Milled flaxseed-added diets ameliorated hepatic inflammation by reducing gene expression of TLR4/NF-κB pathway and altered gut microbiota in STZ-induced type 1 diabetic mice
2022-11-26HuiXiXinglingShiBeijiZhouJingSuiChoYngHechunLiuLigngYngShokngWngGuijuSun
Hui Xi, Xingling Shi, Beiji Zhou, Jing Sui, Cho Yng,Hechun Liu, Ligng Yng, Shokng Wng, Guiju Sun,*
a Key Laboratory of Environmental Medicine and Engineering of Ministry of Education, and Department of Nutrition and Food Hygiene,School of Public Health, Southeast University, Nanjing 210009, China
b Research Institute for Environment and Health, School of Law and Public Affairs, Nanjing University of Information Science and Technology,Nanjing, Jiangsu, 210044, China
Keywords:
Milled flaxseed
Type 1 diabetes
Gut microbiota
TLR4/NF-κB pathway
A B S T R A C T
Flaxseed has displayed the potential beneficial as functional foods.However, most studies focused on effects of flaxseed extracts or ingredients in flaxseed.Besides, few studies showed that flaxseed extracts contributed to anti-type 1 diabetes (T1D), yet the underlying mechanism is still unknown.In the present study, 16.7% of milled flaxseed (MF)-added diet was given to diabetic mice induced by streptozocin for 6 weeks.The results showed that MF feeding 1) slightly decreased blood glucose levels and improved the ability of glucose tolerance by oral glucose tolerance test, 2) decreased liver tumor necrosis factor-α levels and increased liver glycogen levels with significance via down-regulating TLR4/NF-κB pathways, 3) and significantly altered some beneficial bacteria in gut microbiota.In conclusion, the present study showed that milled flaxseed showed the potential on anti-T1D through anti-inflammation via TLR4/NF-κB and altering the gut microbiota in STZ-induced diabetic mice.
1.Introduction
Flaxseed, as the seed of flax (Linum usitassimum), has emerged as the functional foods due to its high content ofα-linolenic acid (ALA),secoisolariciresinol diglucoside (SDG) and dietary fiber [1].The main bioactive components in flaxseed are abundant including 55% of ALA, 30% of protein and 35% dietary fiber [1].It is believed that the potential beneficial function of flaxseed was attributed to ALA and SDG.Plenty of evidence has demonstrated that biological active components in flaxseed showed potential therapeutic benefits in regulation of inflammation, immunity, fecal microbiota, constipation and diarrhea, bone health, cognitive behavioral disorders, etc.[2-6].Therefore, people show the increasing interests of flaxseed and it has been an attractive ingredient designed for specific health benefits.
Type 1 diabetes (T1D) accounts for 5%–10% of diabetes mellitus globally as a group of autoimmune diseases mediated by T cells and characterized by specific damage to pancreatic β-cells [7]and co-regulated by genetic and environmental factors [8,9].Moreover,the decline in the quality of life and increase in mortality in patients with T1D is mainly due to diabetic ketoacidosis and complications of diabetes [10].Although T1D therapy has been gained huge progress, a considerable number of patients are with poor blood glucose control [11]and it brought heavy burden for the country, medical system and diabetic patients.The combination of pharmacological therapy and dietary intervention will be of great significance in the field of T1D management.
Dietary intervention has displayed vital benefits against diabetes and the healthy dietary pattern for prevention and treatment of diabetes mentioned that we should increase the intake of dietary fiber andn-3 polyunsaturated fatty acid [12], which was rich in dietary flaxseed.Lots of studies had displayed the beneficial effects of some ingredients in flaxseed against type 2 diabetes [13,14].Furthermore,only few studies reported that flaxseed extraction or an ingredient of fvlaxseed may decrease the risk of T1D incidence and the treatment of its complications [15-17].However, the effects and potential mechanisms of whole flaxseed on T1D is still unknown.Therefore,more studies are required to ascertain health benefits and specific mechanisms of whole flaxseed on T1D.
Therefore, the present study aims at further investigating the role that the whole flaxseed plays in the progression of T1D.We hypothesized that a milled flaxseed added dietary could improve the symptom of STZ-induced T1D mice through regulation of inflammation and altering gut microbiota.
