香茅油纳米乳剂的构建、表征、抗菌性能和细胞毒性
2023-01-13陈志洋唐雅婷吕泽孟霄汉梁前伟冯建国
陈志洋,唐雅婷,吕泽,孟霄汉,梁前伟,冯建国
扬州大学园艺与植物保护学院,江苏 扬州 225009
1 Introduction
The misuse and abuse of agricultural synthetic bactericides have caused a series of serious ecological and environmental problems, including environmental pollution1, ecological damage2, pathogen resistance3, and toxic influence on many organisms4, including humans.Therefore, natural bactericides with high efficiency and low toxicity are urgently needed5,6.Plant essential oils have bactericidal and antiseptic effects and can be used in preventing and reducing the speed of pest infestation and disease outbreaks caused by fungi and bacteria and have thus become the focus of some studies on fungicides and bactericides7-9.For instance, citronella oil, a natural plant essential oil belonging to the genusCitronellaof Gramineae, is obtained from theCymbopogon nardusthrough steam distillation10.Citronella oil is regarded as an insect repellent11and is effective in refreshing the mind, purifying the room, and softening the skin12.Moreover, it can be used in cleaning oily skin and sweaty feet13.Interestingly, citronella oil possesses antibacterial activities, manifesting its latent capacity as a natural bactericide14,15.However, the use of citronella oil in preventing agricultural pathogen invasion has not been widely studied.
As a plant essential oil, citronella oil is prone to oxidative degradation and volatilization16, which result in the loss of its activity, in practical applications.Additionally, dispersing citronella in water is difficult because of its hydrophobic trait17.To overcome the limitations imposed by the oxidizability and hydrophobicity of citronella oil, researchers have explored various methods that integrate it into different systems18-20.Nanoemulsions are nanometric dispersion systems of oil droplets in external aqueous phases21.Emulsifiers can promote the distribution of hydrophobic plant essential oils in the tiny oil droplets in water by naoemulsions, thereby preventing the adverse effects of external causes, such as pH22, oxidation23,and hydrolysis, and increasing the solubility of bioactive compounds24.Nanoemulsions have exceptional interface traits,such as electrostatic repulsion and viscoelasticity, and have thus been widely used in the delivery systems of various hydrophobic substances25, including essential oils.Compared with other types of colloidal delivery systems, nanoemulsions have the following advantages: (1) simple process, small particle sizes(generally 20-500 nm), and large specific surface areas, which result in good load capacity and water solubility26; (2) excellent physical stability and ability to coalesce and flocculate27; (3)ability to improve the bioavailability of organic matter and change the uniformity, dispersion, and absorption rate of organic matter simultaneously28; (4) low requirement regarding the amount of emulsifier (less than the amounts required by other emulsions)29.The emulsifier dosage in a microemulsion is more than 20%.In summary, oil-in-water nanoemulsions are effective in reducing the hydrophobicity of citronella oil, thereby facilitating it to exert its effects.
In this research, a high-energy emulsification method was used in preparing oil-in-water citronella oil nanoemulsions.The influences of emulsifier type (HLB), emulsifier dosage and emulsifying time on the stability of nanoemulsion were studied.Then, the best citronella oil nanoemulsion formula was determined.In addition, biological activity againstPantoea ananatisand cytotoxicity analysis on the L02 cells of citronella oil nanoemulsions were investigated.The present study may provide novel methods for fabricating stable, efficient, and environmentally friendly nanoemulsions loaded with citronella oil as a natural biological bactericide.
2 Materials and methods
2.1 Materials
Citronella oil was obtained from Shanghai Yuanye Biological Technology Co., Ltd.(Shanghai, China).Castor oil polyoxyethylene ethers (EL-20, EL-40, EL-60, EL-80, and EL-90) and industrial grade emulsifiers were obtained from Tianjin C&S Biochemical Technology Co., Ltd.(Tianjin, China).Although these castor oil polyoxyethylene ethers have the same lipophilic structures, they have different quantities of hydrophilic groups.Their HLB values are 9.5, 13.5, 14.5, 15.5,and 17.0, respectively.In the preparation of nanoemulsions,deionized water is necessary for dilution.
