Safety assessment of monosodium glutamate based on intestinal function and flora in mice
2022-11-26JinzhaoXuMengqiTangYiniLiuJinghanXuXiaoxiXu
Jinzhao Xu, Mengqi Tang, Yini Liu, Jinghan Xu, Xiaoxi Xu*
Key Laboratory of Dairy Science, Ministry of Education/College of Food Science, Northeast Agricultural University, Harbin 150030, China
Keywords:
Monosodium glutamate
Safety assessment
Intestinal flora
Intestinal inflammation
A B S T R A C T
Although monosodium glutamate (MSG) is a widely used food additive, its safety and systemic side effects have not been fully clarified.The intestinal flora is closely associated with human health; however, it remains unclear whether MSG consumption can affect health by acting on the intestinal flora.In this study, serum biomarkers, intestinal structure, intestinal immunity, and intestinal flora were examined to investigate the effects of different doses of sodium glutamate on the body, intestinal function, and intestinal flora of mice.The results showed that 30 mg/kg MSG had no significant effect on serum C-reactive protein, trimethylamine N-oxide, angiotensin II, intestinal interleukin (IL)-1β, IL-6, tumor necrosis factor-α, secretory IgA and fecal albumin in mice, but also promoted intestinal development and regulated the intestinal flora.Moreover,1 500 mg/kg MSG increased the risk of cardiovascular disease and damaged the intestinal structure and flora.In this study, MSG was also found to be healthier than salt at the equivalent sodium concentration.Collectively, these findings suggested that low doses of MSG were safe for mice and may have some health benefits as a probiotic by promoting intestinal development and regulating the intestinal flora.
1.Introduction
Monosodium glutamate (MSG, C5H8NO4Na) is the sodium salt from glutamic acid, the most abundant amino acid in nature [1].Since the discovery of the unique umami flavor of glutamate by Dr.Ikeda in 1908, MSG has been used to improve the palatability and texture of food [2,3].As a flavor enhancer, MSG is usually found in stewed meat products, canned products, soups, and commercially processed foods [4].In the United States, the Food and Drug Administration has suggested that MSG poses no threat to human health, classifying it as a generally-recognized-as-safe food ingredient [1,5], while the FAO and WHO had indicated that the acceptable daily intake (ADI) of MSG should not exceed 120 mg/kg body weight/day [6].According to the latest assessment report issued by the European Union’s Food Safety Authority (EFSA) in July of 2017, the safe intake of MSG is 30 mg/kg body weight/day [7].Moreover, only a small portion of the body’s glutamate intake comes from MSG condiments; the main source of glutamate is foods that are naturally high in protein (e.g.,fish, eggs, meat, and cheese) and vegetables (tomatoes, green beans,and mushrooms) [1,8].
The safety of food substances depends on the route of administration, the dosage used, and the structure of the food consumed.In studies on MSG, abnormalities, including liver and hypothalamus lesions, fat metabolism disorders, and genital and brain injury caused by subcutaneous or intravenous administration,are commonly observed [9,10].However, these studies violated the metabolic pathways involved when glutamate is ingested through the diet and hence are not applicable to human nutrition.Similarly,the amount of MSG required to induce brain lesions in mice is much higher than the level that is tolerable in humans and can result in nausea [11].Indeed, no tissue damage or lesions were observed in studies of normal MSG ingestion, and obesity or body weight changes are not associated with MSG use [12,13].
The intestinal flora is a complex microecosystem in the gut tract and is occasionally referred to as the invisible organ of the gastrointestinal system [14].The balance created by the composition and diversity of intestinal flora is closely correlated with host metabolism, immune development, cognitive neurodevelopment,and some diseases processes [15-17].Studies to date have indicated that dietary composition can affect the homeostasis of the internal environment by affecting the survival and activities of intestinal microorganisms [18,19].For example, artificial sweeteners can change several bacterial taxa associated with human type 2 diabetes and induce glucose intolerance in a microbiota-dependent manner,thereby reducing the beneficial flora [20].Dietary emulsifiers have also been shown recently to alter the diversity of the microbiota and promote the development of low chronic inflammation in mice [21].Thus, analysis of the intestinal flora could help improve our understanding of the safety of minor food constituents and food additives.
