Effects of Tuina on serum creatine kinase and skeletal muscle mitochondria in delayed onset muscle soreness model rats
2022-12-28WEQingbo韦庆波ZHAOQian赵谦GUJialing顾嘉凌Jia林佳ZHUYan朱延SONGZiqi宋子琪Fenglei李风雷
WEⅠ Qingbo (韦庆波), ZHAO Qian (赵谦), GU Jialing (顾嘉凌), LⅠN Jia (林佳), ZHU Yan (朱延), SONG Ziqi (宋子琪),LⅠ Fenglei (李风雷)
1 Nanjing University of Chinese Medicine, Nanjing 210023, China
2 Jiangsu Second Chinese Medicine Hospital, Nanjing 210017, China
3 Jiangsu Haibin Rehabilitation Hospital, Lianyungang 222042, China
Abstract
Keywords: Tuina; Massage; Manual Therapies; Creatine Kinase; Myalgia; Fatigue; Mitochondria; Rats
Delayed onset muscle soreness (DOMS), also known as tenderness, is a myogenic pain that mainly refers to muscle pain induced by mechanical stimulation (such as contraction, stretch, and other eccentric movements).DOMS can lead to joint movement limitation, which is related to the weakening of muscle contraction force and the changes in activation sequence and activation mode. There are many hypotheses about the pathogenesis of DOMS. Researchers have found that the occurrence of DOMS is closely related to the tear of muscles or connective tissues, and the damage of skeletal muscle ultrastructure and contractile structure,and thus put forward the “mechanical injury”hypothesis for the first time[1]. The “ischemia-spasm”hypothesis believes that exercise-induced ischemia,muscle pain, and muscle fiber spasm are mutually causal, forming a vicious circle[2]. In addition, the interruption of the excitation/ contraction coupling process of muscle fibers and the disorder of myofilaments are also possible reasons[3].
Serum creatine kinase (CK) is from muscle tissues.Studies have found that serum CK increase is related to muscle function damage caused by various factors[4].Eccentric exercise leads to a sustained increase in the cytoplasmic Ca2+of muscle cells, thereby impairing muscle function[5]. Tuina (Chinese therapeutic massage)relaxes tendons and collaterals, reduces swelling,relieves pain, and improves tension, strength, and mechanical properties of muscle tissues[6-8]. Much progress has been made in studying the mechanism of Tuina in repairing soft tissue injuries, but there are still controversies over improving local circulation and assisting muscle function recovery[9-11]. Therefore, a rat DOMS model was established in this study. Based on the kinetics of CK clearance and release and the theory of mitochondrial Ca2+transport mechanism, the model rats received Tuina before and after exhaustive exercise,and different sampling time points were designed. By measuring the content of CK in the serum, the concentration of Ca2+in mitochondria of skeletal muscle and the content of Ca2+-adenosine triphosphate(Ca2+-ATPase) in rats, and using transmission electron microscopy to observe the ultrastructural changes of skeletal muscle in rats, the prevention and treatment of acute muscle injury with Tuina were analyzed.Therefore, the study was to provide an experimental basis for clinical intervention of Tuina in sports injuries.
1 Materials and Methods
1.1 Subjects and groups
A total of 130 specific-pathogen-free/virus-antibodyfree healthy male Sprague-Dawley rats with a body weight of 180-200 g (6 weeks old) were provided by Shanghai Sipple-Bike Laboratory Animal Co., Ltd., with the license number SCXK (Shanghai) 2018-0006. All rats were housed in separate cages, with 10 rats per cage,with free access to food and natural light. The temperature was 17-23 ℃, and the relative humidity was 40%-60% in the animal room. The experiment was carried out after 7 d of acclimatization in the animal laboratory. The rats were divided into a blank group(group A), an exercise control group (group B),pre-exercise Tuina group (group C), and a post-exercise Tuina group (group D) by the random number table method. According to different sampling time points,group B was further divided into a 0 h exercise control group (group B1), a 24 h exercise control group (group B2), a 48 h exercise control group (group B3), and a 72 h exercise control group (group B4). Group C was further divided into a 0 h pre-exercise Tuina group (group C1),a 24 h pre-exercise Tuina group (group C2), a 48 h preexercise Tuina group (group C3), and a 72 h pre-exercise Tuina group (group C4). Group D was further divided into a 0 h post-exercise Tuina group (group D1), a 24 h post-exercise Tuina group (group D2), a 48 h postexercise Tuina group (group D3), and a 72 h postexercise Tuina group (group D4). There were 10 rats in group A, and 10 rats in each subgroup of groups B, C,and D. The experimental process strictly followed the relevant regulations of theAdministrative Regulations of the People’s Republic of China on Laboratory AnimalsandInstructive Notions with Respect to Caring for Laboratory Animals.