2.Materials and methods
2.1 Animal model and study design
Six-week-old C57BL/6J male mice were purchased from Shanghai SLAC Laboratory Animal Company and housed in the Specific Pathogen Free (SPF) animal laboratory at the room temperature of 20–26 °C,the relative humidity of 45%–60% with a 12 h light/dark cycle.The protocol for the animal study was also in accordance with the Animal Management Committee and Animal Ethical Committee of Jiangsu Province, and approved by Animal Experimental Ethical Committee of Southeast University.Mice were divided into two groups, the control group (CON) fed with the chow diet (AIN-93G) and T1D model group, which were established by the intraperitoneal injection of a large dose of streptozocin (170.00 mg/kg, STZ).The fasting blood glucose level ≥ 16.7 mmol/L was considered as qualified T1D mice.After 1-week, T1D mice were randomly divided into two groups (n= 12 for each group), the model group (MOD) and the milled flaxseed group (MF).Mice in MOD were fed with the chow diet, and mice in MF was fed with the diet added with 16.7% ground flaxseed for 6 weeks.The ingredients of three groups were shown in Table 1.The ground flaxseed contained 19.90% of protein, 41.99% of fat, 30.01% of total fiber, 5.01% of moisture,2.89% of ash, and 0.2% of carbohydrate.Three days before the end of the experiment, the Oral Glucose Tolerance Test (OGTT)was conducted and the tail veil blood glucose levels of 0, 30, and 120 min were detected after the 2 g/kg body weight of glucose fed intragastrically.At the end of the experiment, mice were sacrificed with deep isoflurane.Meanwhile we collected blood samples,liver tissues, pancreas, and fecal samples with the snap-frozen in liquid nitrogen and storage at –80 °C for subsequent analysis.The pancreas of the mouse was fixed with 4% paraformaldehyde overnight, followed by dehydration, embedding, filming,hematoxylin-eosin staining (H&E, Sigma Aldrich), sectioning and sealing, respectively.The pancreas sections were examined and evaluated under light microscope (BX41, Olympus, Japan).

Table 1The ingredients (g/100 g) of milled flaxseed fortified diet in different groups.
2.2 Glucose metabolism-related index and pro-inflammatory cytokine detection
Fasting blood samples were collected and examined at 0, 4,6-week during the flaxseed intervention period.Liver glycogen was detected and quantified by the anthrone colorimetry.Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 in the liver tissues were examined by the enzyme linked immunosorbent assay (Nanjing Jiancheng Bioengineering Institute).
2.3 Western blotting and RT-PCR analysis
Trizol reagent was used to extract total RNA of the liver tissues.cDNA synthesis was by the reverse transcription kit (Cat:RR036A, Takara Bio, Japan).A Real-Time PCR System (788BRO-7517, Bio-Rad, USA) equipped with the CFX Maestro 1.1 (version 4.1.2433.1219) software was applied to perform the analysis.The relative mRNA levels were calculated by the equation 2-ΔΔCtand presented as the fold change normalized to the internal control,β-actin.Protein concentrations of liver tissues were measured by using the BCA protein assay (Themo Fisher Scientific Company, USA).The following antibodies were purchased from Cell Signaling Technology for Western blotting: primary antibodies for toll-like receptor 4(TLR4), myeloid differentiation factor 88 (MyD88) nuclear factorκB (NF-κB) andβ-actin.The anti-rabbit and anti-mouse antibodies used as the second antibodies were from Santa Cruz.100 μg protein from each sample was subjected to SDS-PAGE, which was produced by the TGX FastCast Acrylamide Kit (Bio-Rad, USA).The proteins were then transferred to the polyvinylidene fluoride membrane under the condition of 70 V for 4 h.Then the membrane bearing the proteins was incubated with the primary antibodies overnight at 4 °C and then incubated with the second antibodies for 2 h at room temperature.The membrane was exposed with Tanon-5200 ECL detection reagent and captured by the Image J Software.
2.4 Fecal sample collection and 16S rDNA sequencing analysis
The fecal sample was collected from the cecum after we scarified the mice, then transferred on liquid nitrogen and finally stored at –80 °C.The fecal DNA was extracted by using the PowerSoil® DNA Isolation Kit (MO BIO, US) followed by the detection of the purity and concentration of the DNA.The PCR amplification and purification of V3-V4 variable regions of DNA (F: 5’-ACTCCTACGGGAGGCAGCA-3’, R:5’- GGACTACHVGGGTWTCTAAT-3’) were detected and then 50 μL DNA of purified and screened fragments was added in amplified products.The supernatant that elution from the amplified products was transferred to enzyme-free EP tubes.In addition,purified amplicons were quantified with the Solexa PCR system and then microbial 16S rDNA was sequenced on the Illumina Hiseq 2500 platform (Norcross, GA, USA).