Recent studies of MSG and the intestinal flora are limited to a single low dose or inappropriate experimental models, and published data are still scarce [22,23].Mice are the most widely used animal model for studying human intestinal nutrition and disease.Therefore, in order to clarify the potential health threats of MSG, we evaluated the effects of different doses of MSG on disease-related biomarkers, intestinal function,and intestinal flora in mice.Table salt (NaCl) was used as a positive control to compare the effects of sodium ions.This study provides insights into the healthy use of MSG and establishes new perspectives regarding the roles of the intestinal flora, thereby facilitating the exploration of the relationships between food additives and health.
2.Materials and methods
2.1 Animals and treatments
Animals were allowed to acclimate to the environment for 1 week before any treatments, and then randomly divided into 5 groups(n= 12/group): The control (Con) group was administered pure water by oral gavage; MSG groups were administered 30 (L-MSG), 300(M-MSG), or 1 500 (H-MSG) mg/kg MSG by oral gavage (in turn corresponds to 0.34, 3.4, or 17 g/day for a 70 kg man) [24]; NaCl(NC-NaCl) group was administered 100 mg/kg NaCl by oral gavage.All treatments were performed once a day for 5 weeks.The doses of MSG were selected based on the ADI provided by the EFSA and daily intake data from the literature [1,5]; the dose of NaCl was calculated according to the sodium ion concentration of 300 mg/kg MSG.
2.2 Determination of serum biomarkers
After the intervention, blood samples were collected from the orbital sinus by removing the eyeballs under deep anesthesia.Samples were maintained at 4 °C overnight, then centrifuged at 3 000 r/min for 20 min at 4 °C to obtain serum and stored at−80 °C for subsequent assays.The concentrations of C-reactive protein(CRP), trimethylamine N-oxide (TMAO), and angiotensin II (AngII)were determined using mouse enzyme-linked immunosorbent assay(ELISA) kits (Jianglai Bioengineering Institute, Shanghai, China).
2.3 Determination of fecal albumin
During the last 2 days of the experiment, fresh fecal pellets were collected and immediately stored at −80 °C.Pellets were weighed,homogenized (1:9,m/V) in 0.1 mol/L phosphate-buffered saline(PBS), and then centrifuged at 3 000 r/min for 20 min at 4 °C.The concentration of albumin in the supernatant was measured using ELISA kits (H127-1-1; Nanjing Jiancheng Bioengineering Institute,Nanjing, China).
2.4 Histomorphology
The abdominal cavity of each animal was opened, and the ileum was separated from the intestine of the animal.The intestines were flushed with precooled PBS to remove intestinal contents, and the ileum was then fixed in 10% neutral-buffered formalin and embedded in paraffin.Wax blocks were cut into 5 μm slices according to the instructions of the microtome, and samples were stained with hematoxylin and eosin to verify the histopathological diagnosis.Next,the sections were observed with a microscope (Zeiss Axioskop 40,Carl Zeiss, Jena, Germany) using a 20 × objective lens, and images were captured with an attached digital camera using NIS-Elements F software.Morphometric analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).The villus height was analyzed from the lamina to the villus apex, and the crypt depth was defined as the depth of emboly (near the villus height end) between adjacent villi.