2.2 Forming of citronella oil nanoemulsions
All nanoemulsions loaded with citronella oil were fabricated through high-energy emulsification at room temperature (25 °C)30.Citronella oil and an emulsifier were mixed in a small beaker and magnetically stirred at 800 r·min-1for 5 min as the oil phase.The water phase (deionized water) was added.The mixture was subjected to high shear at 10000 r·min-1for 3 min for the production of nanoemulsions.Using different emulsifiers and dosages, we analyzed the influences of the emulsifiers on the forming nanoemulsions loaded with citronella oil.After determining the optimal emulsifier type and dosage, we determined the best emulsification time.
2.3 Visual observation of citronella oil nanoemulsions
The newly prepared nanoemulsions loaded with citronella oil(approximately 8 mL) were transferred and packaged into transparent sample bottles, and differences in appearance among the samples were observed after 14 days of storage at a high temperature 54 ± 2 °C.
2.4 Measurement of droplet size in the nanoemulsions
The average droplet size of the citronella oil nanoemulsions was determined using a nano-laser particle size and zeta potentiometer (Zetasizer Nano ZS90, Malvern laser particle size potentiometer, Malvern Instruments Co., Ltd., England) after preparation at 25 ± 2 °C (0 day), low-temperature storage at 0 ±2 °C for 7 days, and high-temperature storage at 54 ± 2 °C for 14 days successively.Before measurement, all the samples were diluted 200 times with deionized water.
2.5 Microstructural analysis of the citronella oil nanoemulsions
Fluorescein was added during the preparation of the citronella oil nanoemulsion.Then, the samples were dropped evenly on glass slides, which were gently covered with glass coverslips.Under an excitation wavelength of 519 nm, a confocal laser scanning microscope (LSM 880 NLO, Carl Zeiss, Germany) and a fluorescence microscopy imaging system were used to capture the microscopic images.
2.6 Biological activity determination of the citronella oil nanoemulsions
Pantoea ananatiswas provided by the Laboratory of Plant Pathology in Yangzhou University.An appropriate amount of deionized water was added to the nanoemulsions loaded with citronella oil samples.The samples were diluted to 62.5, 125,250, 500, and 1000 mg·L-1according to the law of equal proportion.The sample containing deionized water only was used as the blank control.The bacterial solution (OD600= 0.7)was evenly distributed (5 mL) after the culture was shaken for 2 h at a speed of 200 r·min-1and temperature of 37 °C in a centrifuge tube.Then, the bacterial solution was diluted 106times with sterile deionized water.The diluted bacterial solution was placed in a pre-made Petri dish (100 μL for each test and repeated for 3 times) with a pipette and distributed evenly with the coating method.The number of colonies was calculated after 12 h.
2.7 Cytotoxicity assay of the citronella oil nanoemulsions
2.7.1 Cell viability
L02 cells (human normal liver cells) were nurtured in a Dulbecco’s Modified Eagle Medium (DMEM) with fetal bovine serum in an incubator (37 °C with 5% carbon dioxide).The toxicity of the nanoemulsions loaded with citronella oil to cells were analyzed with the CCK-8 analytical method.The L02 cells were exposed to 6.25, 12.5, 25, 50, and 100 mg·L-1citronella oil nanoemulsions for 24 h (a blank test control was used for comparison).Then, 10 µL of the Cell Counting Kit-8 (CCK-8)solution was added to each well in the dark, and the cells were incubated in a 96-well plate at 37 °C (6 × 104cells in each well).A hole was created to prevent bubbles that may affect the OD reading.Absorbance value was measured at 450 nm with a microplate reader.The calculation formula of cell viability is as follows:

2.7.2 Cell apoptosis
Annexin-V-FITC/PI double staining method was used for apoptosis analysis.Dosing and negative control groups were established for comparison.After 24 h, 50 mg·L-1citronella oil nanoemulsions were used in treating the cells of the dosing group.Cells that were not treated with citronella oil nanoemulsions were included in the negative control group cultured in a serum-free medium.After 24 h of culture, the adherent cells were digested with tryptic enzyme for 30 s and centrifuged for 5 min at a speed of 800 r·min-1.The cells were washed three times with precooled PBS, and 5 μL of annexin-VFITC and PI staining solution were added to each tube.The cells were stained for 15 min in the dark at 4 °C.Then, flow cytometry analysis was conducted after filtration with 400 mesh screens.
