give solid evidences of proving the hydrocarbon chains are attached to OX-CQDs.
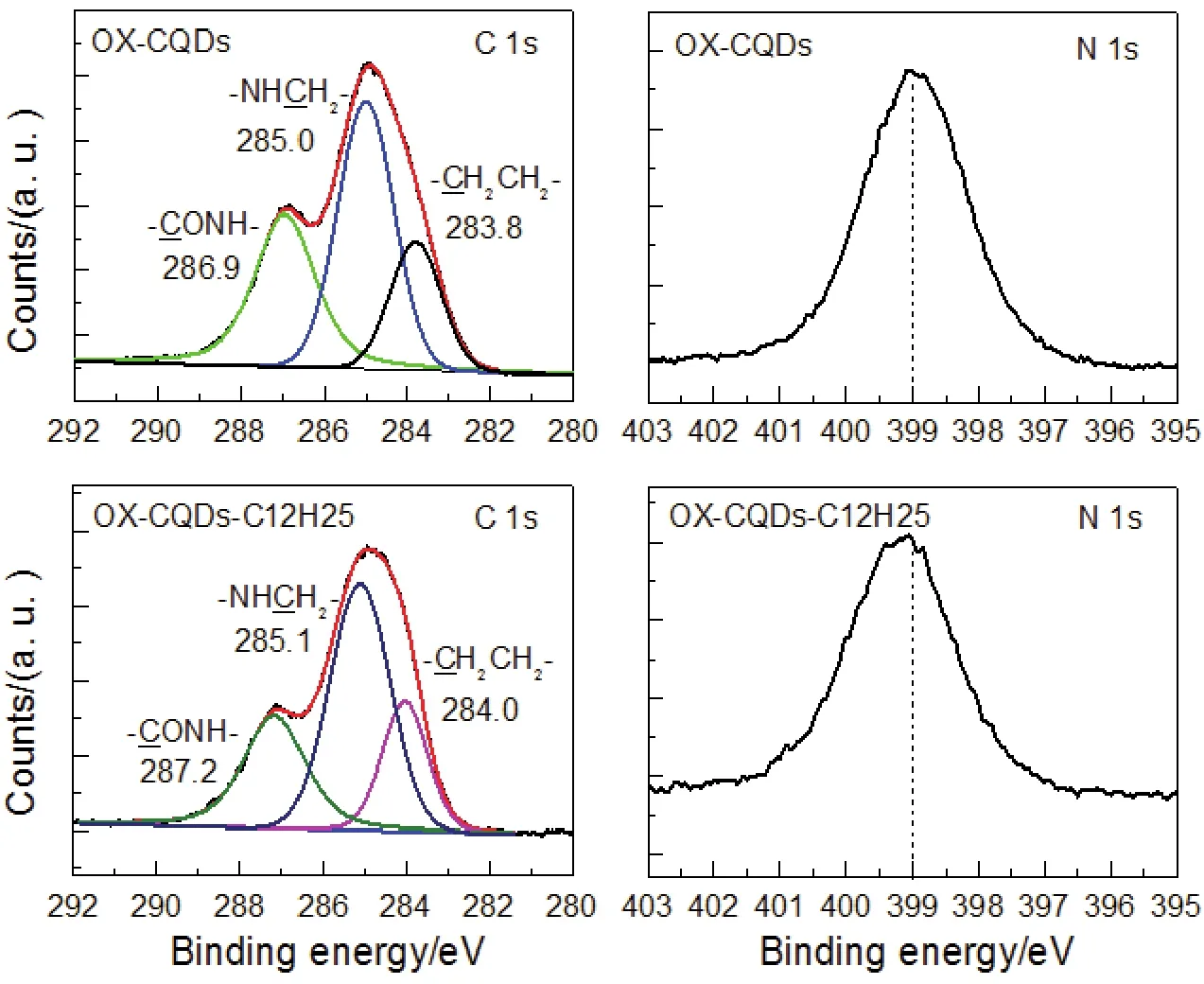
Fig.3 C 1s and N 1s signals of XP spectra of OX-CQDs and OX-CQDs-C12H25.
Additionally, Fig.1b exhibits that the particle sizes of OXCQDs-C12H25are distributed predominately in 3.5-4.5 nm,which are greater than those of OX-CQDs.Such evidence proves that the long hydrocarbon chain is attached to OX-CQDs.