1.2 Modeling method[12]
Except for group A, the other rats were trained to exhaustion. All rats had free access to water and the same food. After randomization, experiments were conducted daily from 7:00 a.m. to 10:00 a.m. The model rats swam under weight-bearing in a glass tank(100 cm × 60 cm × 70 cm) with a still water depth of 55 cm. The water temperature was 31-35 ℃, and the weight-bearing was 3% of the rat’s body weight. The swim session began at 9:00 a.m. During the training,when significantly decreased rat motor coordination and repeatedly falling were found, or the rats floated on the water surface without exercise, the rats would be driven with wooden sticks to maintain their motion.Sinking for more than 10 s or 3 continuous times of sinking fulfilled the standard for taking the rat out of the swimming tank, and rats were dried with a hairdryer at room temperature and put back in the cage.
1.3 Tuina intervention
Rats in group A and group B did not receive Tuina intervention.
Rats in group C were placed in a fixed frame and received professional Nie-Pinching manipulation and finger Nian-Twisting manipulation on bilateral lower limbs. The manipulations were performed by the same operator. Before Tuina, the operator’s manipulation must be evaluated by the manipulation tester to evaluate the strength and frequency. Started the manipulation when the strength and frequency waveform were consistent with the manipulation model without causing contraction or resistance of the rat’s lower limbs. The manipulation strength was 0.4 kg. The operation frequency was 120 times/min, which has the most significant improvement in muscle damage[13].After a 2-minute rest following the Tuina manipulation on bilateral lower limbs for 5 min, the rat was put into a water tank to perform exhaustive exercises, and the status of the rat was observed. The time to finish the exhaustive exercise was recorded for each rat, which was used as the basis for the accurate sampling time point (the samples were collected immediately for the 0 h group, and at 24 h, 48 h, or 72 h for the other groups after the exhaustive exercise, respectively).
The rats in group D received exhaustive exercise first,and each rat was placed in a water tank at an interval of 10 min. The rats were taken out after the exhaustive exercise and rested for 2 min, and then Tuina manipulation was performed. The steps and precautions were the same as in group C. The time to finish the Tuina manipulation was recorded for each rat,which was used as the basis for the accurate sampling time point (the samples were collected immediately for the 0 h group, and at 24 h, 48 h, or 72 h for the other groups after the Tuina manipulation, respectively).
1.4 Sample collection
1.4.1 Serum preparation
Group A was sampled 48 h later after the experiment started. Each subgroup of group B and group C was sampled at 0 h, 24 h, 48 h, or 72 h after the exhaustive exercise. Each subgroup of group D was sampled at 0 h,24 h, 48 h, or 72 h after the Tuina manipulation,respectively. After being sacrificed by cervical dislocation, the rats were quickly decapitated and inverted, and about 5 mL of blood was collected. After standing for 30 min, the blood was centrifuged at 3 500 r/min for 5 min, and then serum was collected for detection.
1.4.2 Mitochondrial preparation
Immediately after blood collection, 1 g of the left gastrocnemius muscle was collected from each rat,washed with the homogenization medium to remove the floating blood, minced in an ice bath, homogenized with 5 mL of normal saline at 4 ℃ for 30 s, and centrifuged at 2 000 r/min for 10 min. The supernatant was collected and centrifuged at 10 000 r/min for 15 min. Removed the supernatant, the precipitate was mitochondria and stored at -20 ℃ for later use.
1.5 Indicator detection
1.5.1 Detection by enzyme-linked immunosorbent assay (ELISA)
The serum CK level was determined by doubleantibody sandwich method. Solid-phase antibodies were prepared by coating purified rat antibodies on microplates. Rat blood samples and each factor standard were sequentially added into the microwells coated with the monoclonal antibody, and combined with the CK antibody labeled with horseradish peroxidase to form an antibody-antigen-enzymeantibody complex. After thorough washing, the matrix tetramethylbenzidine was added to develop color. This kit was provided by Bio Swarm (Product No. RA20686).The absorbance (optical density, OD) value of the samples was measured at a wavelength of 450 nm using an elx800 microplate reader. The CK concentration in the samples was calculated using the standard curve method.