The Flash software (version 1.2.11) was utilized to merge pairedend reads in order to form raw tags, which were further filtered into clean and effective tags by Trimmomatic software (version 0.33)and UCHIME software (version 8.1), respectively.The operational taxonomic units (OTUs) number of each sample was obtained by clustering sequences with 97% similarity using Usearch software(version 10.0).Alpha-diversity and beta-diversity were analyzed to describe species richness and diversity, species composition change over time and space scales.Metastats software was used to determine differences between the groups.
2.5 Statistical analysis
Data are expressed as mean ± standard deviation.One-way ANOVA was used to examine the differences among groups with the SPSS 18.0 version.APvalue < 0.05 was considered statistically significant.GraphPad Prism 5 was used to perform all analyses.
3.Results
3.1 MF feeding regulated body weight, glucose metabolism and islet morphology in T1D mice
According to Fig.1, STZ injection significantly decreased body weight of mice in the continuous observation of 6 weeks.In the present study, MF (16.7%) successfully ameliorated weight loss of mice when comparing with MOD group at the 6th week.In addition,after MF feeding, fast blood glucose (FBG) concentrations showed a significant downward at the 6th weeks.However, we did not observe change of insulin between MOD and MF groups.MF feeding significantly increased the liver glycogen level compared to MOD.The 2 h-OGTT indicated that the MF feeding lowered area under the curve (AUC) with significance.The islet structure was severely damaged with the irregular morphology, severe atrophy, blurred boundaries and compensatory cytoplasmic swelling cells, which were improved after the treatment of MF feeding (shown in Fig.2).

Fig.1 The outcomes of MF feeding on T1D mice on body weight (A), FBG levels (B), AUC of OGTT test (C), liver glycogen levels (D) and serum insulin levels (E).*P < 0.05 for comparison between MOD and CON; #P < 0.05 for comparison between MF and MOD.

Fig.2 The pancreas histopathological evaluation in STZ-induced mice with MF feeding with H&E staining under 400 × magnification of microscope.
3.2 MF feeding played anti-inflammatory roles via TLR4/NF-κB pathway
MF feeding significantly decreased TNF-α levels in liver.However, we did not observe any regulation of MF on liver IL-1β and IL-6 levels (Fig.3A).In addition, we observed MF feeding significantly down-regulated proinflammatory-gene expression includingTLR4,MyD88, andNF-κB(Fig.3B).Furthermore, MF feeding down-regulated protein expression of MyD88 and NF-κB significantly (Fig.3C).
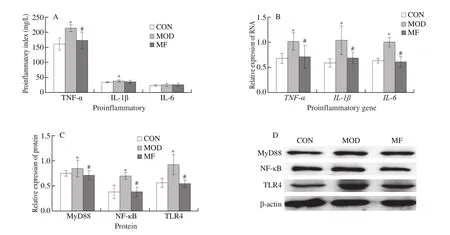
Fig.3 Effects of MF feeding on pro-inflammation cytokines (A), mRNA expression (B), protein expression shown as bar chart (C) and western blotting image (D)in diabetic mice.*P < 0.05 for comparison between MOD and CON; #P < 0.05 for comparison between MF and MOD.β-actin, used as the internal control.
3.3 MF feeding changed the composition of gut microbiota in T1D mice
We preformed 16S rDNA sequencing to explore the changes of gut microbiota composition in T1D mice after MF feeding.MF feeding significantly decreased the richness of gut microbiota by detecting ACE and Chao1 index.In addition, a statistical change of Simpson and Shannon index was observed after MF feeding and it suggest that the microbiota diversity increased after MF feeding(Figs.4A, 4B).β-diversity was evaluated by PCoA (weighted Unifrac)analysis and the results showed that MF, CON and MOD groups were remarkably separate, which indicated that MF feeding did change the overall phyla distribution in feces with the 47.77% contribution of PC1 and 13.96% contribution of PC2 (Fig.4C).

