Effects of moxibustion on miRNA-133b, Pitx3/TH,and neurotransmitters in the midbrain of rats with diarrhea-predominant irritable bowel syndrome
2022-12-28CHENJinyu陈进雨WANGJiaojiao王娇娇ZOULing邹玲ZHUShanshan祝姗姗Kuiwu李奎武AOLumin廖路敏RUANJingru阮静茹CHUHaoran储浩然
CHEN Jinyu (陈进雨), WANG Jiaojiao (王娇娇), ZOU Ling (邹玲), ZHU Shanshan (祝姗姗), LⅠ Kuiwu (李奎武),LⅠAO Lumin (廖路敏), RUAN Jingru (阮静茹), CHU Haoran (储浩然)
1 Graduate School of Anhui University of Chinese Medicine, Hefei 230038, China
2 The Second Affiliated Hospital of Anhui University of Chinese Medicine, Hefei 230061, China
3 Clinical Ⅰnstitute of Acupuncture and Moxibustion, Anhui Academy of Chinese Medicine, Hefei 230038, China
Abstract
Keywords: Moxibustion Therapy; Mild Moxibustion; Irritable Bowel Syndrome; Diarrhea; MicroRNA-133b; Pituitary Homeobox Family Factor 3; Tyrosine Hydroxylase; Neurotransmitter
Irritable bowel syndrome (IBS) is a common functional bowel disease in clinical practice, with a prevalence of about 9%-23% worldwide and 6.5% in the Chinese population. The most common IBS subtype is diarrhea-predominant IBS (IBS-D)[1-2]. At present, the pathogenesis of IBS has not been conclusively established, and most relevant studies have suggested that it may be related to abnormal gastrointestinal dynamics, visceral hypersensitivity, intestinal inflammation and immune response, abnormal braingut axis interaction, and psychosomatic factors[3-4].Western medicine treatment mainly improves the symptoms of IBS, including the application of antispasmodics, antidiarrheal drugs, microbial agents,and antidepressants such as pinaverium bromide and rifaximin, which showed a high relapse rate, many side effects, and a poor overall efficacy[5-6]. Studies have reported that moxibustion has good clinical therapeutic efficacy in treating IBS-D with few adverse effects[7-10].
The mechanism of moxibustion for IBS-D has been recently widely reported, such as reducing the abnormally high pain threshold in IBS rats[11]and inhibiting the Toll-like receptors 4 (TLR4)/myeloid differentiation factor 88 (MyD88)/nuclear factor kappa B (NF-κB) pathway in rats[12]. Dopamine (DA),noradrenaline (NE), and 5-hydroxytryptamine (5-HT) act abnormally in the central nervous system and the gastrointestinal tract. They are neurotransmitters that play bridging and regulatory roles in the brain-gut interface and are thought to be closely associated with IBS gastrointestinal motility, visceral hypersensitivity,and psychosomatic manifestations[13]. There are many preliminary observations on neurotransmitters (DA, NE,and 5-HT)[14-15], but observations on the substances through which moxibustion affects neurotransmitters are rare and deserve to be conducted.
MicroRNA-133b (miRNA-133b) binds to the 3’non-coding region (3’UTR) of pituitary homeobox family factor 3 (Pitx3) to form a negative feedback regulatory pathway that affects the maturation and function of midbrain DAergic neurons[16]. Pitx3 is a key specific transcription factor that determines the phenotype,differentiation, and survival maintenance of midbrain DAergic neurons[17]. Also, Pitx3 is a transcription factor that activates tyrosine hydroxylase (TH) and dopamine transporter protein[18]. In contrast, TH is a DA, NE, and 5-HT rate-limiting enzyme that directly affects DA, NE,and 5-HT production[19-21].
So, is the mechanism of moxibustion for IBS-D related to modulating miRNA-133b’s regulation on Pitx3 and TH to affect DA, NE, and 5-HT levels, thus improving the diarrhea symptoms and visceral hypersensitivity of IBS-D? By establishing a rat model of IBS-D and observing the effects of moxibustion on miRNA-133b,Pitx3/TH, and neurotransmitters, this study aimed to provide a new scientific basis for the mechanism, thus promoting the clinical application of moxibustion in IBS-D treatment.
1 Materials and Methods
1.1 Laboratory animals
Six specific-pathogen-free grade Sprague-Dawley pregnant rats [body mass (360±20) g, gestational age(18±1) d] were provided by the Experimental Animal Center of Anhui Medical University [Animal Certificate No. SCXK (Wan) 2017-001]. The rats were housed in clean cabinets at the Experimental Animal Center of Anhui University of Chinese Medicine under Class Ⅱconditions, with (23±2) ℃, 50%±10% humidity, and a 12 h/12 h light/dark cycle. Fifty-four pups were born under normal standard feeding conditions.
The handling and disposal of all animals in this experimental protocol were in accordance with theGuiding Opinions on the Treatment of Experimental Animalsissued by the Ministry of Science and Technology of the People’s Republic of China and approved by the Laboratory Animal Ethics Committee of Anhui University of Chinese Medicine (Ethics No.AHUCM-rats-2020011).