2.5 Determination of cytokine levels
The ileum was chopped into small pieces and thoroughly washed with ice-cold PBS.Homogenates were then prepared using a pestle in glass tissue grinders with ice-cold PBS and diluted to 10% (V/V)with PBS.After centrifugation at 3 000 r/min for 15 min at 4 °C, the supernatants were recovered and stored at −80 °C until analysis.The levels of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α,and secretory IgA (SIgA) were determined using mouse ELISA kits (H002,H007,H052,H108-2; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6 DNA extraction and 16S rRNA gene sequencing for microbiome analysis
Cecal contents were collected in a sterile environment and stored in a freezer at −80 °C after flash-freezing in liquid nitrogen.After extraction and quantification, the integrity of total bacterial genomic DNA samples was examined using agarose gel electrophoresis.Then,the 16S rDNA gene region V3-V4 was sequenced using the Illumina MiSeq platform, as described previously [25].Sequence data analyses were mainly performed using QIIME (version 1.8; Flagstaff, AZ,USA) and R packages (v3.2.0) and classified according to operational taxonomic units (OTUs) at a similarity level of 97%.OTU-levelα-diversity indices for each sample, such as the Chao1 richness estimator, observed species, Shannon diversity index, and Simpson index, were calculated using the OTU table in QIIME.The relative abundance at the phylum and genus levels was determined in the intestinal flora using the RDP classifier (v2.2).Linear discriminant analysis (LDA) and effect size (LEfSe) analyses were performed to determine differences among groups.
2.7 Statistical analysis
All results were processed using descriptive statistics, such as measures of central tendency means ± SEMs.Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA).Data were analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s tests for multiple group comparisons.Results withP-values < 0.05 were considered statistically significant.
皇权伴随帝国梦的破灭出现断层,统治美学却并未终止,它因长期存在而留下巨大惯性,如记忆和基因一般流传下来,幻化成陶瓷本身所携带的思维样式,它有别于西方陶艺的情趣化表现,更多体现的是对空间的占有或渲染。这的确是一种非常神奇的感受,就像皇权赐予的物件一般,拥有当代语境下的魔力。
3.Results
3.1 Effects of MSG on biomarkers in serum
As an overall measure of animal health, the concentrations of CRP, TMAO, and AngII were evaluated as key biomarkers.As shown in Fig.1, the levels of CRP, TMAO, and AngII did not increase in the L-MSG group compared with that in control mice.Only secretion of AngII in the M-MSG group was higher than that in the Con group.Notably, the concentrations of all 3 biomarkers in serum were significantly increased in the H-MSG group.Moreover, serum CRP and TMAO levels were higher in the NC-NaCl group than in the M-MSG group.The results showed that excess supplementation with MSG caused increased CRP, TMAO, and AngII levels in the serum.Sodium ions in MSG may be the main factor leading to increased biomarker levels and risk of cardiovascular disease.

Fig.1 (A) CRP, (B) TMAO and (C) AngII in mice.Values are expressed as means ± SEMs; n = 5.*P < 0.05 compared to the Con group, #P < 0.05 compared to the M-MSG group.
3.2 Effects of MSG on albumin leakage in feces
To observe the effects of MSG administration on intestinal permeability, the amount of albumin leakage in mouse feces was measured.Any increase in albumin leakage at this concentration indicated an increase in intestinal permeability.As shown in Fig.2A,compared with control mice, there were no differences in the amount of albumin leakage in the feces of mice in the L-MSG and M-MSG groups.In contrast, mice in the NC-NaCl group showed significantly higher albumin leakage than mice in the M-MSG group, indicating that sodium ions had a significant effect on intestinal permeability.Similarly, mice in the H-MSG group were found to have significantly increased levels of albumin content in the feces.
3.3 Effects of MSG on the histomorphology of the ileum in mice
The height of the small intestinal villi and the depth of crypts are essential indicators of the functions of intestinal digestion and absorption.The ratio of villus height/crypt depth (V/C ratio) is the most frequently used index for evaluation of overall intestinal function [26].As shown in Fig.2C, the control mice and mice in the M-MSG group showed normal histological morphology of the ileum, without edema,inflammatory cell infiltration, and other pathological changes.In mice in the L-MSG group, the ileum showed a healthy histological morphology, but the V/C ratio was significantly increased (Fig.2B).The epithelial cell structure and the ileum villi were severely destroyed in the H-MSG group, with a significant downward trend in the V/C ratio.In addition, compared with the M-MSG group, the NC-NaCl group showed a significantly lower V/C ratio,accompanied by slight villous epithelial cell edema and inflammatory cell infiltration.These data indicate that low levels of MSG supplementation promoted small intestine development.