1.5.2 Mitochondrial Ca2+concentration and Ca2+-ATPase determination
Mitochondria were suspended in 1 mL of normal saline, ground in an ice bath for 3 min, and centrifuged at 2 500 r/min for 10 min. The supernatant was collected, and evenly mixed with methylthymol blue sodium salt reagent, alkaline solution, deionized water,2.5 mmol/L calcium standard solution, and protein clarifying agent. After standing for 5 min, the OD value was measured at a wavelength of 610 nm and an optical diameter of 1 cm.
The calcium level in the tissue was calculated according to the following formula. Calcium level in tissue (mmol/gprot) = (OD value of test tube - OD value of blank tube) ÷ (OD value of standard tube - OD value of blank tube) × Concentration of standard tube(2.5 mmol/L) ÷ Protein level of test sample (gprot/L).
Mitochondrial Ca2+-ATPase determination was processed as follows. The mitochondria were suspended in 1 mL of normal saline, ground in an ice bath for 3 min, and centrifuged at 2 500 r/min for 10 min. Collected the supernatant, mixed it with reagents, centrifuged it at 3 500 r/min for 10 min, and collected 100 μL of the supernatant for phosphorus determination. The solution was placed in a water bath at 45 ℃ for 20 min, and then cooled to room temperature. The OD value of each tube was measured by a spectrophotometer at a wavelength of 660 nm(1 cm in optical path, balanced with distilled water). The amount of 1 μmol of inorganic phosphorus generated by the decomposition of ATP per milligram of protein per hour was defined as an ATPase activity unit, namely μmolPi/(mgprot·h). The Ca2+-ATPase activity was calculated according to the following formula.Ca2+-ATPase activity = (Test tube OD - Control tube OD) ÷ Standard tube OD × Standard tube concentration(1 μmol/mL) × Sample dilution factor in the reaction system (2.8) × 6* ÷ Protein level (mgprot). Note: * Since the reaction time was 10 min, which became 1 h after being multiplied by 6 in the above formula.
1.5.3 Electron microscope observation
Two rats were randomly selected from each group,and the right gastrocnemius muscle tissue (without pulling the tissue) with a size of 1 mm × 1 mm × 1 mm was cut with a blade immediately within 1 min after sacrifice. The muscle tissues were fixed with 4%glutaraldehyde. The fixed muscle tissue was washed 3 times with phosphate buffer (10 min/time), fixed with 1% osmic acid for 2 h, and then washed with phosphate buffer solution 3 times (10 min/time). Subsequently,ethanol dehydration was carried out with a gradient of 50%, 70%, 90% (15 min each), and 100% (3 times,30 min/time). Replaced with acetone 3 times(3 min/time). After immersion for more than 10 h, the samples were embedded with epoxy resin, polymerized at 40-60 ℃, trimmed and ultrathin-sectioned (50-90 nm in thickness) after 48 h. Double staining with uranyl acetate and lead citrate was performed for 5-10 min,and muscle tissue structure was observed by the transmission electron microscope.
1.6 Statistical analysis
The SPSS version 22.0 statistical software was used for data analysis. The measurement data were in normal distribution and had homogeneity of variance,and expressed as mean ± standard deviation (±s).The one-way analysis of variance was used for the comparisons among multiple groups, andt-test was used for the comparisons between the two groups.P<0.05 indicated the statistical significance.
2 Results
2.1 Ultrastructural changes of skeletal muscle
The rat skeletal muscles in group A showed normal morphology, complete sarcomere structure, and tight arrangement of myofibrils. In group B, sarcomeres and myofibrils of skeletal muscle were contracted, squeezed,twisted, and disintegrated with myofilament edema between fibers. The sarcomeres were slightly contracted and squeezed, and some muscle fibers were twisted in groups C1 and C2. Among them, the damage of muscle fiber structure in group C1 was more significant. The skeletal muscle morphology of group D3 and group D4 was basically normal, and the myofibrils were closely arranged. The morphology of group D4 was closer to that of group A (Figure 1).