1.2 Main reagents and instruments
Fine moxa strips (Enterprise Standard: Q/JXFF001-2015, Yueyang Aijintang Biotechnology Co., Ltd., China);sevoflurane [State Food and Drug Administration (SFDA)Approval No. H20080681, Lunanbetter Pharmaceutical Co., Ltd., China]; rifaximin (SFDA Approval No.H20040030, Chongqing Sano Bio-Pharmaceutical Co.,Ltd., China); glacial acetic acid (Lot No. 20190601JN,Shanghai R&J Chemical Reagent Co., Ltd., China); DA(Lot No. JYM0237Ra), 5-HT (Lot No. JYM0326Ra),and NE (Lot No. JYM0587Ra) enzyme-linked immunosorbent assay (ELISA) kits (Wuhan Genome Biotechnology Co., Ltd., China); trizol (Lot No. 251804,Life Technogies, USA); goat anti-rabbit immunoglobulin G (IgG, Lot No. 206820103, Beijing Zhongcein Bridge Biotech Co., Ltd., China); β-actin (Lot No. 19C10511,Beijing Zhongsun Jinqiao Biotechnology Co., Ltd., China);TH primary antibody (rabbit anti-rat, Lot No.AG08047220, Beijing Bioss Biotechnology Co., Ltd.,China); Pitx3 primary antibody (rabbit anti-rat, Lot No.AI06581265, Beijing Bioss Biotechnology Co., Ltd.,China); goat anti-mouse IgG (Lot No. 140193, Beijing Zhongsun Jinqiao Biotechnology Co., Ltd., China); RIPA cell lysate (Lot No. 09271919023, Shanghai Beyotime Biotechnology Co., Ltd., China); PAGE gel procoagulants(Lot No. 1017M021, Beijing Solebro Technology Co., Ltd.,China); DAPI staining solution (Lot No. 071618190213,Shanghai Beyotime Biotechnology Co., Ltd., China);reverse transcription kit (Lot No. AJ51485A, Takara,Japan); ECL ultrasensitive luminescence kit (Lot No.UE283595A, Thermo Scientific, USA).
RT-6000 multifunctional enzyme labeler (Jinan Redu,China); CX41 optical microscope (Olympus, Japan); K960 general polymerase chain reaction (PCR) instrument(Hangzhou Jingge Scientific Instruments Co., Ltd., China);PIKOREAL 96 fluorescent quantitative PCR instrument(Thermo Scientific, USA); BA410E fluorescence microscope (Motic, China); EPS300 type electrophoresis instrument and VE-186 type film transfer instrument(Shanghai Tennant Technology Co., Ltd., China);JW-3021HR type high-speed benchtop frozen centrifuge(Anhui Jiawen Instrument Equipment Co., Ltd., China).
1.3 Model preparation
Mother-offspring separation and acetic acid enema combined with chronic restraint stress stimulation were applied to produce the IBS-D rat model[22-24]. The modeled rats were separated from their mothers for 3 h per day at 2-14 d of age. Weaning was performed at 24 d of age, and acetic acid enema was performed at 35 d of age. The rats fasted for 24 h before the acetic acid enema. After anesthesia with sevoflurane inhalation, a 1 mm diameter single-lumen central venous catheter lubricated with paraffin oil was inserted into the intestine for about 4 cm through the anus, then a syringe was connected to slowly inject 1 mL of 4% acetic acid solution. The catheter was slowly pulled out, the anus was pressed, and then the rat’s tail was raised for 60 s to prevent the outflow of acetic acid.Finally, the colon was flushed with 1 mL of 0.01 mol/L PBS dilution. After 3 d of rest, restraint stress was performed at 38 d of age. The upper body of the rat was restrained with tape to restrict the front forelimbs from scratching the head and face. The rats were returned io cages. The binding duration was 2 h/d for 7 d. The detailed schedule is shown in Figure 1. Criteria for successful modeling: 60% or higher loose stool rate and 0.2 mL or more reduced minimum volume threshold for the abdominal withdrawal reflex (AWR)with 3 points in the modeled rats.
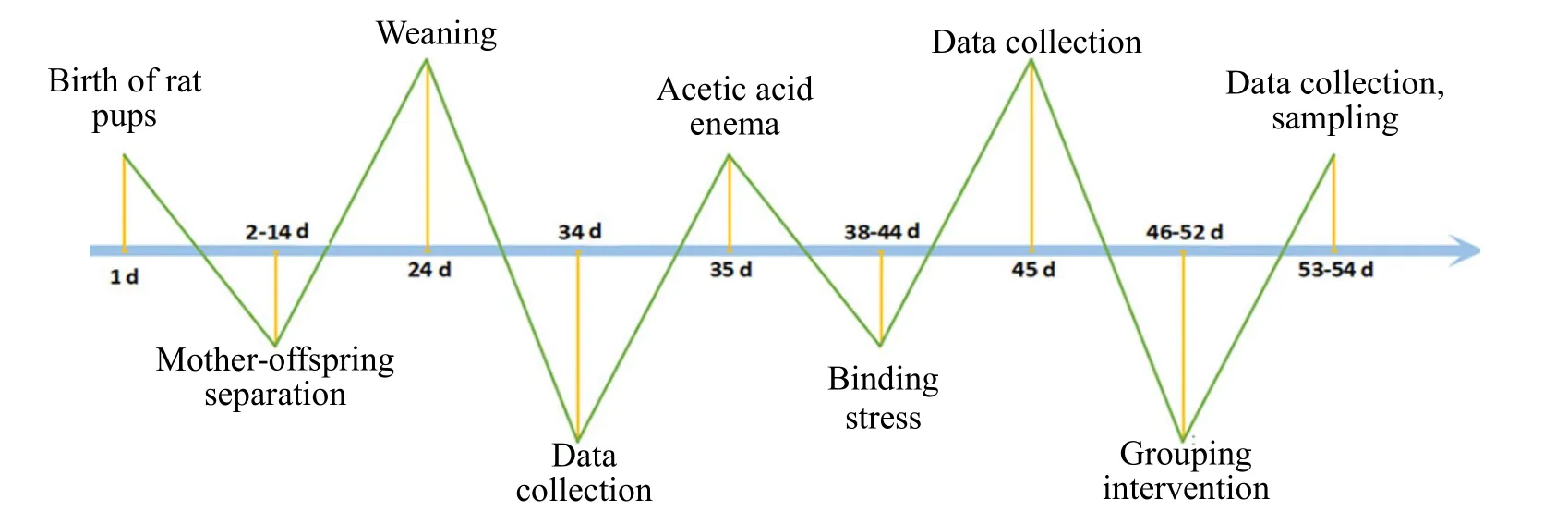
Figure 1 Schematic diagram of the experimental timeline
1.4 Grouping and intervention
Fifty-four newborn rat pups were numbered and labeled. Twelve rat pups were selected as the normal group according to the random number table method,and the remaining 42 pups were subjected to establishing the IBS-D rat model. Four rats died and 2 rats failed to be modeled during the modeling process,with a success rate of approximately 86%. The 36 successfully modeled rats were divided into a model group, a moxibustion group, and a Western medicine group by the random number table method, with 12 rats in each group. All rats were routinely housed with no intervention in the normal group and the model group. Moxibustion intervention was performed in the moxibustion group, and rifaximin gavage was performed in the Western medicine group.