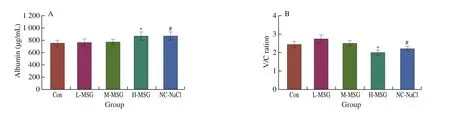
Fig.2 Effects of MSG supplementation on levels of (A) albumin leakage in feces, (B) V/C ratio and (C) morphology of terminal ileum (× 200).Values are expressed as means ± SEMs; n = 5.*P < 0.05 compared to the Con group, #P < 0.05 compared to the M-MSG group.

Fig.2(Continued)
3.4 Effects of MSG on IL-1β, IL-6, TNF-α, and SIgA levels in the ileum of mice
To clarify the influence of MSG on intestinal immunity, the levels of IL-1β, IL-6, TNF-α, and SIgA were evaluated in mouse ileum tissue.As shown in Fig.3, the intervention dose of MSG in the L-MSG group did not cause substantial changes in the expression of the three pro-inflammatory cytokines.Compared with the Con group, mice in the M-MSG group showed only slight increases in TNF-α expression(Fig.3C).In contrast, the secretion of all three pro-inflammatory cytokines was substantially increased in the ileum of mice in the H-MSG group compared with that in the Con group.Compared with the M-MSG group, the levels of IL-1β and IL-6 were significantly increased in the NC-NaCl group (Figs.3A, 3B).Moreover, secretion of SIgA was decreased in the ileum of mice in the M-MSG and H-MSG groups and was significantly lower in the NC-NaCl group than in the M-MSG group (Fig.3D).

Fig.3 Effects of MSG supplementation on intestinal immunity.The levels of (A) IL-1β, (B) IL-6, (C) TNF-α, and (D) SIgA after 5 weeks.Values were expressed as means ± SEMs; n = 5.*P < 0.05 compared to the Con group, #P < 0.05 compared to the M-MSG group.
3.5 Modulation of the intestinal flora by MSG
The intestinal flora compositions of mice were determined by high-throughput sequencing of the 16S rRNA gene.In total, 2.2 × 106sequence reads were obtained using Illumina MiSeq.As shown in Table 1, the M-MSG group had the highest OTU number, whereas the NC-NaCl group had the lowest OTU number.Within-sample(alpha) diversity was assessed using 4 indices: Chao1, observed species, Shannon, and Simpson indices (Table 2).Chao1, observed species, and Shannon indices were higher in mice supplemented with MSG than in the Con group.The results showed that MSG could increase the species diversity of bacteria, and the medium dose of MSG supplementation (300 mg/kg) had the strongest effect.Compared with the other groups, the NC-NaCl group had the lowest Chao1, observed species, Shannon, and Simpson indices, indicating that supplementation with salt further reduced species richness.In addition, the clean tags (Table 1) of intestinal flora were not markedly different among all groups, suggesting that microbiota abundance and diversity were comparable between groups [27].
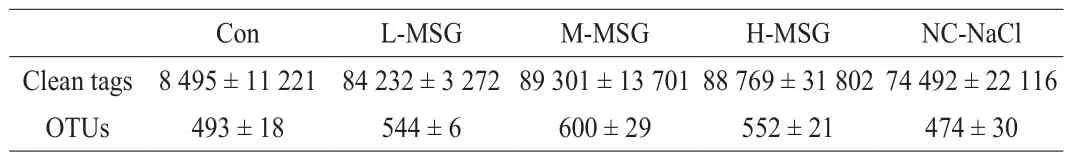
Table 1Quality statistics for sequences.
