Chinese Torreya grandis cv.Merrillii seed oil affects obesity through accumulation of sciadonic acid and altering the composition of gut microbiota
2022-11-26HuanWangYeLiRuiWangHuaifeiJiChenyangLuXiurongSu
Huan Wang, Ye Li*, Rui Wang, Huaifei Ji, Chenyang Lu Xiurong Su
a State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo 315211, China b Collage of Food and Pharmaceutical Sciences, Ningbo University, Ningbo 315800, China
c Collage of Marine Science, Ningbo University, Ningbo 315800, China
Keywords:
Torreya grandis cv. Merrillii seed oil
Obesity
Lipid metabolism
Gut microbiota
A B S T R A C T
Torreya grandis cv. Merrillii (Taxace, Torreya) is mainly distributed across the hilly areas of subtropical China and is well known for its nutritional value.In this study, the ameliorative effects of T.grandis seed oil on lipid metabolism were investigated, and the underlying mechanism was explored from the perspective of gut microbiota.Mice experiments showed that the rate of body mass gain in the group where the mice were fed a high-fat diet (HFD) and supplemented with 550 mg/(kg·day) T.grandis seed oil (HFD + TO550 group) was 42.27%, while it was 62.25% in the HFD group.Compared with the HFD group, the liver and fat indices, total cholesterol, triglycerides, and low-density lipoprotein cholesterol were reduced in the oil-supplement groups.Moreover, the oil supplement significantly changed the fatty acid composition and alleviated pathological damage to the liver caused by the high-fat diet.Additionally, the distinct clustering of bacteria in the composition of gut microbiota was observed in the oil treatment group compared with that in the HFD group.T.grandis seed oil significantly increased the abundance of the beneficial bacteria and short-chain fatty acid producers, including Lactobacillus, Bifidobacterium, Faecalibaculum and Allobaculum.Our results suggest that the supplements of T.grandis seed oil could alleviate hyperlipidemia caused by HFD.These positive effects are considered to be related with sciadonic acid (SCA) and are partially mediated by alterations in gut microbiota composition and functionality.
1.Introduction
Obesity is considered to be a major and growing health problem globally.Its worldwide prevalence has tripled between 1975 and 2016, and the global prevalence of overweight or obesity in both men and women is 39%, according to the National Health Survey 2016—2017 [1,2].Obesity is one of the risk factors for an expanding set of chronic diseases, including cardiovascular disease (CVD), diabetes,chronic kidney disease, nonalcoholic fatty liver disease, metabolic syndrome, and many cancers, among other co-morbid conditions [3].Recently, obesity is included among the global noncommunicable disease targets identified by the World Health Organization [4];however, it is seldom addressed as a discrete medical problem.
Some studies have shown that not only the amount of fat but also the types of fat in food have a significant relationship with obesity.Fatty acids generally include saturated fatty acids (SFA),monounsaturated fatty acids (MUFA), as well as polyunsaturated fatty acids (PUFAs).Consuming PUFAs instead of SFA may lower coronary heart disease risk and improve blood lipid profile.A typical example is fish oil, which is rich inn-3 PUFAs and has hypolipidemic and anti-obesity effects [5].Moreover, krill oil,which contains bioactiven-3 PUFAs in the form of phospholipids, is capable of modulating lipid metabolism by lowering plasma levels of triacylglycerols (TAG) and cholesterol [6].In recent years, it has been found that some oils extracted from plants, most of which contain specific fatty acids, have positive effects on obesity and hyperlipidemia.For example, a dietary supplement of pomegranate seed oil, containing high levels of punicic acid (9c,11t,13coctadecatrienoic acid), can significantly decrease serum triglycerides and phospholipids levels [7].Yang et al.[8]found that garlic oil and onion oil, containing several organosulfur compounds that possess numerous biological activities, have anti-obesity properties.
Torreya grandiscv.Merrillii(Taxace,Torreya) is the only grafted and thoroughbred species of Chinese torreya, which is mainly distributed across the hilly areas of subtropical China, especially in Zhuji, Zhejiang province [9,10].T.grandisis well known for its edible seeds, which have a unique nutty flavor and high nutritional value.The consumption ofT.grandisseeds has been found to have several positive benefits, including anti-atherosclerosis, antihelminthic, antifungal, and anti-tumor effects [11-13].T.grandisseed oil is a good source of unsaturated fatty acids, particularly oleic and linoleic acids [9,14].Besides, a non-methylene-interrupted unsaturated fatty acid-sciadonic acid (5c,11c,14c-eicosatrienoic acid,SCA), which possesses anti-inflammatory and antioxidative activities,has been also found in this kind of oil product [15,16].Several studies have shown that feeding rodents SCA-containing seed oils affected lipid metabolism in the liver [17,18]and gene expression of lipid metabolism-related enzymes [19].However, the ameliorative effects ofT.grandisseed oil on gut microbiota and the underlying mechanisms remain unclear.