1.4.1 Moxibustion group intervention method
The points were positioned according to the commonly used points for rats combined with the anthropomorphic comparison method in theExperimental Acupuncture Science[25].
Tianshu (ST25): At the umbilicus horizon and 5 mm away from the anterior median line. The umbilicus is the intersection of the lower 1/4 and upper 3/4 of the line connecting the sternoclavicular and pubic symphysis.
Shangjuxu (ST37): On the lateral side of the lower extremity and 10 mm below the capitulum fibulae.
The points were positioned, shaved, and marked after the rats were fixed, and then the rats were placed on a special rat moxibustion stand in a prone position and relatively static state for the moxibustion treatment.Thin moxa strips of 0.7 cm in diameter were placed under the moxibustion frame 2 cm from the points for 15 min once daily for 7 d.
1.4.2 Intervention methods in the Western medicine group
Rats were treated with rifaximin by gavage at a dose of 150 mg/(kg·bw) once a day, which was calculated from the equivalent dose conversion ratio between experimental animals and humans based on the body surface area ratio. The drug was ground into powder and weighed by electronic scale according to the body weight of each rat, and diluted with purified water to make 2 mL of suspension. The rats were then treated by gavage using a gavage needle once daily for 7 d[26].
1.5 Testing indicators and methods
Body weight, loose stool rate, and minimum volume threshold of AWR in rats of each group were observed and examined at pre-modeling (day 34), post-modeling(day 45), and post-intervention (day 53).
1.5.1 Loose stool rate
Rats in each group were placed in a steel cage with a stainless-steel grid at the bottom, and a tray with filter paper was placed at the bottom of the cage. The total defecated pellet number of rats and the loose stool number were counted 6 h later. The loose stool rate was calculated by the equation below.
Loose stool rate (%) = Loose stool number ÷ Total defecated pellet number × 100%.
The distinction between the dry stools and the loose stools was based on the presence or absence of stains on the filter paper.
1.5.2 Minimum volume threshold of AWR
The colorectal visceral sensitivity was determined using the non-injurious colorectal balloon distention reflex (CRD) volume threshold method after the loose stool rate test. All rats in each group were fixed after being fasted for 12 h with water intake. A paraffin oil-lubricated F6 catheter was slowly inserted into the intestine through the anus and secured to the tail with medical tape, and then the rats were placed in a clear glass box. After adaptation, the rats were injected with 26-28 ℃ saline via balloon, and the minimum volume threshold (3 min and above of water injection) that caused AWR was observed. The mean value of triplication was used as the minimum volume threshold of AWR caused by rectal dilatation. The AWR scoring criteria are shown in Table 1[27].

Table 1 Reflex scoring criteria of abdominal retraction
After the above items were collected, 6 rats were randomly selected from each group and subjected to intraperitoneal anesthesia. The abdomen was dissected,and 4 mL of blood was collected from the abdominal aorta for measurement. The rat colon (7 cm from the anus) was collected after blood collection. Two segments of 2 cm rat colon were separated, rinsed with ice-cold saline. One segment was fixed in 10%formalin, and the other segment was placed in a cryopreservation tube and stored in liquid nitrogen at-80 ℃ for later measurement. The skin of the severed head was removed on an ice bath to fully expose the rat skull. The tissue scissors reached the large foramen of the occipital bone toward the ipsilateral orbit, cut till the orbit against the bone, and then split the bone by the middle of the orbits. The skull was fixed with hooked forceps by inserting into the rat orbit and then raised by clamping the bent forceps at the occipital tuberosity. The brain was separated after the cerebral nerve was cut. The left and right midbrains were detached with a razor blade, put in a cryopreservation tube and stored in liquid nitrogen at -80 ℃ for future testing.
1.5.3 Histopathological examination of the colon tissue
Colon tissue was dehydrated after 24 h of fixation,paraffin-embedded, and uniformly serially sectioned at a thickness of approximately 3 μm. The colon histomorphology was observed under 200 × microscope and photographed after being dewaxed to water,hematoxylin-eosin staining, dehydration transparency,and neutral gum sealing.
1.5.4 ELISA for the determination of DA, 5-HT, and NE in plasma, colon, and midbrain tissue
The blood was naturally coagulated at room temperature for 10-20 min and then centrifuged at 4 ℃and 2 000 r/min for 20 min. The supernatant was transferred to a 1.5 mL EP tube and stored at -20 ℃.The operation was carried out strictly following the ELISA reagent instructions. The colonic and left midbrain tissues in the cryopreservation tubes were thawed and suspended with PBS (pH 7.4). The specimens were homogenized with a homogenizer,centrifuged at 2 000 r/min for 20 min, and the supernatant was used for DA, 5-HT, and NE detection according to the kit instructions.