Fig.4 Effects of MF feeding on fecal microbial diversity and composition in diabetic mice.(A) OTUs, Chao1 and ACE, representing community richness; (B) Simpson and Shannon, representing community diversity; (C) PCoA of three groups; (D) The Firmicutes/Bacteroidetes ratio of 3 groups; (E–G) The relative abundance of major bacteria at phylum, genus and species levels.*P < 0.05 for comparison between MOD and CON; #P < 0.05 for comparison between MF and MOD

Fig.4(Continued)
Firmicutes/Bacteroidetes ratio was shown in Fig.4D, gut dysbiosis at the phylum level was observed in T1D and flaxseed feeding reversed the situation to some extent.Analysis at the phylum level indicated that the gut microbiota was dominated by 4 major phyla: Firmicutes, Proteobacteria, Bacteroidetes and Patescibacteria (Fig.4E) and comprised > 98% in all groups.The 10 kinds of dominant genera at the genus levels wereEscherichia-Shigella,Faecalibaculum, uncultured_bacterium_f_Muribaculaceae,Lactobacillus,Candidatus_Saccharimonas, Ruminococcaceae_UCG-014, uncultured_bacterium_f_Lachnospiraceae,Desulfovibrio,Bacteroides, Lachnospiraceae_NK4A136_group in CON, MOD and MF groups (Fig.4F).The distribution of gut microbiota in MOD and MF groups at the genus level were disturbed due to the injection of STZ, and to some extent, MF feeding reversed the genus distribution(Fig.4F).In CON group uncultured_bacterium_g_Escherichia-Shigella, uncultured_bacterium_g_Faecalibaculum, uncultured_bacterium_f_Muribaculaceae, uncultured_bacterium_g_Lactobacillus,and uncultured_bacterium_g_Candidatus_Saccharimonasare the 5 dominant species.However, the homeostasis of species was broken along with the statistical increase of uncultured_bacterium_g_Ruminococcaceae_UCG-014 and uncultured_bacterium_g_Bacteroidesand decrease of uncultured_bacterium_g_Lactobacillusand uncultured_bacterium_g_Candidatus_Saccharimonasin MOD and MF groups (Fig.4G).MF feeding seems change the overall species distribution compared to MOD group (Fig.4G).
Then we performed the analysis of differences between groups at phylum, genus and species levels, respectively.Among themBacteroidesportion was the lowest in MOD group and it showed a rise trend after MF feeding when comparing with MOD group.Patescibacteria portion decreased significantly after STZ injection in MOD and MF groups(Fig.5A).MF feeding showed a statistic regulation of increasing the relative abundance ofBacteroidesand UBA1819 and decreasing the relative abundance ofEnterococcusandFamily_XIII_AD3011_group when comparing with MOD group at genus levels (Fig.5B).Furthermore, MF feeding significantly upregulated the relative abundance of uncultured_bacterium_g_Bacteroidesand uncultured_bacterium_g_UBA1819 and down-regulated the relative abundance ofEnterococcus_faecalisand uncultured_bacterium_g_Family_XIII_AD3011_group at species levels (Fig.5C).
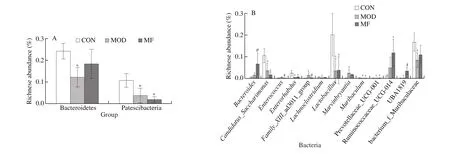
Fig.5 Effects of MF feeding on specific bacteria at phylum, genus and species levels (A, B, C, respectively) in diabetic mice.*P < 0.05 for comparison between MOD and CON; #P < 0.05 for comparison between MF and MOD.
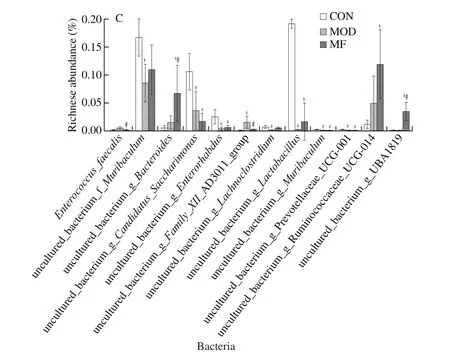
Fig.5(Continued)
In addition, the correlation analysis was conducted between improved physiological parameters and phyla at the phylum level.The relative abundance of increased Firmicutes was significantly correlated with the increased liver glycogen levels and body weight.The relative abundance of decreased Proteobacteria was significantly correlated with the increased liver glycogen levels and the decreased blood glucose concentrations (Table 2).