Gut microbiota, a diverse and complex microbial ecosystem viewed as a hidden metabolic organ, is composed of trillions of bacteria and plays a critical role in regulating host metabolism and immunity [20].Accumulating evidence suggests that obesity and its complications, such as insulin resistance, hyperlipidemia,and atherosclerosis caused by high-fat, high-calorie diet are often accompanied with alterations in the gut microbiota [21,22].Besides,a decrease in probiotic bacteria (such asBifidobacteriumspp.andPediococcus pentosaceus) and increase in pro-inflammatory/pathogenic bacteria (such asAlistipes) were associated with the development of obesity and metabolic co-morbidities [23,24].Studies have shown that diet can modify gut microbiota, which in turn can have a profound impact on overall health.For example, a report suggested that treatment with fish oil, krill oil, and their mixtures lead to obesity alleviation and increased the abundance of the generaAllobaculum,Oscillibacter, andBarnesiellain the gut microbiota, but decreased the proportion ofLactobacillus[25].Given this association, there may be a significant therapeutic application of such oils, which alter microbial composition through diet, in certain diseases, such as obesity.
2.Materials and methods
2.1 Animals and experimental design
A total of 48 ICR male mice (4-weeks-old, weighing (26.03 ±1.13) g) were purchased from Zhejiang Laboratory Animal Center(Hangzhou, Zhejiang, China) and housed in specific pathogenfree conditions at Ningbo University, China.Animal experiments were performed in accordance with the National Guideline for Experimental Animal Welfare and approved by the Ningbo University Laboratory Animal Center.During the experiments, mice had free access to water and feed, and the room temperature was maintained at (24 ± 1) °C; night and day intervals were set to 12 h.After acclimation to a standard diet for 2 weeks, all the mice were randomly divided into 6 groups (n= 8 for each group), and mice from the same groups were housed in individual cages.Mice in control group were fed normal chow (proteins 20% kcal, carbohydrates 70% kcal, fats 10% kcal, purchased from Laboratory Animal Center of Ningbo University, Ningbo, China).The other 5 groups were fed high-fat diet(HFD) (proteins 25% kcal, carbohydrates 55.8% kcal, fats 18% kcal,cholesterol 1% kcal, bile salts 0.2% kcal).At the same time, these 5 groups were administered either 0.2 mL normal saline (HFD group),simvastatin (3 mg/(kg·day), HFD + S group), orT.grandisseed oil(250 mg/(kg·day), HFD + TO250 group; 550 mg/(kg·day), HFD+TO550 group; 850 mg/kg·day, HFD + TO850 group) by oral gavage.
Body mass and food intake were measured weekly and fecal samples were collected individually and stored at –80 °C until further analysis.After 8 weeks of feeding, the mice were sacrificed and the liver and adipose tissues were excised, weighed, and immediately frozen in liquid nitrogen.Serum was isolated by centrifugation at 3 500 ×gat 4 °C for 15 min and stored at –80 °C for subsequent biochemical testing.
2.2 Fatty acids analysis
Total lipids were extracted from the livers of mice as previously described [26].After beingtrans-esterified to methyl esters with sulfuric acid-methanol solution (1:9,V/V) [27], the composition of fatty acids of theT.grandisseed oil, as well as the livers of mice was measured by a gas chromatography-mass spectrometer (GC-MS).
The gas chromatograph (Agilent, Japan) system, attached to a mass spectrometer (Persee, Beijing, China), was equipped with a DBWAX column (60.0 m × 250 μm, 0.25 μm, Agilent Technologies Inc., Santa Clara, CA, USA), as well as a hydrogen flame ionization detector.The sample injection volume was set to 1 μL with a split ratio of 1:30.The carrier gas was helium at the rate 1 mL/min.The injection temperatures of the port and detector were 250 and 260 °C,respectively.The oven temperature program was set initially at 50 °C for 2 min, then increased to 230 °C at a rate of 20 °C/min, and further maintained for 15 min.Finally, the temperature was increased to 250 °C at a rate of 3 °C/min and maintained for 9 min to complete the process.