1.5.5 Real-time fluorescence quantitative PCR(RT-qPCR) for miRNA-133b, Pitx3 mRNA, and TH mRNA expression in the midbrain tissue
Cut and ground 50-100 mg of the right midbrain tissue sample in the cryopreservation tube stored in liquid nitrogen. Total RNA was then extracted using the Trizol reagent. The cDNA produced by reverse transcript reaction in a 0.2 mL EP tube was used as the template for fluorescence quantification. The PCR reaction system: 5 μL of 2×SYBR green mixture, 1 μL of upstream and downstream primers for each gene, 1 μL of cDNA template, and 2 μL of RNase-free water. PCR reaction conditions: 95 ℃ for 1 min, 95 ℃ for 20 s, 60 ℃ for 1 min, 40 cycles. Finally, the relative expression levels were calculated by the Relative Quantification Study analysis method and the 2-ΔΔCtmethod. The primer sequences are shown in Table 2.
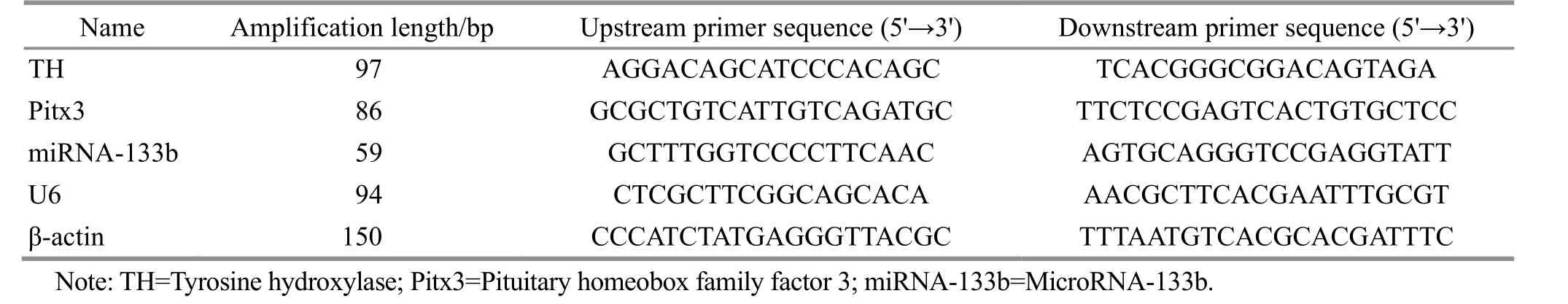
Table 2 Primers for mRNA detection
1.5.6 Detection of Pitx3 and TH protein expression in the midbrain tissue by Western blotting
The remaining right midbrain tissue stored in the cryopreservation tube was lysed with 1 mL of RIPA lysate to homogenize and extract proteins. Protein samples were processed and loaded into SDS-PAGE gel wells for electrophoretic separation, followed by constant-flow membrane transfer in a membrane transfer machine. The 32 kDa Pitx3 was transferred for 30 min. The 60 kDa TH was transferred for 55 min. The membrane was blocked with block buffer (5% skim milk powder) with a slow shake on a shaker at room temperature for 2 h. Primary antibody was diluted with the primary antibody dilution buffer in proportion(rabbit anti-Pitx3 antibody was 1:1 000 dilution; rabbit anti-TH antibody was 1:300 dilution) and incubated overnight at 4 ℃ with a slow shake. The horseradish peroxidase-labeled secondary antibody was then diluted with the secondary antibody dilution for Pitx3(1:20 000) and TH (1:5 000) and incubated for 1.5 h at room temperature. The proteins were detected using the ECL luminescence kit, and the protein bands were analyzed by Image J software. The β-actin was used as the internal reference, and the target protein to β-actin ratio was used as its relative expression level.
1.5.7 Positive expression of Pitx3 and TH in the midbrain tissue detected by immunofluorescence
The thorax of the remaining 6 rats in each group was opened after anesthesia. First, 250 mL of 0.9% normal saline was used to perfuse through the left ventricle to the organs until the paw nail turned white, then 250 mL of 5% paraformaldehyde was used to perfuse until the tail was hard and the body was as hard as cardboard.The right midbrain tissue was dissected and fixed in paraformaldehyde to be measured.
The paraformaldehyde-fixed midbrain tissue was dehydrated, paraffin-embedded, and sectioned. Paraffin sections of 5 μm thickness were blocked with goat serum for 30 min after sequential xylene (15 min for each time) and sequential ethanol (100%-95%-80%,5 min for each time) exposures, 3 times each. Shaked off, added primary antibody (1:200 dilution of rabbit anti-rat Pitx3 and TH) dropwise, and incubated for 1 h at 37 ℃. Added fluorescent secondary antibody and incubated for 30 min at 37 ℃ avoiding light, followed by DAPI re-staining for 5 min. Anti-fluorescence quenching sealer was used to seal the sections. The sections were rinsed with PBS 3 times before each of the three steps mentioned above. Photographs were taken under a fluorescent microscope, a digital scanner was used to scan the entire section, and three fields of view screenshots were randomly selected for each section using the CaseViewer reading software. The average absorbance values of positive cell expression were calculated using the Jetta JD801 series medical image analysis system.
1.6 Statistical analysis
The data were analyzed using the SPSS version 23.0 statistical software. Measurement data conforming to normal distribution were expressed as mean ± standard deviation (±s), and those not conforming were expressed as median (lower quartile, upper quartile)[M (QL, QU)]. One-way analysis of variance (ANOVA) was used if the data of each group met the normal distribution and the homogeneity of variance. The Kruskal-WallisH-test was used if the normal distribution or the homogeneity of variance was not satisfied.Repeated-measure data were compared using repeated-measure ANOVA. Greenhouse-Geisser method was used for correction if the sphericity test was not satisfied. A further simple effect analysis was performed if there was an interaction. Pearson bivariate correlation analysis was used if both groups met normal distribution. Spearman correlation analysis was used if the two groups did not meet normal distribution.P<0.05 indicated a statistical difference.
2 Results
2.1 Comparison of rats’ general conditions among groups
Before modeling, rats in each group showed normal stools, smooth hair, sensitive activity, and normal feeding and water intake. After modeling, the model rats had soft or unformed stools, increased defecation frequency, more feces stain around the anus, less lustrous fur, sluggish activity, depression, and reduced food and water intake. After intervention, rats in the moxibustion and Western medicine groups showed decreased loose stools, increased coat glossiness and activity, and improved diet and mental conditions compared with the model group.