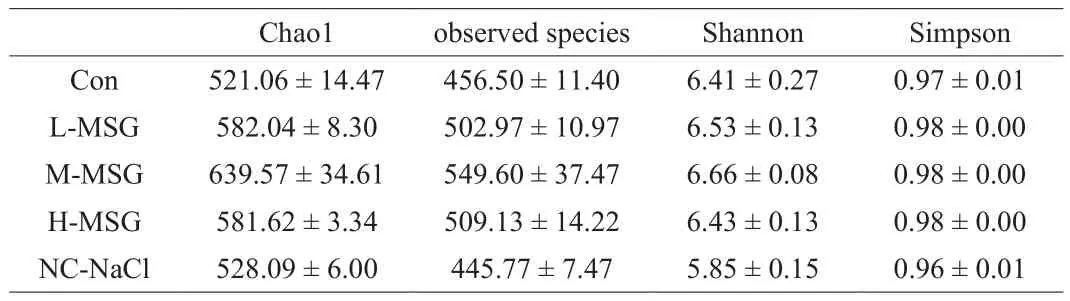
Table 2Alpha diversity.
As a result of the relative abundance of the intestinal flora at the phylum level, Bacteroidetes and Firmicutes were dominant in all groups (Fig.4A).Multiple studies have shown that changes in the Bacteroidetes to Firmicutes ratio (B/F ratio) are associated with the development of some diseases; thus, we calculated the B/F ratio for all groups (Fig.4B).The B/F ratios in the Con and L-MSG groups were both 0.46, lower than those in the M-MSG and H-MSG groups(0.68 and 0.63, respectively), but the difference was not significant.Compared with the M-MSG group, the B/F ratio in the NC-NaCl group was significantly increased to 1.26.In particular, in mice whose diets were supplemented with MSG, we found that the proportion of the microbiota from Patescibacteria increased compared with those in the Con and NC-NaCl groups.Thus, these findings suggested that Patescibacteria may be a key factor mediating the effects of MSG on the body.Additionally, compared with the Con group, the level of Proteobacteria decreased in the H-MSG and NC-NaCl groups and increased in the M-MSG group.This shows that the glutamate in MSG could improve the ability of the microbiota to resist the interference.However, excessive sodium consumption (H-MSG and NC-NaCl groups)would cause a decrease in the abundance of certain microbiota.
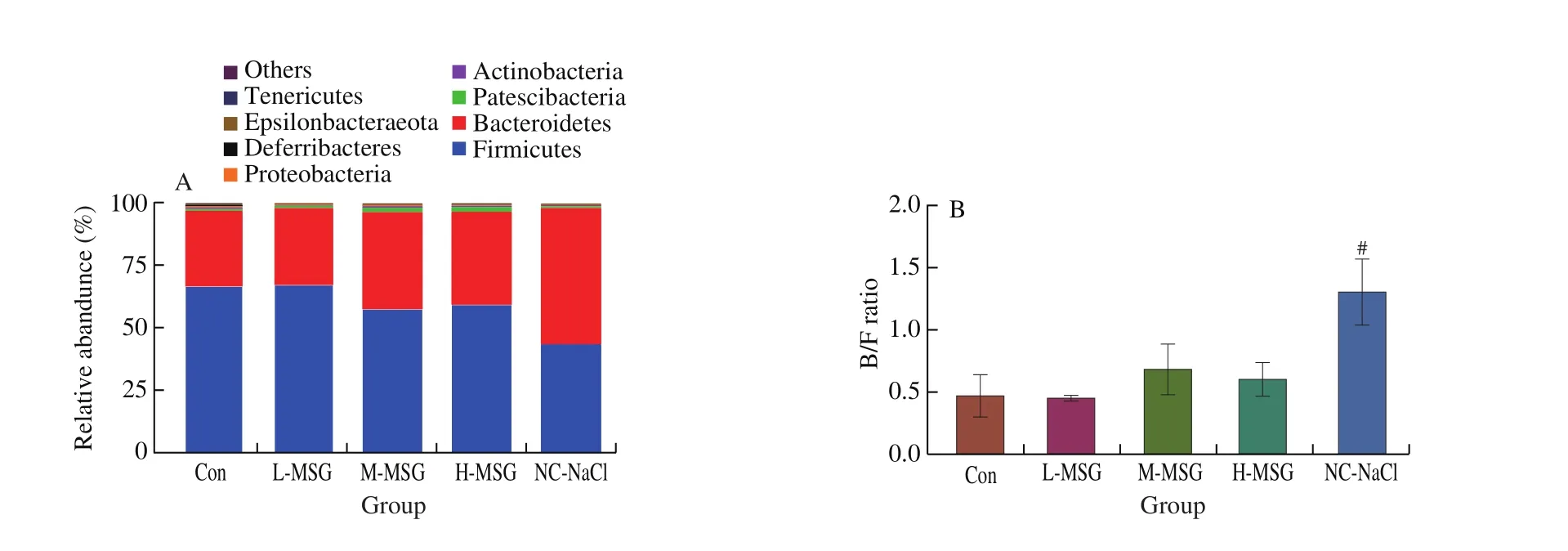
Fig.4 Effects of MSG supplementation on the intestinal microbiome at phylum and genus level.The intestinal flora was characterized by 16S rRNA gene sequencing.(A) Relative abundance at phylum level, (B) B/F ratio, and (C) heatmap cluster at genus level after 5 weeks.Values were expressed as means ± SEMs;n = 5.#P < 0.05 compared to the M-MSG group.