3.2 Fluorescent performances of CQDs, OX-CQDs and OX-CQDs-C12H25
Integrated areas of fluorescent peaks of CQDs, OX-CQDs and OX-CQDs-C12H25varied with the absorbance are recorded in Fig.4a, b.The slopes of the regressed lines corresponding to CQDs and OX-CQDs are 3731.62 and 5006.59, respectively.Thus the quantum yields are 6.09% and 8.17% respectively.That illustrates hydrogen peroxide is contributive for the enhancement of quantum yield.Intensive oxidation of hydrogen peroxide may result in more oxygen-contained functional groups in the CQDs.Some of them may be involved in conjugated structuresviap-πorπ-πinteractions.The quantum yield of OXCQDs-C12H25is 6.44% according to the slope of regressed line shown in Fig.4b, which declares that fluorescent quantum yield of OX-CQDs decreases after the quaternization.The fluorescence spectra in Fig.4c indicate the fluorescent emissions of both OX-CQDs and OX-CQDs-C12H25are 414 nm.Quaternization on OX-CQDs with 1-chlorododecane does not alter the emission.This declares the tertiary amino group does not belong to fluorophore of OX-CQDs.Additionally the excited electrons are disturbed by positive charge of quaternary ammonium of OX-CQDs-C12H25, which impedes the back transition to ground state that declines the fluorescent quantum yield.
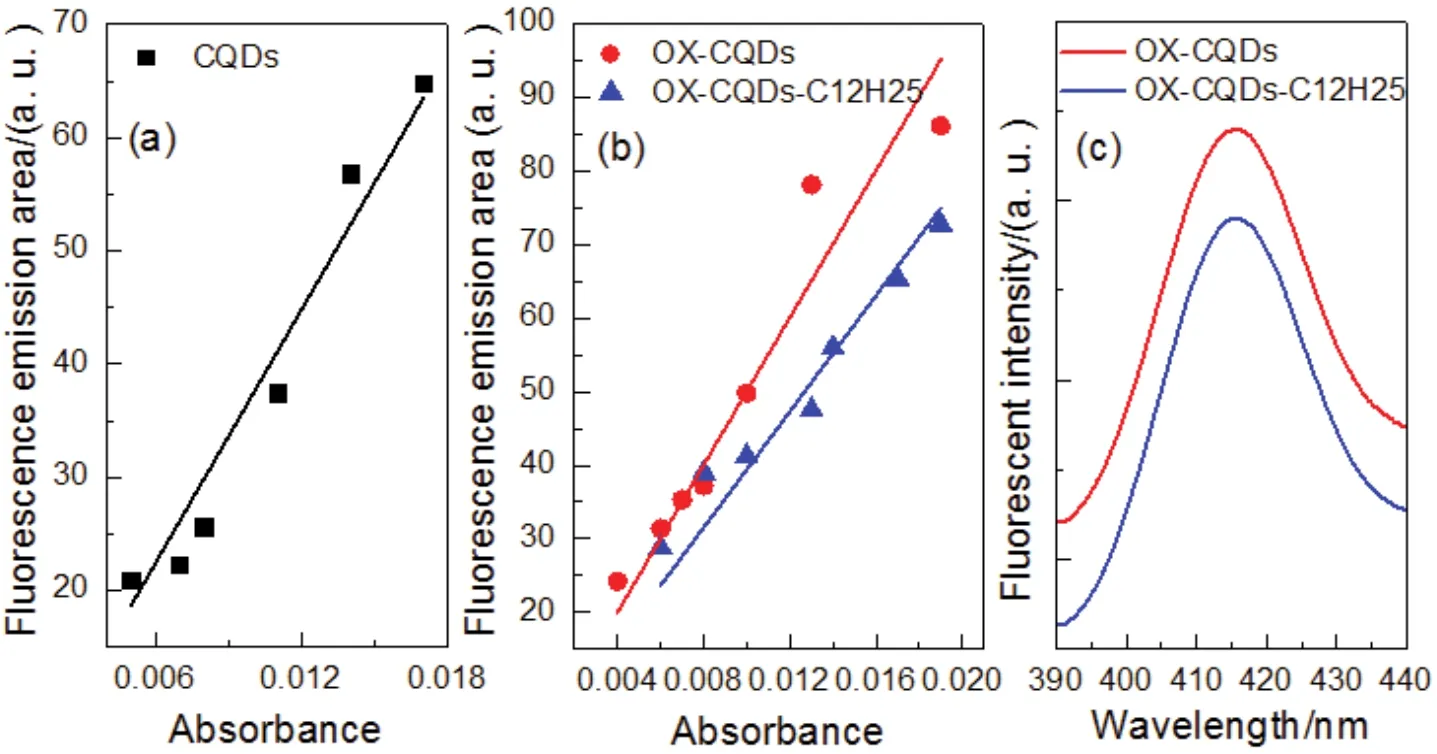
Fig.4 (a, b) Fluorescent emission area versus the corresponding absorbance of the measured solutions as well as the regressed lines(fixed intercept at 0); (c) fluorescence spectra of OX-CQDs and OX-CQDs-C12H25 (excitation wavelength = 340 nm).