2.2 Comparison of rats’ body weight among groups
Repeated-measure ANOVA showed statistically significant within-group differences in rat body weight at 3 time points in all groups (F=1 094.687,P<0.001),and the body weight was significantly correlated with the time factor (F=13.660,P<0.001). The comparison among groups was also statistically significant (F=33.008,P<0.001). Before modeling, there was no significant difference in rat body weight among groups (P>0.05).After modeling, the weight of the normal group was significantly higher than that of the model, moxibustion,and Western medicine groups (P<0.01). There was no statistical difference among the model, moxibustion,and Western medicine groups (P>0.05). After intervention, the body weight of the normal group was significantly higher than that of the model group(P<0.01), and the body weight of the model group was significantly lower than that of the moxibustion and Western medicine groups (P<0.01). There was no statistical difference in body weight between the moxibustion and Western medicine groups (P>0.05).Rats in the normal group gained more weight over time(Table 3).
2.3 Comparison of the minimum volume threshold of AWR and loose stool rate in rats among groups
Repeated-measure ANOVA showed that the minimum volume thresholds of AWR at the 3 time points were statistically different in each group(F=862.380,P<0.001), and the minimum volume threshold was significantly correlated with the time factor (F=187.880,P<0.001), which was statistically significant among groups (F=129.562,P<0.001). There was a significant difference in the rat loose stool rate at 3 time points among groups (F=2 451.926,P<0.001),and the rat loose stool rate was significantly correlated with the time factor (F=353.446,P<0.001) with statistically significant differences among groups(F=261.041,P<0.001). Simple effect analysis showed that the minimum volume threshold of AWR and the loose stool rate in rats were not statistically different among groups before modeling (P>0.05). After modeling, the minimum volume threshold of AWR was significantly lower (P<0.01), and the loose stool rate was significantly higher (P<0.01) in the model, moxibustion,and Western medicine groups compared with the normal group. There was no statistical difference among the model, moxibustion, and Western medicine groups (P>0.05). After the intervention, the minimum volume threshold of AWR was significantly lower(P<0.01), and the loose stool rate was significantly higher (P<0.01) in the model group compared with the normal group. Compared with the model group, the minimum volume threshold of AWR was significantly higher (P<0.01) and the loose stool rate was significantly lower (P<0.01) in the moxibustion and Western medicine groups. There was no statistically significant difference between the moxibustion group and the Western medicine group (P>0.05). See Figure 2.
2.4 Histopathological changes in rats’ colons
The colonic and mucosal tissue of rats were structurally intact, and the glands in the mucosal layer were well arranged in the normal group. Compared with the normal group, mild inflammatory cell infiltration was seen locally in the rat colonic mucosa with slight mucosal destruction in the model group.Compared with the model group, the moxibustion and Western medicine groups showed significantly fewer inflammatory responses, with more intact colonic and mucosal tissue structures. See Figure 3.
Table 3 Comparison of rat body weight among groups ( ±s) Unit: g

Table 3 Comparison of rat body weight among groups ( ±s) Unit: g
Note: Compared with the same group before modeling, 1) P<0.01; compared with the same group after modeling, 2) P<0.01; compared with the normal group at the same time point, 3) P<0.01; compared with the model group at the same time point, 4) P<0.01.
Group n Before modeling After modeling After intervention Normal 12 78.67±4.91 116.93±9.501) 181.46±7.832)Model 12 77.67±4.52 98.80±7.241)3) 110.46±11.312)3)Moxibustion 12 77.71±3.91 101.95±6.871)3) 131.00±13.852)4)Western medicine 12 78.08±4.24 102.46±8.881)3) 130.38±13.872)4)
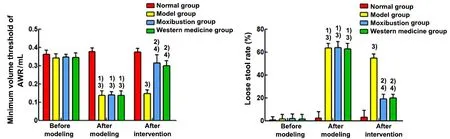
Figure 2 Comparison of the minimum volume threshold of AWR and the loose stool rate in rats among groups (n=12)
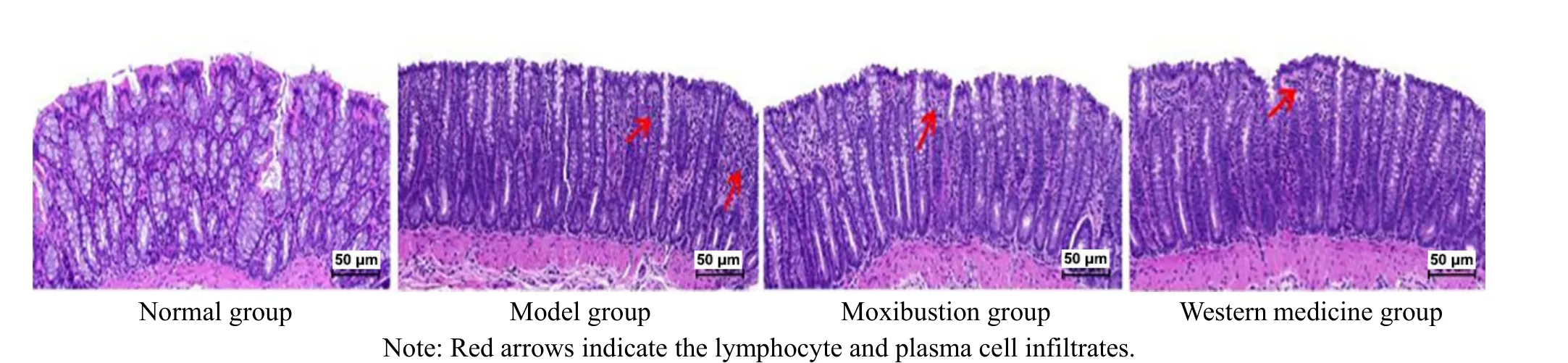
Figure 3 Microscopic observation of the colon tissue (hematoxylin-eosin staining, ×200)
2.5 Comparison of the DA, NE, and 5-HT levels in the serum, colon, and midbrain tissue of rats among groups
Compared with the normal group, the levels of DA,NE, and 5-HT in rat serum, colon, and midbrain tissue were significantly increased in the model group (P<0.01).Compared with the model group, the levels of DA, NE,and 5-HT in the serum, colon, and midbrain tissue of the moxibustion and Western medicine groups were significantly reduced (P<0.01). Compared with the Western medicine group, the NE level in the colon tissue and the 5-HT level in the midbrain tissue of the moxibustion group were significantly reduced (P<0.05),and the inter-group differences of the remaining items were not statistically significant (P>0.05). See Figure 4.