3 Results and discussion
3.1 Influence of emulsifier type on the stability of the citronella oil nanoemulsions
An appropriate emulsifier plays an important role in the preparation of stable nanoemulsions31,32.As an essential trait of emulsifiers, HLB value is an important basis for selecting emulsifiers for nanoemulsions33,34.Fig.1 shows the appearances of the citronella oil nanoemulsions prepared with different emulsifiers after new preparation and storage at a high temperature for 14 days.The nanoemulsions prepared by different emulsifiers obviously showed different levels of stability in terms of appearance.After preparation, the citronella oil nanoemulsions prepared with EL-20, EL-40, EL-60, EL-80,and EL-90 were fairly homogeneous in appearance but had different colors.The nanoemulsions prepared with EL-40 and EL-60 were slightly bluer than those prepared with EL-20, EL-80, and EL-90 (ivory), showing a translucent state.After 14 days of high-temperature storage at 54 ± 2 °C, the citronella oil nanoemulsions prepared with EL-40 became bluer and transparent.By contrast, the sample prepared with EL-60 changed from having a bluish and translucent appearance to having a milky white and opaque appearance, but both had no unstable phenomena (such as sedimentation and creaming).The citronella oil nanoemulsions prepared with EL-20, EL-80, and EL-90 had greater variations in appearance.The nanoemulsions prepared with EL-20 changed from an opaque phase to a transparent phase accompanied by a clear yellow oil layer (about 15%).The nanoemulsions containing EL-80 and EL-90 were opaquer and slightly creamy.

Fig.1 Appearance of citronella oil nanoemulsions prepared with different emulsifiers (with various HLB values) after preparation and high-temperature (54 ± 2 °C) storage for 14 days.
Fig.2 reveals the influence of the emulsifier type on the average droplet size of the citronella oil nanoemulsions.The nanoemulsions prepared with EL-40 (HLB = 13.5) had the smallest droplet size (30 nm), whereas the nanoemulsions prepared with EL-20 (HLB = 9.5) had the largest (543 nm).The droplet size of the nanoemulsions containing EL-40 changed slightly after low-temperature storage 0 ± 2 °C and hightemperature storage 54 ± 2 °C, but the droplet size of the nanoemulsions made of other emulsifiers changed significantly and generally increased.
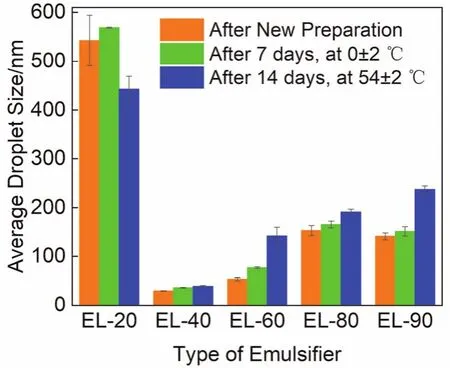
Fig.2 Variations in the average droplet size of citronella oil nanoemulsions prepared with different emulsifiers under different storage periods.
In accordance with droplet size, the laser confocal microscopic images of the nanoemulsions were captured, as shown in Fig.3.Significant differences among the microstructures of the samples were clearly observed.The citronella oil nanoemulsions prepared with EL-40 showed the smallest droplets and the most uniform particle distribution,followed by the samples prepared with EL-60 (HLB = 14.5).The nanoemulsions prepared with EL-20, EL-80, and EL-90 showed tightly packed droplets, which were bulky and unevenly distributed.The nanoemulsions prepared with EL-20 were the worst sample.In terms of the above indicators, EL-40 had an HLB value (13.5) suitable for the production of oil-in-water nanoemulsions loaded with citronella oil, which belong to a stable delivery system with small droplet size and uniform distribution.Therefore, EL-40 was used in preparing citronella oil nanoemulsions.

Fig.3 Photomicrograph of droplets in citronella oil nanoemulsions prepared using different emulsifiers after new preparation.
3.2 Influence of emulsifier dosage on the stability of the citronella oil nanoemulsions
The dosage of the emulsifier is another crucial factor in the fabrication of nanoemulsions and affects the stability of nanoemulsions to a certain extent35,36.Fig.4 shows changes in the appearances of the citronella oil nanoemulsions prepared with different EL-40 dosages after new preparation and storage at a high temperature for 14 days.The nanoemulsions prepared with a high dosage of EL-40 presented a bluish translucent shape(indicating that the droplet size was small) when it was newly prepared, and it was uniform without precipitation.As the dosage of EL-40 decreased, the newly prepared nanoemulsions gradually showed an opaque milky white appearance.After 14 days of high-temperature storage, the samples with a high EL-40 dosage became transparent and showed deep blue colors.The samples with low doses of EL-40 were creamed, and the degree of creaming of the nanoemulsions prepared with 3% (w) EL-40 reached approximately 30%.