3.3 Surface tensions of CQDs-C12H25, OX-CQDs-C12H25, OX-CQDs-C14H29 and OX-CQDs-C16H33 solutions
The dilute solution of OX-CQDs-C12H25performs wonderful capability of declining surface tension of water, which exhibited in Fig.5a.Surface tension of pure water declines rapidly with the increased concentrations of OX-CQDs-C12H25.When the concentration is greater than 10.0 mg·mL-1, surface tension of the solution remains almost unchanged.Minimum of the surface tension reaches 26.7 mN·m-1.At pH = 6.0 or 8.0 such performance is affected scarcely, which indicated in Fig.S2(Supporting Information).Alkalescent medium at pH = 8.0 achieves a better performance of declining surface tension of water compared with that at pH = 6.0, which equals to the result shown in Fig.5a approximately.CQDs-C12H25possesses the similar performance.However, the minimum of surface tension is greater than that of OX-CQDs-C12H25.Hydrogen peroxide plays a role of oxidizer that increases the amount of oxygencontained groups which is favorable for the hydrophilicity of OX-CQDs.Thus hydrophile-lipophile balance is optimized.Additionally quaternization on OX-CQDs withn-C14H29Cl orn-C16H33Cl can not lead to a more decline of surface tension compared with that withn-C12H25Cl, illustrated in Fig.5b.The value of critical micelle concentration is also much greater than that of OX-CQDs-C12H25.These results exclaim thatn-C12H25Cl provide a proper length of hydrocarbon chain that results in a better performance of reducing surface tension of water.
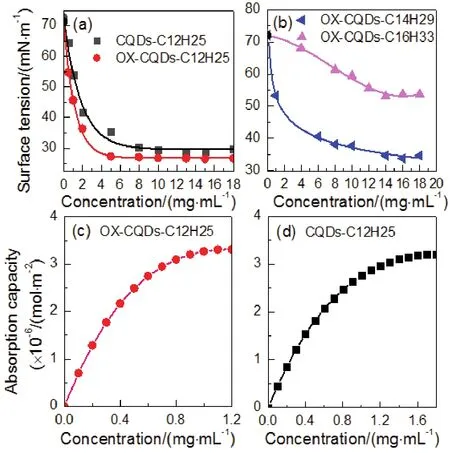
Fig.5 (a, b) Surface tensions of CQDs-C12H25, OX-CQDs-C12H25, OX-CQDs-C14H29 and OX-CQDs-C16H33 solutions varied with the concentrations;(c, d) surface absorption capacities of OX-CQDs-C12H25 and CQDs-C12H25 solutions via the theoretical calculations.
Contact angle detections on solutions of CQDs-C12H25and OX-CQDs-C12H25are exhibited in Fig.6.CQDs-C12H25solutions with 1.0, 5.0 and 10.0 mg·mL-1own contact angles of 75.5°, 50.1° and 44.6° respectively.Contact angles of OXCQDs-C12H25solutions of 70.4°, 43.4° and 38.0° corresponding to 1.0, 2.5 and 5.0 mg mL-1demonstrate more excellent performance of OX-CQDs-C12H25in declining surface tension of water than that of CQDs-C12H25because of the smaller contact angles and lower concentrations.This is also in accordance with the measurements of the surface tension of the solutions shown in Fig.5a.