2.6 Comparison of the relative expression of miRNA-133b, Pitx3, and TH mRNAs in the midbrain tissue of rats among groups
Compared with the normal group, the relative miRNA-133b expression was significantly lower (P<0.01),and the relative expression levels of Pitx3 and TH mRNAs were significantly higher in the rat midbrain tissue of the model group (P<0.01). Compared with the model group, the relative miRNA-133b expression was significantly higher (P<0.01,P<0.05), and the relative expression levels of Pitx3 and TH mRNAs were significantly lower (P<0.01) in the midbrain tissue of the moxibustion group and the Western medicine group.See Figure 5.
2.7 Comparison of the relative protein expression of Pitx3 and TH in the midbrain tissue among groups
The relative protein expression levels of Pitx3 and TH were significantly higher in the rat midbrain tissue of the model group compared with the normal group(P<0.01). Compared with the model group, the relative protein expression levels of Pitx3 and TH in the rat midbrain tissue of the moxibustion and Western medicine groups were significantly lower (P<0.01). See Figure 6.

Figure 4 Comparison of the DA, NE, and 5-HT levels in the serum, colon, and midbrain tissue (ELISA, n=6)
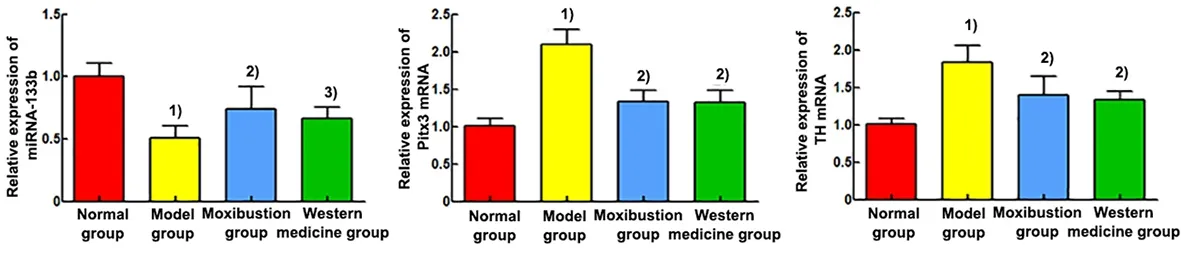
Figure 5 Comparison of the relative expression of miRNA-133b, Pitx3 mRNA, and TH mRNA in the brain tissue (n=6)
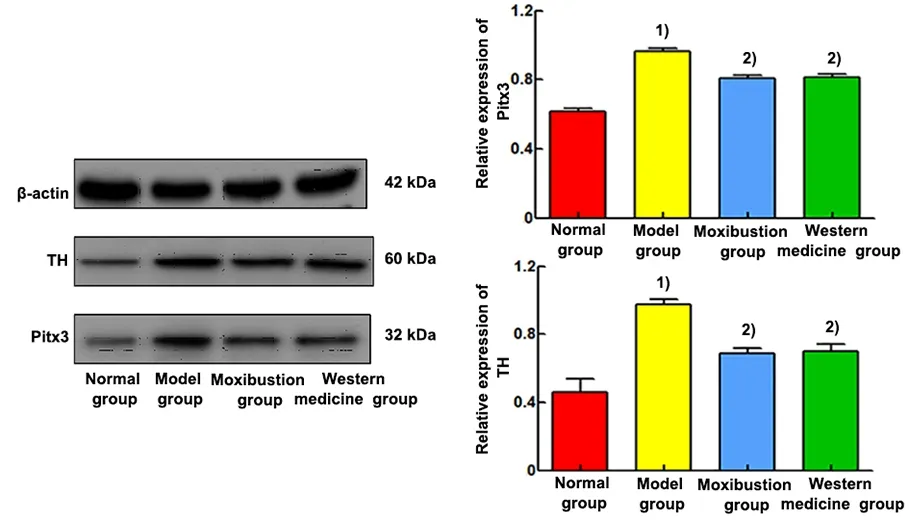
Figure 6 Comparison of the relative mRNA expression and protein electrophoresis bands of Pitx3 and TH in the midbrain tissue (Western blotting, n=6)
2.8 Comparison of the positive expression of Pitx3 and TH in the midbrain tissue among groups
Nucleus was colored blue by DAPI. Pitx3 and TH were target proteins and colored fluorescent green. Merge was the overlap of DAPI to the color of the target proteins of Pitx3 and TH, which was the overlap of fluorescent green with blue. See Figure 7 and Figure 8.
Positive expression levels of Pitx3 and TH proteins were seen in the ventral tegmental area of the midbrain.The positive expression levels of Pitx3 and TH were significantly increased in the midbrain tissue of the model group compared with the normal group (P<0.01).Compared with the model group, the positive expression levels of Pitx3 and TH were significantly reduced in the midbrain tissue of the moxibustion group and the Western medicine group (P<0.01). See Figure 9.