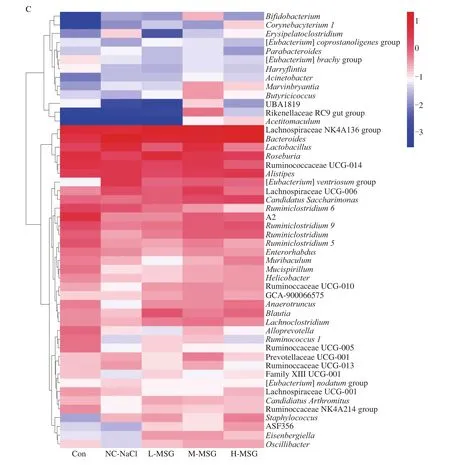
Fig.4(Continued)
At the genus level (Fig.4C, Table 3), the intestinal flora compositions of the mice in each group differed.Following supplementation with low-dose MSG, the populations of Lachnospiraceae NK4A136 group,Roseburia, andBlautiawere significantly increased to 15.44%, 6.39%, and 1.23%, respectively,compared with those in the Con group.Similar findings were observed in the M-MSG and H-MSG groups, with only minor differences among MSG doses.Notably, the populations ofLactobacillusandCandidatus Saccharimonasincreased to 5.07% and 1.58%,respectively, in the M-MSG group.In addition, the H-MSG and NCNaCl groups showed similar trends in changes in some bacterial abundances.We found that supplementation with salt increased the populations ofBacteroidesandAlistipesto 20.34% and 3.07%,respectively, and decreased the population ofAnaerotruncusto 0.08%.These abnormal changes related to salt intake were alleviated in the M-MSG group.The results indicated that a low and safe dose of MSG could improve the abundance and alter the structure of the intestinal flora.Moreover, even at high level of MSG, the deleterious effects were lower than those produced by salt.

Table 3Relative abundance at genus level.
LEfSe analysis was then performed to explore differences in the microbiota profiles between the Con, NC-NaCl, and M-MSG groups.As shown in Fig.5, from a comparison among the three groups, 34 key phylotypes were enriched within the Con group, and 13 key phylotypes were enriched in the NC-NaCl group.Compared with the other two groups, the medium dose of MSG significantly increased the abundances of 21 key phylotypes with discriminative features, such asRuminiclostridium,Methylobacterium, andLachnoclostridium.

Fig.5 (A) Cladogram generated by LEfSe analysis showing the enriched taxa, (B) LDA scores of the enriched taxa.Group A (red): Con group; Group B (green):NC-NaCl group; Group M (blue): M-MSG group.