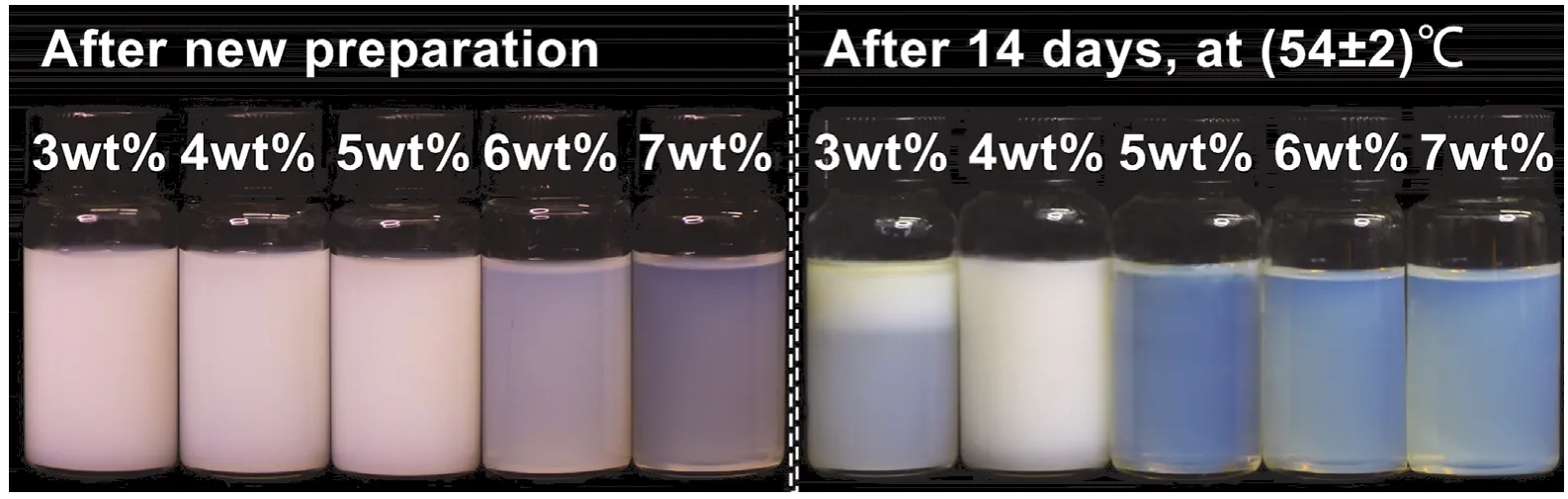
Fig.4 Appearances of citronella oil nanoemulsions prepared with different EL-40 dosages after new preparation and high-temperature (54 ± 2 °C) storage for 14 days.
Fig.5 shows changes in the droplet size of nanoemulsions at different EL-40 dosages under different storage periods.As the dosages of EL-40 increased, the size of the nanoemulsion droplets gradually increased from about 120 nm (3% (w) EL-40)to 22 nm (7% (w) EL-40).The nanoemulsions made of EL-40 showed obvious changes in droplet size after storage at high and low temperatures except the samples made of 6% (w) and 7%(w) EL-40.