Table 2The correlation analysis between improved physiological parameters and phyla at the phylum level.
4.Discussion
Flaxseed as one kind of functional foods has displayed health benefits in various food products that is not only rich in ALA and dietary fiber but also emerged as an essential source with high quality protein and phenolic compounds [18,19].The nutrients of flaxseed in milled formation has a greater bioavailability than in the whole seed [20].Little was known about the effects of milled flaxseed on the STZ-induced diabetic mice.This experiment was designed to evaluate the effects of milled flaxseed on glucose homeostasis and its underlying mechanism including anti-inflammation and altering gut microbiota.This experiment showed that flaxseed regulated body weight, decreased blood glucose levels and improved glucose tolerance and islet morphology in T1D mice.We also found that milled flaxseed adding in the diet of mice has a significant impact against the liver inflammationviaTLR4/NF-κB pathway.Furthermore, flaxseed feeding changes gut microbiota composition by significantly decreasing the richness and increasing microbiota diversity of gut microbiota along with regulation of the phyla distribution at different levels.In conclusion, the present study showed that milled flaxseed showed the potential on anti-T1D through anti-inflammationviaTLR4/NF-κB and altering the gut microbiota in STZ-induced diabetic mice.
The metabolic dysregulation of glucose accompanies the progress of T1D and becomes the major cause of diabetic morbidity and mortality [21].Functional food has displayed potentials in the prevention and treatment of chronic diseases [22].In this study, MF feeding significantly decreased the fasting blood glucose levels and improved the glucose tolerance after 4 weeks’ treatment.In addition,liver glycogen levels increased as well.Abnormal hepatic glycogen metabolism could be observed in T1D patients [23].Al-Ani et al.[16]found that flaxseed extracts could decrease the blood glucose levels in rat with T1D.SDG and dietary fiber that extracted from flaxseed have been proved with regulation of blood glucose levelsviaregulation of insulin sensitivity and inhibition of carbohydrate in gut [14,24].A meta-analysis showed that higherω-3 polyunsaturated fatty acid intake improved insulin and insulin resistance, thereby regulating glucose metabolism in diabetic patients [25].Abovementioned publications further support our results and our study indicated that milled flaxseed itself not only is the ingredients of flaxseed, but also could be engaged in regulation of glucose metabolism.However, the glucose and insulin levels were still significantly higher in the MF group than in the CON group.The present study may provide the potential that flaxseed may be the adjuvant therapy along with the conventional therapy in future.
The liver is an important organ for the metabolism of nutrients and drugs, as well as for detoxification and immune response [26-28].Recently, chronic liver inflammation was gained more attention in the study of T1D due to its contribution to the occurrence of liver injury[29], in which dysregulation of glucose metabolism may promote.The liver plays vital roles in glucose homeostasis [21]and patients with T1D have issues with increased hepatic glucose production [30].It is reported that TNF-α level was abnormally elevated in the liver of STZ-induced diabetic mice [31].Besides, increased NF-κB and NOD-like receptor protein 3 (NLRP3) protein expression explained the IL-1β protein expression in the T1D liver [32].Studies have shown that activation of NF-κB increased TNF-α and IL-6 expression,which could result in the hepatocyte apoptosis in the liver of T1D rats and aggravation of inflammation response [33,34].Therefore,anti-inflammation could be an effective approach for attenuating the progression of T1D in order to improve their quality of life and prolonging the survival.TLR4 is belonged to the TLRs family, the pattern recognition receptors in the innate immune system and it could activate downstream inflammatory signaling pathways [35,36].NF-κB is a downstream gene of TLR4 signaling pathway that mediates several inflammatory processes [36].In previous publications,researches payed more attention to the flaxseed extractions and its ingredients [15-17].However, few studies investigated the effects of whole flaxseed on T1D.In our study, MF deeding significantly downregulated TLR4 gene and protein expression and its downstream gene expression includingMyD88,NF-κB compared to the MOD group,which indicated that MF attenuated the liver inflammation through regulation of TLR4/NF-κB.