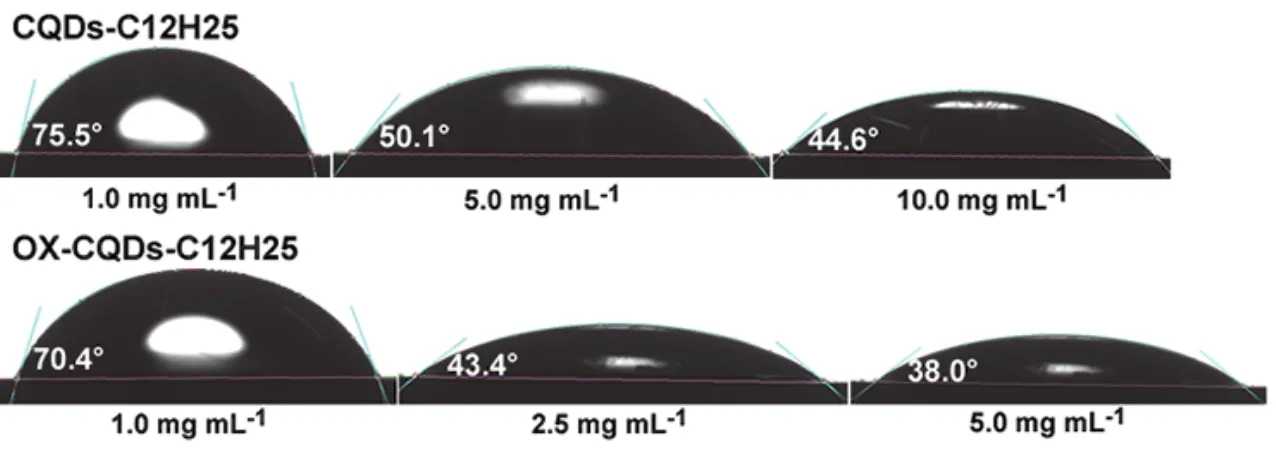
Fig.6 Contact angles of CQDs-C12H25 and OX-CQDs-C12H25 solutions with different concentrations.
Such an outstanding performance of OX-CQDs- C12H25is superior to several of Gemini cationic surfactants reported recently, which belong to new type of one with wonderful property in decreasing surface tension of water.For example, the minimum surface tensions of the solutions of 1,3-2(alkyl amide propyl dimethyl ammonium chloride)iso-propyl alcohol42,N,N-dimethyl-N-[3-(gluconamide/lactobionamide)] propyl-N-alkylammonium bromides43, [CH2=C(CH3)COO(CH2)11N+(CH3)2CH2]2·Br-44and dendritic cationic tetrameric surfactants with different alkyl chains (C12, C14 and C16)45are 34.78,28.36, 32 and 30.29 mN·m-1respectively.All the values are greater than that of OX-CQDs-C12H25.Additionally the procedures of synthesizing these Gemini cationic surfactants are more complicate than the one of preparing OX-CQDs- C12H25.The technique of synthesizing such a novel cationic surfactant shows more superiority in the cheap raw materials and the convenient manufacture.
The regressed curve of Fig.5a represents the relationship between surface tensions of CQDs-C12H25and OX-CQDs-C12H25solutions and the concentrations (see the descriptions in Supporting Information).Hence, the surface absorption amount(Γ) can be calculated by Gibbs absorption isothermal equation as long as the derivation of this curve is obtained, which depicted in the equation:
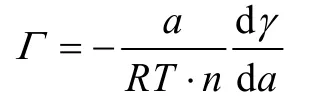
wherea,Tandγrepresent activity of the surfactant, temperature(Kelvin) and surface tension (N·m-1) respectively;Ris gaseous Constance (8.314 J·mol-1·K-1);nis the number of ionic species;n= 2 is generally accepted38.According to the theory of solution surface adsorption, surface adsorption amount enhances gradually with the increased concentration of OX-CQDs-C12H25until it reaches a maximum which predicts absorbing saturation.When the concentration is rather low the variableain Gibbs absorption isothermal equation can be replaced by the concentration.Higher concentrations lead to errors of calculatedΓ.Fig.5c reveals that when the concentration is less than 1.2 mg·mL-1the surface absorption capacities calculated by Gibbs isothermal equation satisfy the rule stated above (the data are exhibited in Table S2 (Supporting Information)).Therefore the maximum of surface absorption capacity is 3.31 × 10-6mol·m-2.Thus the sectional areas of hydrophobic chains can be calculated according to
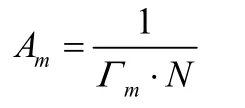
whereAmandΓmrepresent sectional areas of hydrophobic chains of every OX-CQDs-C12H25molecule and saturated absorption capacity respectively;Nis Avogadro constant (6.022 × 1023mol-1).ThusAm= 0.502 nm2.Since sectional area of a hydrophobic chain is 0.205 nm2every OX-CQDs-C12H25particle possesses 2.447 hydrocarbon chains averagely.Such hydrocarbon chains along with the hydrophilic groups of OXCQDs such as carboxyl, amide and amino groups devote the well performance in declining surface tension of water.The oxidation of hydrogen peroxide is contributive for producing more hydrophilic groups, which regulates a proper value of hydrophile-lipophile-balance.That is helpful for the surface activity.