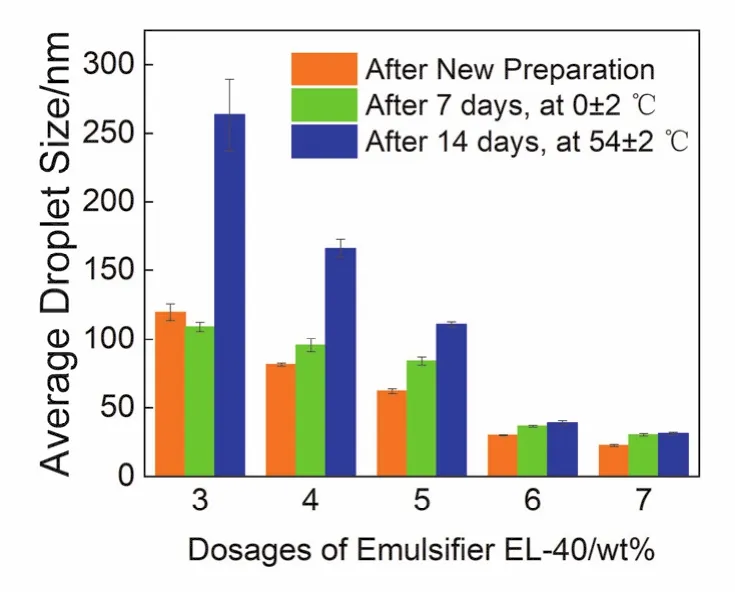
Fig.5 Variations in the average droplet sizes of the citronella oil nanoemulsions prepared with different EL-40 dosages under different storage periods.
Fig.6 shows the fluorescence microscopy images of the newly prepared nanoemulsions with different doses of EL-40.In general, the droplet distributions of the nanoemulsions prepared with high concentration of EL-40 were relatively uniform.As the amount of EL-40 decreased, the nanoemulsion droplets gradually increased in size and showed the characteristics of close packing and unevenness.The results of fluorescence imaging were generally consistent with the particle size data.
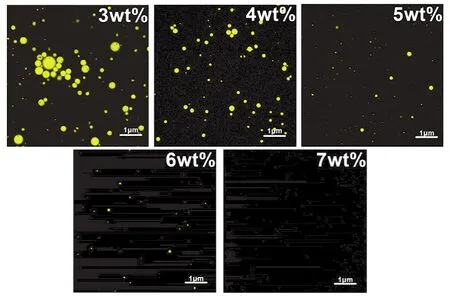
Fig.6 Photomicrograph of the droplets in the citronella oil nanoemulsions prepared with different EL-40 dosages after new preparation.
As can be seen from the experimental results, with the increase of emulsifier dosage, the nanoemulsion has a smaller droplet size, better storage stability and the droplets are more evenly distributed.Similar results have been reported in other studies where the average droplet size of nanoemulsions decreases with increasing emulsifier dosage, it is likely due to the increase in interfacial area and the decrease in interfacial tension with the increase of emulsifier.More emulsifier is adsorbed near the oil/water interface, forming a thick interfacial film that improves the stability of the nanoemulsion and prevents flocculation37,38.At the same time, considering cost and environmental friendliness, this study determined 6% (w) (the result of 6% (w) was similar with 7% (w)) as the appropriate EL-40 dose for making nanoemulsions loaded with citronella oil.
3.3 Influence of emulsifying time on the stability of the citronella oil nanoemulsions
In addition to the type and dosage of emulsifiers, the emulsifying time is an indispensable factor in high-energy emulsification39,40.
Fig.7 shows the appearances of the citronella oil nanoemulsions prepared at different emulsifying times after new preparation and storage at a high temperature for 14 days.The results indicated differences in stability among the nanoemulsions.The nanoemulsions prepared after 3 min of emulsification appeared translucent and slightly blue after new preparation, without any precipitation or creaming.It became increasingly transparent after high-temperature storage.Conversely, the nanoemulsions prepared after 5 and 7 min of emulsification appeared milky white without precipitation after preparation.However, after high-temperature storage for 14 days, the upper layers of the samples showed slight creaming.