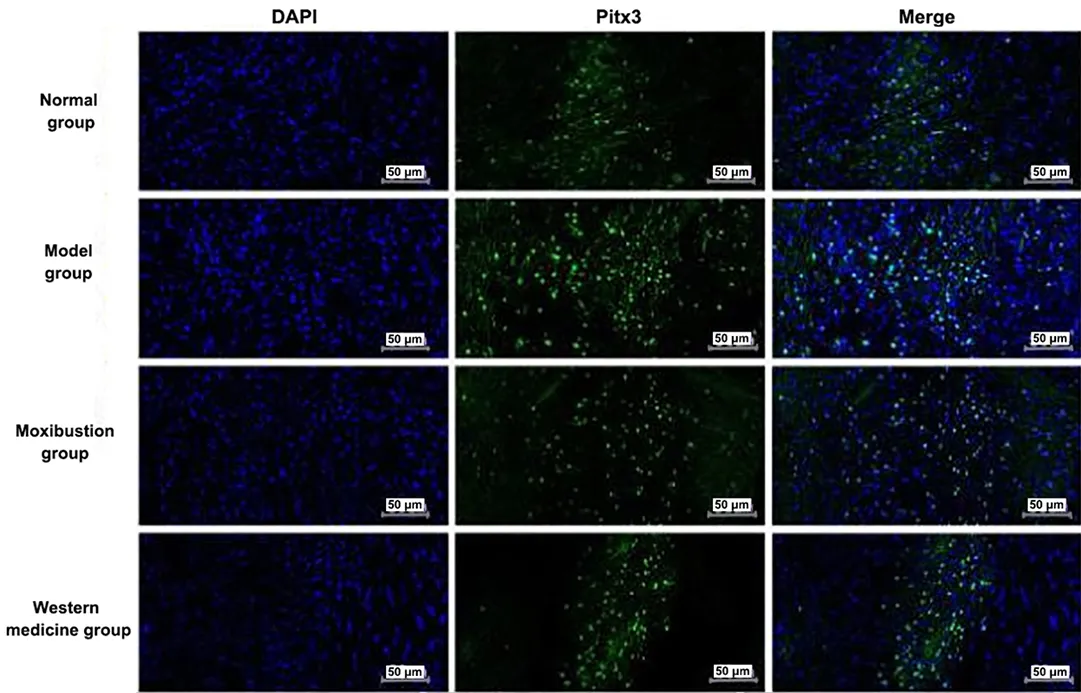
Figure 7 Immunofluorescence results of Pitx3 expression in the brain tissue of each group (×200)
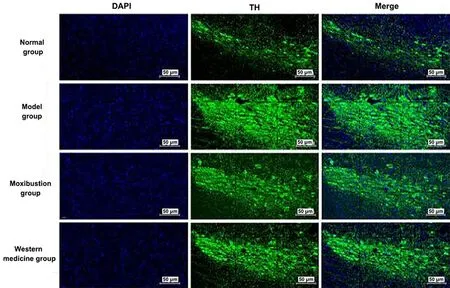
Figure 8 Immunofluorescence results of TH expression in the brain tissue of each group (×200)
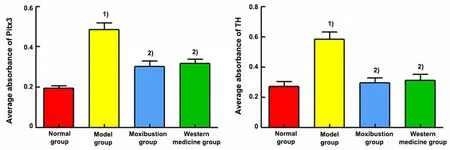
Figure 9 Comparison of the mean absorbance of Pitx3 and TH immunofluorescence results
2.9 Correlation of miRNA-133b with Pitx3, Pitx3 with TH, and TH with neurotransmitters of DA, NE, and 5-HT in the brain tissue of each group
The correlations of miRNA-133b, Pitx3, TH, DA, NE,and 5-HT in the midbrain tissue of the normal, model,moxibustion, and Western medicine groups were analyzed using Spearman’s combined analysis. Based on the scatter plot, we tentatively determined that there were linear relationships between miRNA-133b and Pitx3, Pitx3 and TH, TH and DA, as well as NE and 5-HT in the midbrain tissue. Linear correlation analysis showed that miRNA-133b was negatively correlated with the Pitx3 expression (r<0,P<0.01). Pitx3 with TH,TH with DA, and NE with 5-HT were positively correlated (r>0,P<0.01). See Figure 10.
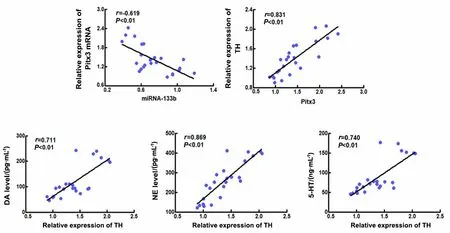
Figure 10 Correlation scatter plots between miRNA-133b and Pitx3,Pitx3 and TH, TH and DA, as well as NE and 5-HT (n=24)
3 Discussion
In recent years, with the continuous development of neurogastroenterology, more and more studies have suggested that the pathogenesis of IBS is closely related to the neuroendocrine bidirectional loop between the central nervous system (CNS) and the enteric nervous system (ENS), which leads to visceral sensory hypersensitivity and gastrointestinal motor dysfunction[28]. Gastrointestinal symptoms, on the other hand, are counteracted in the CNS through the ENS,affecting mood and psychological behavior, which is referred to as “abnormal brain-gut axis interaction”.
The neurotransmitters of DA, NE, and 5-HT play roles in building bridges and regulating the brain-gut interface, which is important in the pathogenesis of IBS.DA, as an inhibitory neurotransmitter, enhances colonic motility and sensitivity by binding to its receptors that block the coordinated movement of the gastric sinus and duodenum[29]. NE is a classical neurotransmitter that regulates intestinal function and plays a major inhibitory role in gastrointestinal motility[19]. 5-HT is also an important neurotransmitter that regulates gastrointestinal motility and visceral sensitivity. The release of large amounts of 5-HT in the intestine of patients with IBS cause abnormal enhancement of gastrointestinal dynamics and diarrhea[30-31].