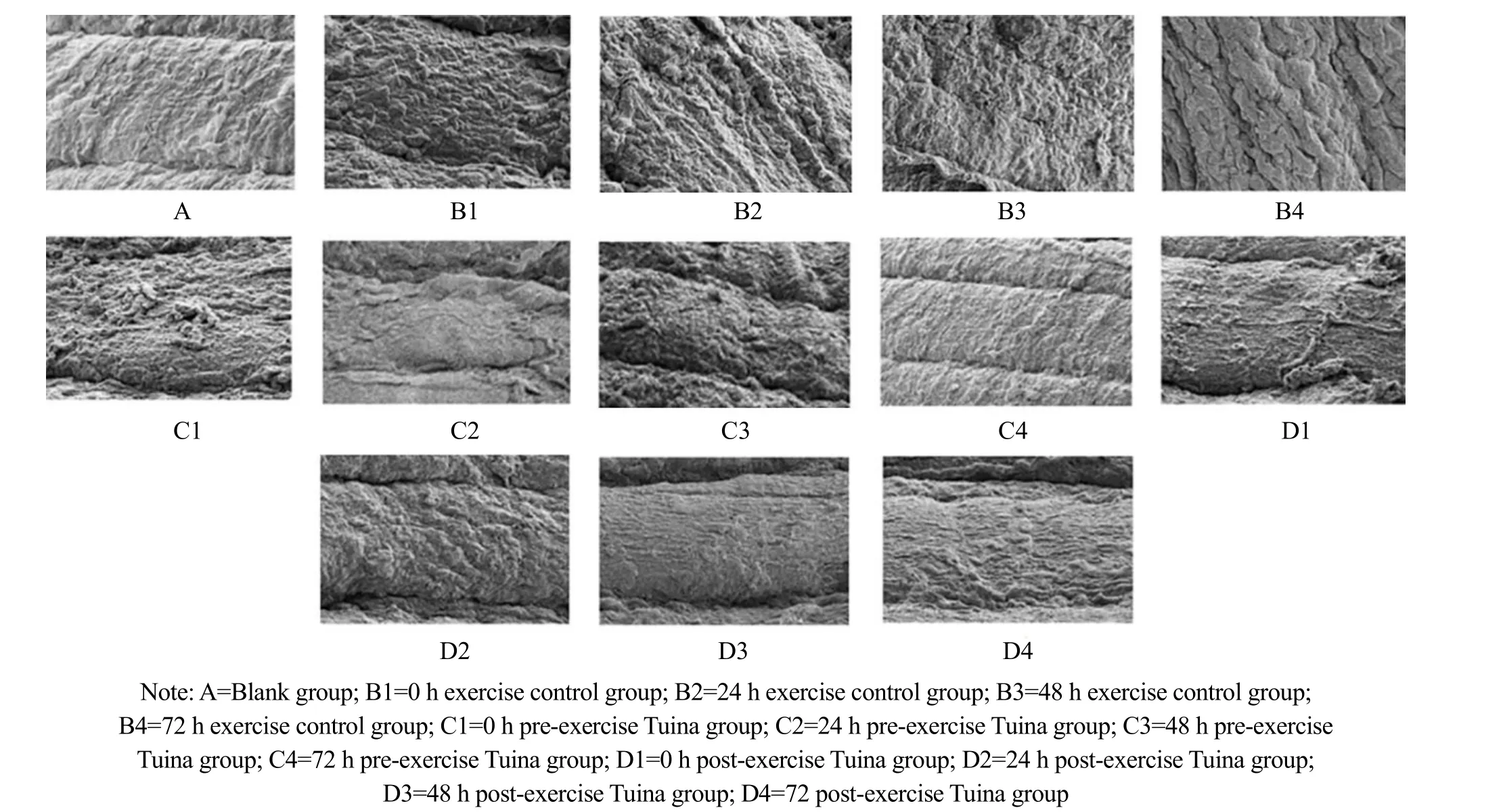
Figure 1 Ultrastructural changes of the gastrocnemius muscle (×1 500)
2.2 Change in mitochondrial Ca2+ concentration in the skeletal muscle
Compared with group A, the concentration of mitochondrial Ca2+in the skeletal muscle of groups B, C,and D was significantly increased (P<0.01). The differences among subgroups in group C were statistically significant (P<0.01), among which group C1 had the lowest value, group C2 had the highest value,and groups C2, C3, and C4 showed a downward trend.At the same time point, the concentration of mitochondrial Ca2+in skeletal muscle of group C1 was significantly lower than that of group B1 (P<0.01), and that of group C2 and group C3 was higher than that of group B (P<0.01); there was no statistical difference between group C4 and group B4 (P>0.05). At the same time point, the concentration of mitochondrial Ca2+in the skeletal muscle of group D1 was significantly lower than that of group B1 (P<0.01), and that of groups D2,D3, and D4 was significantly higher than that of group B(P<0.01). The difference between group D2 and group B2 was the most obvious. At the same time point, the concentration of mitochondrial Ca2+in the skeletal muscle of group C1 was higher than that of group D1(P<0.05), and the concentration of groups C2, C3, and C4 was significantly lower than that of group D, among which the difference between group C2 and group D2 was the most obvious (P<0.01). See Table 1.