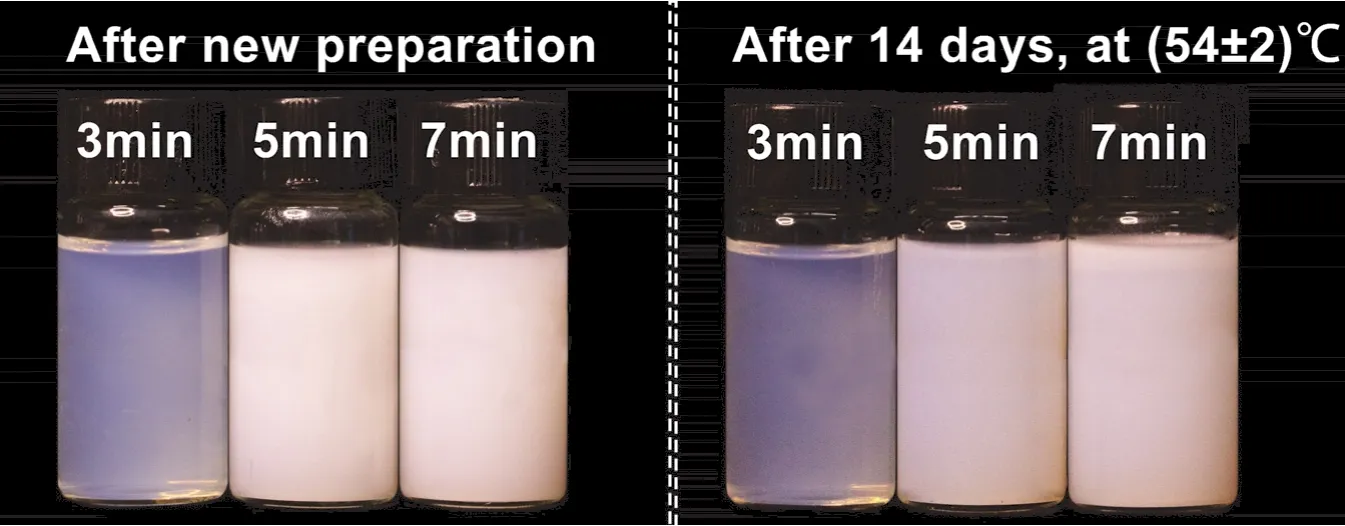
Fig.7 Appearance of citronella oil nanoemulsions prepared at different emulsifying time after new preparation and high-temperature (54 ± 2 °C) storage for 14 days.
The droplet sizes of the nanoemulsions prepared at different emulsifying time are displayed in Fig.8.The average droplet size of the nanoemulsion prepared through 3 min of emulsification was significantly smaller than that of the nanoemulsions prepared through 5 and 7 min of emulsification.After a period of low- or high-temperature storage, the droplet sizes of the nanoemulsions prepared through 3 min of emulsification did not change obviously in contrast to the nanoemulsions prepared through 5 and 7 min emulsification.It is thus determined that the nanoemulsion prepared by emulsifying in 3 min was the most stable sample.

Fig.8 Variations in the average droplet sizes of the citronella oil nanoemulsions prepared at different emulsifying time and different storage periods.
Fig.9 shows the fluorescence microscopy images of nanoemulsions prepared at different emulsifying time after new preparation.The sample droplets prepared for 3 min had the smallest size and the most uniform distribution.With the extension of emulsifying time, the size of the sample droplets (5 and 7 min) gradually increased and the distribution was uneven.The nanoemulsions prepared in 7 min showed close packing.

Fig.9 Photomicrograph of the droplets in citronella oil nanoemulsions prepared under different emulsifying time after new preparation.
In summary, the nanoemulsions prepared in 3 min had the most uniform droplets, thus exhibiting the best stability.By contrast, the nanoemulsions prepared at 5 and 7 min showed creaminess and poor stability.As the emulsifying time increases,the continued high energy input may have negative consequences41, such as a decrease in the thickness of the interface film between the oil and water phases, and a breakdown of the mechanical strength, leading to film rupture, droplet coalescence, and irreversible damage.Thus, resulting in the imbalance of nanoemulsion system.Therefore, stable citronella oil nanoemulsions can be produced by appropriately reducing emulsifying time.
3.4 Bacterial activities of the citronella oil nanoemulsion
The bacteriostatic effect of the citronella oil nanoemulsion againstPantoea ananatisis shown in Fig.10.When the concentrations of the citronella oil nanoemulsions were increased, the number of colonies decreased significantly, and the inhibitory effect was gradually strengthened.The toxicity regression equation of the citronella oil nanoemulsions againstPantoea ananatiswasY= 1.0996 + 2.0811X(R2= 0.9817).The EC50was 74.85 mg·L-1, and the 95% confidence interval was 53.21-105.30 mg·L-1.Therefore, the prepared citronella oil nanoemulsion is a bactericide with excellent activity.
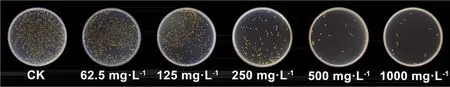
Fig.10 Bacteriostatic effect of the citronella oil nanoemulsions against Pantoea ananatis.