According to the similar way of treating the fitted curve of CQDs-C12H25shown in Fig.5a, the calculatedΓvaried with the concentrations are plotted in Fig.5d (the data are exhibited in Table S3 (Supporting Information)).Every CQDs-C12H25possesses 2.529 hydrocarbon chains averagely that equals approximately with that of OX-CQDs-C12H25.However, the surface activity of CQDs-C12H25is weaker than that of OXCQDs-C12H25.This illustrates that oxidation of hydrogen peroxide increases the amount of hydrophilic groups of OXCQDs which is helpful for the surface activity.Hence, hydrogen peroxide plays a positive role in enhancing both the fluorescent quantum yield and the surface activity of the surfactant.
3.4 Antibacterial activities of of CQDs, OX-CQDs,CQDs-C12H25 and OX-CQDs-C12H25
Antibiosis of CQDs, OX-CQDs, CQDs-C12H25and OXCQDs-C12H25onEscherichia coliis indicated in Fig.7.The minimal inhibitory concentration for OX-CQDs-C12H25or CQDs-C12H25is 0.41 mg·mL-1, at which the antibacterial percentage still approaches 100% approximately.The concentration is far less than critical micelle concentration (10.0 mg·mL-1) revealed in Fig.5a.That declares OX-CQDs-C12H25solution has wonderful antibiosis during exerting the surface activity.OX-CQDs or CQDs solution without chemical modifications has the similar performance46.However, the power of antibiosis is far weaker than that of OX-CQDs-C12H25or CQDs-C12H25since the minimal inhibitory concentration reaches 3.30 mg·mL-1.Quaternization of OX-CQDs or CQDs favors the enhancement of antibiosis.The quaternary ammonium group of OX-CQDs-C12H25or CQDs-C12H25with positive charged interacts with negative charges on surface of cellular cytoplasmic membrane.The long hydrocarbon chain may insert into the membrane.Thus the selective permeability of the membrane is disturbed.
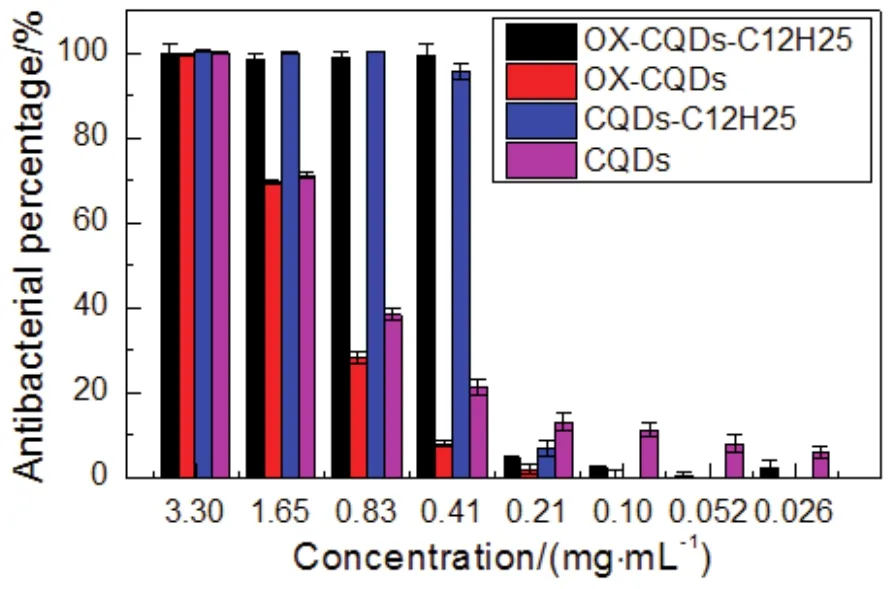
Fig.7 Histogram of antibacterial activities of CQDs, OX-CQDs,CQDs-C12H25 and OX-CQDs-C12H25.
As a new type of surfactant, OX-CQDs-C12H25possesses not only wonderful performance of reducing surface tension of water but also well antibacterial activity.These properties are beneficial for being as a component of making detergent.
4 Conclusions
The work provides economical techniques of synthesizing cationic surfactant with more inexpensive raw materials and more convenient approaches.Hydrothermal approach of preparing OX-CQDs satisfies the demands of green chemical synthesis.OX-CQDs-C12H25prepared in the presence of hydrogen peroxide performs better than that of CQDs-C12H25obtained without hydrogen peroxide.The invented OX-CQDs-C12H25, as a novel CQDs cationic surfactant, owns versatility in fluorescent emission, surface activity and antibiosis.Hence, it is superior to some conventional surfactants with sole function.It is believed that OX-CQDs-C12H25can be as a component of a compounded detergent, in which it exerts the versatility.The fluorescent emission endows function of fluorescent whitening.This may avoid use of organic fluorescence dyes which is advantageous for health of the public such as in paper making.The antibiosis contributes antibacterial function without needing extra addition of bactericide.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