4.Discussion
After ingestion, MSG will be broken down into sodium ions and glutamate.Sodium ions increase the burden of blood pressure and the load on the kidneys to maintain osmotic pressure, and increase the risk of hypertension and cardiovascular and cerebrovascular diseases.In this study, we measured the relevant indicators of hypertension,cardiovascular disease, and metabolic syndrome to explore whether intervention with different doses of MSG impacts health status in mice.As a sensitive inflammatory marker, CRP is often used to symbolize inflammatory and disease activity [28].Patients with hypertension and diabetes have higher CRP levels than normal individuals [29].In this study, we found that compared with the Con group, mice treated with 30 mg/kg MSG did not show increased expression of the inflammation marker CRP, whereas mice treated with 1 500 mg/kg MSG had increased CRP.At the equivalent amount of sodium intake, the salt-supplemented diet increased CRP levels more than MSG.Our findings were consistent with a study by Qing et al.[30]found that a high-salt diet could induce overproduction of reactive oxygen species, leading to increased inflammation.TMAO is formed by the oxidation of trimethylamine, a metabolite of intestinal microorganisms, in the liver [31].Our results showed that supplementation with 300 and 1 500 mg/kg MSG could cause TMAO to accumulate in the blood.A study had concluded that TMAO can interfere with reverse cholesterol transportation and promote the release of inflammatory cytokines, which could increase the risk of cardiovascular disease [32].High salt intake increases TMAO in the plasma by controlling TMAO clearanceviaurinary excretion [33].In addition, we found that the amount of sodium ions ingested was the main factor affecting the secretion of AngII.AngII plays essential roles in the regulation of blood pressure, body fluid, and electrolyte balance.Dietary salt intake can activate the renin-angiotensin system, which in turn affects the production of AngII [34].Therefore, moderate intake of MSG does not increase the risk of hypertension and cardiovascular diseases.
The integrity of the intestinal structure and epithelial barrier is critical to maintaining the healthy state of the organism.The small intestine is the main place for the digestion and absorption of MSG,and MSG can most directly affect the structure and function of the intestine.The villi and crypts are the main structures of the intestinal epithelium, and the V/C ratio can reflect the functions of intestinal digestion and absorption [26].In these experiments, lower doses of MSG (30 mg/kg) were found to promote villi growth, which could be associated with the metabolism of MSG by intestinal epithelial cells to provide energy.Moreover, consistent with the study by Rezaei et al.[35], dietary supplementation with 1%–4% MSG increased villi height and reduced crypt depth in piglets, thereby improving growth performance.Zhang et al.[36]found that orally administered MSG beneficially stimulated intestinal function in piglets by increasing the expression of intestinal glutamate receptors and transporters.Moreover, 1 500 mg/kg MSG clearly decreased the V/C ratio, suggesting that high doses of MSG were not conducive to healthy intestinal development.Intestinal epithelial impairment indicates intestinal barrier dysfunction, and impairment of the epithelial barrier permits lipopolysaccharides and endotoxins to enter the bloodstream [37].Similarly, albumin can penetrate the mucosal interstitium and intestinal lumen from the blood [38].Our results showed that 1 500 mg/kg MSG resulted in an increase in albumin leakage and impaired intestinal permeability.This may be related to disruption of the balance of intestinal osmotic pressure by the presence of excess sodium ions, leading to damage to the intestinal barrier structure.
Intestinal epithelial injury and barrier dysfunction can cause intestinal inflammation and immune disorders.Consumption of a high-salt diet can upregulate the expression of genes encoding inflammatory pathway components [39].The influence of MSG on intestinal structure and epithelial barrier can also be reflected in intestinal inflammation.IL-1β, IL-6, and TNF-α are primary proinflammatory cytokines and are thought to be molecular markers of inflammatory responses.IL-1β and TNF-α are regulated by the nuclear factor-κB pathway in the early stages of inflammation and promote the production and release of other cytokines [40].IL-6 is a quintessential pro-inflammatory cytokine produced by Th2 cells,fibroblasts, and macrophages [41].Moreover, SIgA is the main defense mechanism of the intestinal innate and adaptive immune responses [42].Our study showed that as MSG dose increased,intestinal inflammation in mice increased, whereas intestinal immunity decreased.Similar changes have been reported in many patients with enteritis [43].It has been previously confirmed that high salt levels can disrupt intestinal immune homeostasis by affecting the functions of intestinal Th17 cells and type 3 innate lymphoid cells,thereby increasing the risk of gastrointestinal diseases [44].This suggests that foods with high salt content may be a potential threat to health.All in all, the intake of sodium glutamate will cause an upregulation of intestinal inflammation, which is a common feature of salty foods, and sodium glutamate cannot be avoided.However, in our research, it was found that the dose of sodium glutamate when it damages the intestinal immunity exceeds the daily level.Furthermore,glutamate has a nutritional effect on the small intestine, so the damage is less than the problem caused by salt intake with the same sodium ion concentration.