3.5 Viability and apoptosis of the L02 Cells
3.5.1 Cell viability
The citronella oil nanoemulsions showed high stability and antibacterial ability, but its cytotoxicity also needs to be explored.Cytotoxicity analysis is one of the crucial methods for evaluating the safety and quality of bactericides.Fig.11 shows the cytotoxicity test results for the citronella oil nanoemulsions(6.25, 12.5, 25, 50, and 100 mg·L-1) against L02 cells after incubation.Below 100 mg·L-1, the cell viability of the citronella oil nanoemulsions was above 83% after 24 h of incubation.After 48 h, although the cell viability decreased, it was still above 63%.The experiment showed that the citronella oil nanoemulsions had slight effect on normal human liver cells, exhibiting good biocompatibility.
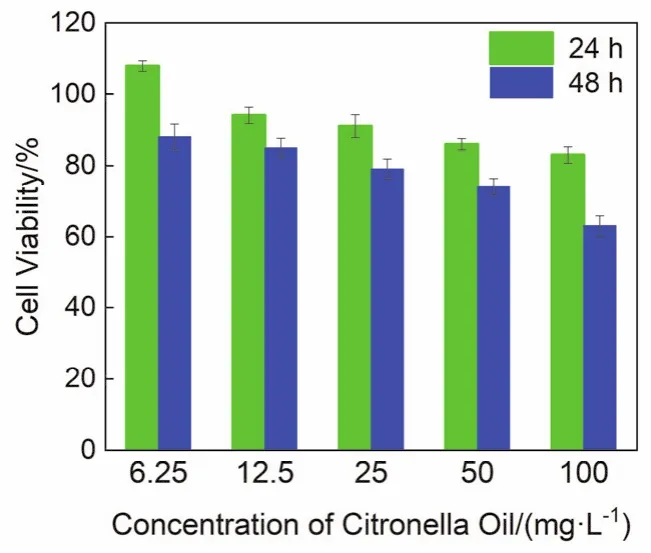
Fig.11 Cytotoxicity of citronella oil nanoemulsions against L02 cells after incubation for 24 and 48 h, as estimated by CCK-8 assay.
3.5.2 Apoptosis detection
The potential harm of using the citronella oil nanoemulsions in the human body was investigated.L02 cell line from the human liver was used.As shown in Fig.12a and Fig.12b, the total apoptosis levels in the L02 cells of the control group and nanoemulsions treatment group were 5.85% and 6.93%,respectively, and no significant difference were observed between them.Both in the control group and citronella oil nanoemulsions treatment group, the percentages of late apoptotic cells were lower than the early apoptotic cells.The distribution of living cells treated with citronella oil nanoemulsion (92.80%) was similar to that of the control group(93.80%).Meanwhile, the percentage of necrotic cells in citronella oil nanoemulsion treatment group was lower, only 0.29%.These results indicated that the self-prepared citronella oil nanoemulsions had a low toxic effect on the L02 cells.
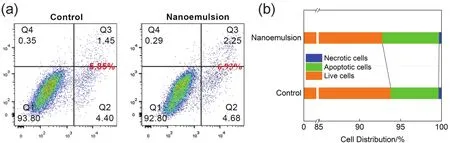
Fig.12 (a) Flow cytometry was performed for the analysis of the ratio between apoptotic and necrotic cells after exposure to citronella oil nanoemulsions (Q1: live cells; Q2 and Q3: apoptotic cells; Q4: necrotic cells).(b) Accumulation histogram of apoptosis rate.
4 Conclusions
The type and dosage of an emulsifier and emulsifying time have significant influences on the stability of nanoemulsions loaded with citronella oil.Among the emulsifiers studied (castor oil polyoxyethylene ether), EL-40 (HLB = 13.5) was found to be the optimal emulsifier for preparing citronella oil nanoemulsions with long-term stability.In a certain range (3%-7% (w)), the stability of the nanoemulsions decreased with emulsifier dosage.Regarding emulsification time, appropriately less high emulsifying time prepare the way for the more stable nanoemulsions.Hence, the optimal formula of a citronella oil nanoemulsion is as follows: 5% (w) citronella oil, 6% (w) EL-40, and 89% (w) deionized water.Finally, the EC50of citronella oil nanoemulsions againstPantoea ananatiswas 74.85 mg·L-1and low cytotoxicity.The present experimental findings are useful in the fabrication of stable citronella oil nanoemulsions as natural and safe bactericides for bacterial control in agriculture.