2.3 Change in mitochondrial Ca2+-ATPase concentration in the skeletal muscle
Compared with group A, the concentration of Ca2+-ATPase in skeletal muscle mitochondria of groups B,C, and D was significantly increased (P<0.01), and the intra-group differences in group C and group D were statistically significant (P<0.01). Group C1 and group D1 had the lowest values, while group C2 and group D2 had the highest values, and the 24 h, 48 h, and 72 h subgroups of group C and group D all showed a downward trend. The comparison between group C and group B showed that the concentration of skeletal muscle mitochondrial Ca2+-ATPase in group C1 was lower than that in group B1 (P<0.01), and the concentrations in groups C2, C3, and C4 were significantly higher than those in group B at the same time point (P<0.01), and the difference between group C2 and group B2 was the most obvious. The concentration of mitochondrial Ca2+-ATPase in the skeletal muscle of group D1 was lower than that of group B1 (P<0.01), and the concentration of groups D2,D3, and D4 was significantly higher than that of group B at the same time point (P<0.01), and the 24 h group had the most significant difference. The concentration of mitochondrial Ca2+-ATPase in the skeletal muscle of group C2 and group C4 was significantly lower than that of group D at the same time point (P<0.01). See Table 1.

Table 1 Comparison of skeletal muscle mitochondrial Ca2+, Ca2+-ATPase, and serum CK concentrations among groups
2.4 Change in the serum CK concentration
ELISA detection showed that there were significant differences in the CK levels of subgroups in group C and group D at each time point, showing a downward curve(P<0.01); the serum CK levels in group C were statistically significantly lower than those in group B at the same time point (P<0.01). Among them, the decreasing trend of serum CK in group C1, group B1,group C2, and group B2 was more significant; the serum CK levels in group D were lower than those in group B at the same time point, and the difference between group D1 and group B1 was statistically significant (P<0.01),but the level in group D4 was higher than that in group B4. The levels of group D at each time point were significantly higher than those of group C at the same time point (P<0.01), and the difference between the 0 h and the 48 h subgroups was statistically significant(Table 1).
3 Discussion
Based on thein vivoexperiments, it has been found that the data from samples collected at 7 h, 24 h, and 48 h after muscle tissue injury were relatively in line with the release and clearance kinetics of CK[14]. Based on the results, this experiment designed the sampling time and further observed the influence of Tuina on serum CK by adding the 72 h group. Intercellular fluid CK enters the blood circulatory system through lymphoid tissues after muscle cell injury. Therefore,muscle tissue injury after exercise will lead to an increased CK activity in serum and muscles, which is proportional to the degree of muscle injury[15]. The mechanisms of serum CK activity increase by different exercise time and intensities are also different. The increased serum CK activity after short-term highintensity exercise is mainly caused by the mechanical damage of muscle fibers. Although long-term endurance exercise causes less mechanical damage,abnormal metabolism of cell membranes causes muscle cells to lose their normal functions, showing permeable cell membranes. Meanwhile, a large amount of CK leakage from muscle cells due to the increased permeability of the cell membrane results in increased CK activity[16]. Our results showed that compared with group A, the rat serum CK concentrations in groups C, D,and B were all increased. The serum CK level in group B1 was significantly higher than that in group A.Although the serum CK levels in 24 h, 48 h, and 72 h groups gradually decreased, they were still significantly higher than the level in group A. The serum CK value in group C decreased significantly at each time point.Although the lowest value did not return to the level of group A, the peak value was significantly reduced. This indicates that Tuina can prevent exercise-caused muscle damage, reduce CK leakage from muscle cells, increase CK clearance in lymph-blood circulation, and reduce the activity of serum CK[17]. This is basically consistent with our findings of the ultrastructure of gastrocnemius muscles under the electron microscope.