Currently, a growing body of research has indicated that diet is closely related to intestinal flora.High consumption of MSG may affect the survival of some bacteria due to the different salt tolerance among strains.Bacteroidetes and Firmicutes are the dominant phyla and the main contributors in human and mouse intestinal flora [45].In this study, different doses of MSG did not significantly affect the B/F value of mice, while salt did.This indicated that MSG had little influence on the composition of microbiome.Likewise, some researchers have shown that addition of MSG alone in pigs had no influence on the B/F ratios [23].Patescibacteria can be found widely in seawater, soil, and the digestive tracts of animals, although only a few studies have described these organisms [46].Notably,MSG can promote the growth of Patescibacteria, but the specific mechanisms have not yet been fully elucidated.At the genus level,changes in intestinal bacteria were also observed.Importantly,Lachnospiraceae NK4A136 group,Roseburia, andBlautiaare short chain fatty acid (SCFA)-producing bacteria that can decompose cellulose and hemicellulose and provide substrates for the production of SCFAs [47,48].SCFAs play critical roles in ameliorating obesity, hypertension, and dyslipidemia.The abundance of these taxa increased after MSG supplementation compared with that of controls.Accordingly, MSG can promote the production of SCFAs through regulating bacteria.Hu et al.[49]found that glutamic acid can affect the composition of intestinal flora and prevent obesity by increasing the production of SCFAs.Interestingly, the administration of 300 mg/kg MSG significantly increased the abundance ofLactobacillus, which are beneficial for the immune system and exert antidiabetic effects [50].In addition, excessive MSG intake increases the abundance ofAlitipesandBacteroides.Alistipescan activate inflammatory reactions in hosts and has been found to be abundant in patients with type 2 diabetes [51].Wan et al.[52]found that a highfat diet could increase the abundance ofAlistipesandBacteroides,concomitant with a decrease in the total SCFA concentration.
Taken together, our findings suggested that MSG may be helpful for promoting healthy intestinal flora by enhancing the energy and amino acid supply, but that high doses could cause microecological dysbiosis and increase the abundance of disease-related microbiota.
5.Conclusion
In summary, our findings demonstrated that low-dose MSG intake was safe and even beneficial for the health of mice, promoting intestinal development and regulating the structure of the intestinal flora by increasing the abundance of probiotics.Moreover, increased serum biomarkers, damaged intestinal morphology, aggravated intestinal inflammation, and altered bacteria suggested that excessive MSG could increase the risk of cardiovascular disease and cause intestinal dysfunction and microbiome disorders in mice.Furthermore,MSG showed less harmful effects than salt at the equivalent sodium concentration in this study.Overall, our findings provided a new reference and theoretical basis for future research on the influence of MSG on human intestinal health and may contribute to eliminating public concerns on MSG safety.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Inner Mongolia Autonomous Region Science and Technology Achievement Transformation Project(CGZH2018035).
猜你喜欢
杂志排行
食品科学与人类健康(英文)的其它文章
- Effects of dietary fiber on human health
- Tea polyphenol - gut microbiota interactions: hints on improving the metabolic syndrome in a multi-element and multi-target manner
- Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways
- Milled flaxseed-added diets ameliorated hepatic inflammation by reducing gene expression of TLR4/NF-κB pathway and altered gut microbiota in STZ-induced type 1 diabetic mice
- Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice
- Effects of soy hull polysaccharide on dyslipidemia and pathoglycemia in rats induced by a high-fat-high-sucrose diet