In this study, we found that DA, NE, and 5-HT levels in plasma, colon, and midbrain tissue of IBS-D rats were significantly increased, AWR was decreased and the loose stool rate was increased. The levels of DA, NE, and 5-HT were significantly reduced, the AWR was increased,and the loose stool rate was reduced after the moxibustion intervention, suggesting that moxibustion may improve gastrointestinal dynamics and visceral hypersensitivity to treat IBS-D by modulating neurotransmitters. The results of the present study are consistent with related reports[32-34].
MiRNA-133b is a microRNA that has received much attention in recent years and is widely involved in digestive diseases and neurological disorders[35-37]. It is abundantly expressed in the midbrain dopamine neurons and downregulates the Pitx3 transcription to regulate the differentiation, maturation and function of dopaminergic neurons; on the other hand, Pitx3 activates the gene transcription required for the differentiation of dopaminergic neurons, such as the synthesis of the rate-limiting enzyme TH[38]. In this study,the miRNA-133b expression level was significantly decreased in IBS-D rats; the Pitx3 and TH expression levels were significantly increased in the midbrain tissue;the DA, NE, and 5-HT levels were significantly increased in plasma, colon, and midbrain tissue. After moxibustion intervention, miRNA-133b expression level was significantly increased; Pitx3 and TH expression levels in the midbrain tissue were significantly decreased; the DA, NE, and 5-HT levels in plasma, colon,and midbrain tissue were significantly decreased. This suggests that moxibustion intervention down-regulates the neurotransmitter levels in plasma, colon, and midbrain tissue, up-regulates miRNA-133b expression,and inhibits midbrain Pitx3/TH expression in IBS-D rats.
Combined with the results of correlation analysis, this study also demonstrated that after moxibustion intervention, the miRNA-133b expression was increased,and the Pitx3 expression was decreased, with a negative correlation between them. The Pitx3 and TH expression levels were reduced, which were positively correlated.The TH expression was increased. The DA, NE, and 5-HT levels were increased. TH was positively correlated with DA, NE, and 5-HT. It has been reported that miRNA-133b affects the neurotransmitters, DA, NE, and 5-HT, by regulating the transcription factor Pitx3 and the rate-limiting enzyme TH[39-41]. Therefore, we speculate that moxibustion regulates neurotransmitters in IBS-D rats and improves diarrhea symptoms and visceral hypersensitivity in IBS-D rats, which may be related to up-regulating miRNA-133b and suppressing midbrain Pitx3/TH expression.
Moxibustion has the effect of warming Yang and dissipating cold, circulating blood and transforming stasis, unblocking and harmonizing meridians[42].Ling Shu(Spiritual Pivot) records, “The large intestine and small intestine both belong to the stomach.” So the main points for the treatment of large intestine pathologies should be selected from the Stomach Meridian of Foot Yangming. Tianshu (ST25) is from the Stomach Meridian and also is the Front-Mu Point of the large intestine, serving as the hub of ascending the nutrients and descending the turbid. Tianshu (ST25) has the effect of regulating the intestinal organs,transforming the dampness, harmonizing the middle Jiao (energizer), and stopping diarrhea and pain.
According toZhen Jiu Jia Yi Jing(A-B Classic of Acupuncture and Moxibustion), Shangjuxu (ST37), the Lower He-Sea Point of the large intestine, has a special therapeutic effect on diseases of Fu organs.Moxibustion at Tianshu (ST25) and Shangjuxu (ST37),the compatibility of the He-Sea Point and the Front-Mu Point, can smooth and downbear Qi movement of the intestine to stop diarrhea and relieve pain. The effect of moxibustion’s warmth then helps to enter the brain through the meridians and regulates the levels of central neurotransmitters. This improves gastrointestinal motility and visceral hypersensitivity, thus achieving the effect of treating IBS-D.
Rifaximin, a broad-spectrum antibiotic that acts locally in the intestine, inhibits bacterial protein synthesis by blocking bacterial RNA synthesis and RNA transcription process, thereby correcting intestinal flora imbalance[43-45]. A rifaximin control was set up in this study to provide a reference for the effect of the moxibustion intervention. The results of the present study showed that rifaximin improved visceral hypersensitivity and diarrhea symptoms in IBS-D rats,which is consistent with related reports[46-47]. The interventional effects of moxibustion in IBS-D rats are similar to those of rifaximin, but treatment of IBS-D with moxibustion is superior to that with the Western medicine rifaximin in terms of non-dependence and adverse effects[42,48].
In conclusion, the results of this study indicate that moxibustion intervention improves diarrhea symptoms and visceral hypersensitivity in IBS-D rats. The mechanism may be associated with the up-regulated miRNA-133b suppressing the Pitx3/TH expression in the midbrain and thus reducing the neurotransmitter expression. However, this study is only a preliminary observation and further in-depth studies will be considered in the future by setting a control group or using miRNA-133b inhibitors.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Project of National Natural Science Foundation of China (国家自然科学基金项目, No. 81774399); Construction Project of Famous Senior Doctor of Traditional Chinese Medicine in Anhui Province: CHU Haoran Studio (安徽省名老中医储浩然工作室建设项目).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 8 June 2021/Accepted: 29 January 2022
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Grain-sized moxibustion inhibits the progression of Alzheimer disease in 5XFAD transgenic mice
- Effects of Tuina on serum creatine kinase and skeletal muscle mitochondria in delayed onset muscle soreness model rats
- Effects of herbal cake-partitioned moxibustion on the expression of thyroid autophagy-related factors LC3B and Beclin-1 in rats with autoimmune thyroiditis
- Clinical study of acupuncture combined with medication for the elderly with Alzheimer disease
- Clinical observation of acupuncture and moxibustion for functional dyspepsia due to Yang deficiency of the spleen and stomach
- Clinical observation of acupuncture combined with sitting-position knee-adjustment manipulations for patellofemoral arthritis