Gut microbiota plays an important role in regulation of metabolism and immunity [37].Liver, as the portal circulation, has the strongest relationship with the intestinal tract by maintaining balanced bacterial composition and metabolites [38].Recently, a systematic review showed that a significant association was confirmed between alterations in intestinal microbial composition and T1D, although the distribution of microbiota composition differed in different populations [39].T1D patients exhibited a similar richness of gut microbiota compared to healthy population yet a significant decrease of diversity of gut microbiota and proinflammatory cytokine and lipopolysaccharide levels [40].Another study showed milled flaxseed supplementation regulated the composition of gut microbiota in the healthy population and increased Shannon index significantly [41].In our present study, MF feeding significantly decreased richness of gut microbiota and increased the diversity of gut microbiota, which was partially consistent with previous studies.Theβ-diversity showed that MF significantly changed the gut microbiota distribution generally,which further confirmed the potential flora alteration of grounded flaxseed.In our study we found two kinds of flora (Bacteroides and Patescibacteria) changed with significance at phylum level.Bacteroideteswas found decreased in a case-control of T1D patients in Italy [42], which was consistent with our study.A study exhibited that the reduced glycemic was correlated to an increase in hepatic glycogen content and it also showed that the relative abundance of Firmicutes increased significantly after treatments, which is consistent with our study that the positive co-relationship between Firmicutes and liver glycogen levels after flaxseed feeding [43].Furthermore, we also found that the relative abundance increased Firmicutes was significantly correlated with the elevated body weight.According to previous studies, Firmicutes was related with elevated body weight in mice and population, respectively [44,45].The overgrowth of Proteobacteria was observed in T1D patients and the Chinese traditional extracts could regulate Proteobacteria and increase the hepatic glycogen levels [46,47].Furthermore, the similar results were observed in another hyperglycemic study that the relative abundance of decreased Proteobacteria was related with hyperglycemia [48].However, the study of biological properties of Patescibacteria in T1D have not been characterized yet.As for the bacteria at genus level, we found that MF significantly up-regulated the relative abundance ofBacteroidesand UBA1819.Bacteroidesstrains has displayed the potential as therapeutics to prevent intestinal inflammatory and intestinal permeability disorders [49].UBA1819 belongs toFaecalibacterium, one previous study showed a prebiotic treatment demonstrated increased abundance of UBA1819, which has not been reported in the literature so far [50].The increased relative abundance ofEnterococcusspp.was observed in the gut microbiota in T1D patients [51,52].In addition,Enterococcusspp.was closely associated with the colon inflammation [53].Therefore,in the present study, MF decreased the expression ofEnterococcusat genus level with significance, which indicated MF could attenuated the inflammation through regulation of gut microbiota.Enterococcus faecalishas shown the potential with modulating inflammation through MAPK signaling pathways [54].However, in the present study we found that MF down-regulated the relative abundance ofEnterococcus_faecalis.We assume that T1D mice may have a totally different composition of gut microbiota that compared to other diseases, which needs more studies to further confirm.Together,MF feeding regulated the richness and diversity of gut microbiota and some beneficial bacteria, which in consequently may help regulating hepatic inflammation by gut-liver interaction.One of our limitations is that we did not apply knock-out mice of TLR4 to further confirm the effects of MF against T1D.In addition, the fecal bacteria transplant could be investigated in our future work.Another limitation of the study was that single dosage of 16.7% milled flaxseed was applied, thus we cannot observe the doseresponse relationship.Furthermore, although flaxseed decreased the glucose levels with significance, the glucose levels did not drop as the normal level, it may be related to the intervention duration and it also may provide the potential that flaxseed can be used as the adjuvant therapy method, which will be further investigated in our future study.
Conflict of interests
None.
Acknowledgements
The authors also thank the support from National Key Research and Development Program of China (NO.2016YFD400604-02),the National Natural Science Foundation of China (NO.82003457),Jiangsu Province Science Foundation for Youths (NO.BK20200366),the Fundamental Research Funds for the Central Universities and“Zhishan” Scholars Programs of Southeast University.
杂志排行
食品科学与人类健康(英文)的其它文章
- Effects of dietary fiber on human health
- Tea polyphenol - gut microbiota interactions: hints on improving the metabolic syndrome in a multi-element and multi-target manner
- Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways
- Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice
- Effects of soy hull polysaccharide on dyslipidemia and pathoglycemia in rats induced by a high-fat-high-sucrose diet
- Chinese Torreya grandis cv.Merrillii seed oil affects obesity through accumulation of sciadonic acid and altering the composition of gut microbiota