At the same time, this study found that although the serum CK value in group D was decreased, it was higher than that in group C. Therefore, we believe that Tuina therapy after high-intensity exercise cannot prevent damaged cells from leaking CK in time, and can even increase the level of serum CK activity by accelerating CK entering the blood circulation from lymph. Taken together, Tuina can reduce the CK activity level, the muscle injury marker, but further investigation is required to determine whether Tuina can restore muscle injury based on serum CK value[18].
Ca2+is a coupling factor of skeletal muscle excitation and contraction, with the main function of promoting ATP hydrolysis to provide energy for the sliding of muscle contractile fibrils. Relying on the energy generated by the Ca2+-ATPase hydrolysis to transport Ca2+in the cytoplasm into mitochondria, the mitochondria regulate Ca2+through inflow and outflow.The Ca2+uptake by skeletal muscle mitochondria is affected by the level of cytoplasmic Ca2+. The increase of cytoplasmic Ca2+level after exercise stimulates the hydrolysis of Ca2+-ATPase to release energy, which enables mitochondria to intake Ca2+actively, reducing the muscle damage caused by increased cytoplasmic Ca2+. When exercise fatigue occurs, the activity of skeletal muscle mitochondrial Ca2+-ATPase decreases,which reduces the ability of Ca2+transport into mitochondria, and a large number of Ca2+accumulate in the cytoplasm, resulting in decreased exercise capacity.Current studies suggest that plasma membrane permeability is increased and Ca2+release is decreased after DOMS injury[19]. Ca2+supports cellular uptake of Ca2+through Na+-Ca2+exchange and plasma membrane Ca2+-ATPase binding with cytoplasm[20].
We found that Ca2+and Ca2+-ATPase of rat skeletal muscle mitochondria in group B were increased,reached the peak at 24 h, and then were decreased,which was synchronized with exercise fatigue.Compared with group B, the activities of mitochondrial Ca2+and Ca2+-ATPase in skeletal muscles of group C and group D were enhanced, which was more significant in group D. We believe that Tuina can interfere with Ca2+and Ca2+-ATPase in the skeletal muscle mitochondria.Except for the 0 h time point, the level of skeletal muscle mitochondrial Ca2+-ATPase in group C and group D was higher than that in group B, which indicates that Tuina enhances skeletal muscle mitochondrial Ca2+-ATPase activity and the ability of mitochondria to transport Ca2+, thereby protecting skeletal muscle tissue.The 24 h post-exercise Tuina group showed the most significant effect. In addition, DOMS leads to the increase in Ca2+level in skeletal muscle mitochondria and the aggregation of Ca2+in vacuoles, which may be an effective mechanism for the body to reduce the content of Ca2+in fiber cytoplasm, so as to maintain the activity of muscle fibers[18]. Whether Tuina can promote the generation of this mechanism is worthy of further study.
The ultrastructure changes of gastrocnemius muscles observed by electron microscope in group B were basically consistent with the changes in serum CK and skeletal muscle mitochondrial Ca2+at different time points. Compared with group B, the changes of skeletal muscle structure in group C and group D were improved,and the overall result of group C was more similar to that of group A. It can be considered that the preventive effect of Tuina before exercise on the structural changes of skeletal muscles in DOMS model rats is better than the intervention effect of Tuina after exercise.
In conclusion, Tuina can reduce the serum CK activity in the DOMS rat model, decrease muscle damage, and protect skeletal muscles by enhancing the transport capacity of skeletal muscle mitochondrial Ca2+.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Science and Technology Project of Jiangsu Bureau of Traditional Chinese Medicine(江苏省中医药局科技项目, No. YB201849).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 8 June 2021/Accepted: 29 January 2022
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Grain-sized moxibustion inhibits the progression of Alzheimer disease in 5XFAD transgenic mice
- Effects of moxibustion on miRNA-133b, Pitx3/TH,and neurotransmitters in the midbrain of rats with diarrhea-predominant irritable bowel syndrome
- Effects of herbal cake-partitioned moxibustion on the expression of thyroid autophagy-related factors LC3B and Beclin-1 in rats with autoimmune thyroiditis
- Clinical study of acupuncture combined with medication for the elderly with Alzheimer disease
- Clinical observation of acupuncture and moxibustion for functional dyspepsia due to Yang deficiency of the spleen and stomach
- Clinical observation of acupuncture combined with sitting-position knee-adjustment manipulations for patellofemoral arthritis