Mass spectrograms were recorded in the EI mode at 70 eV with a mass scan range of 45–450 amu.The temperature of the ion source was 200 °C, and each mass spectrogram corresponding to its chromatographic peak was qualitatively determined by a computer chart-base.The relative content of each component was calculated by the peak area normalization method according to its total ion current chart.
2.3 Biochemical analysis
The levels of high-density lipoprotein cholesterol (HDL-C), lowdensity lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triacylglycerols (TG) in serum were measured using corresponding assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China).
2.4 Liver histopathology
Liver tissues were fixed in 4% paraformaldehyde, dehydrated in graded alcohol, rendered transparent with xylene, soaked in wax, and then embedded in paraffin.Tissue sections (4 μm) were stained with hematoxylin and eosin (H&E).Next, the sections were dewaxed by xylene, decolorized in graded alcohol, stained by H&E, dehydrated,transparentized, and then examined under a light microscope(RM2235, Leica, Germany) for histopathologic observation.
2.5 16S rDNA sequencing for gut microbiota
Total bacteria DNA was extracted from fecal samples by using the E.Z.N.A.®Stool DNA Kit (D4015-02, Omega, Inc, USA) in accordance with the manufacturer’s instructions.The quality of extracted DNA was measured using Qubit (Invitrogen, USA).
PCR primers were designed based on the V3 and V4 hypervariable regions of the bacterial 16S rRNA gene.The primers, 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R(5’-GGACTACHVGGGTWTCTAAI-3’) [28]were designed with a barcode sequence that was unique to each sample.PCR amplification was performed in a total volume of 25 μL reaction mixture containing 25 ng of template DNA, 12.5 μL PCR Premix, 2.5 μL of each primer;PCR-grade water was used to adjust the volume.The PCR conditions used to amplify the prokaryotic 16S fragments were as follows: an initial denaturation at 98 °C for 30 s; 35 cycles of denaturation at 98 °C for 10 s; annealing at 54 °C for 30 s; extension at 72 °C for 45 s;and final extension at 72 °C for 10 min [29,30].
The PCR products were confirmed using 2% agarose gelelectrophoresis, purified by means of AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA), and quantified by Qubit(Invitrogen, USA).Sequencing was performed using an Illumina MiSeq instrument (Illumina, Inc., San Diego, CA, USA) using two 300-bp paired-end sequencing runs according to the manufacturer’s instructions.
2.6 Data analysis for gut microbiota
Samples were sequenced on an Illumina MiSeq platform provided by LC-Bio Technology Co., Ltd.(Hangzhou, Zhejiang Province,China) according to the manufacturer’s recommendations.Pairedend reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence.Pairedend reads were merged using FLASH.Quality filtering of the raw tags was performed under specific filtering conditions to obtain high-quality clean tags using FastQC (version 0.10.1).Chimeric sequences were filtered using the VerSearch software (version 2.3.4).Operational taxonomic units (OTUs) were clustered using VerSearch(version 2.3.4), and 97% was chosen to define a similarity level in OTU analysis.Redundancy analysis (RDA) was performed using Canoco for Windows 4.5 (Microcomputer Power, Ithaca, NY, USA)to identify distinct and significant phylotypes associated with HFD-treated andT.grandisseed oil co-treated groups according to the manufacturer’s instructions.Alpha diversity was applied in analyzing the complexity of species diversity for each sample through 4 indices,including Chao1, Shannon, Simpson, and Observed species.Beta diversity was calculated by principle co-ordinates analysis (PCoA).All the indices in our samples were calculated employing QIIME(version 1.8.0).
2.7 Statistical analysis
All the data are presented as mean ± standard deviation (SD).Analysis of variance (ANOVA) and Tukey’s post hoc test (SPSS,version 19.0, Chicago, IL, USA) were used to analyze the data with normal distribution.Statistical significance was defined asP< 0.05.
3.Results
3.1 Fatty acid compositions of T.grandis seed oil
The fatty acid composition of theT.grandisseed oil is presented in Table 1.The result show thatT.grandisseed oil is a good source of unsaturated fatty acids (UFA) (90.79%) and mainly include oleic acid((20.63 ± 0.48)%) and linoleic acid ((57.32 ± 0.73)%).Specifically,the percentages of SCA and juniperonic acid (allcis-5,11,14,17-eicosatetraenoic acid), which belong to non-methylene-interrupted polyunsaturated fatty acids (NMIFAs), reached (8.85 ± 0.14)% and(0.28 ± 0.11)%, respectively [9].
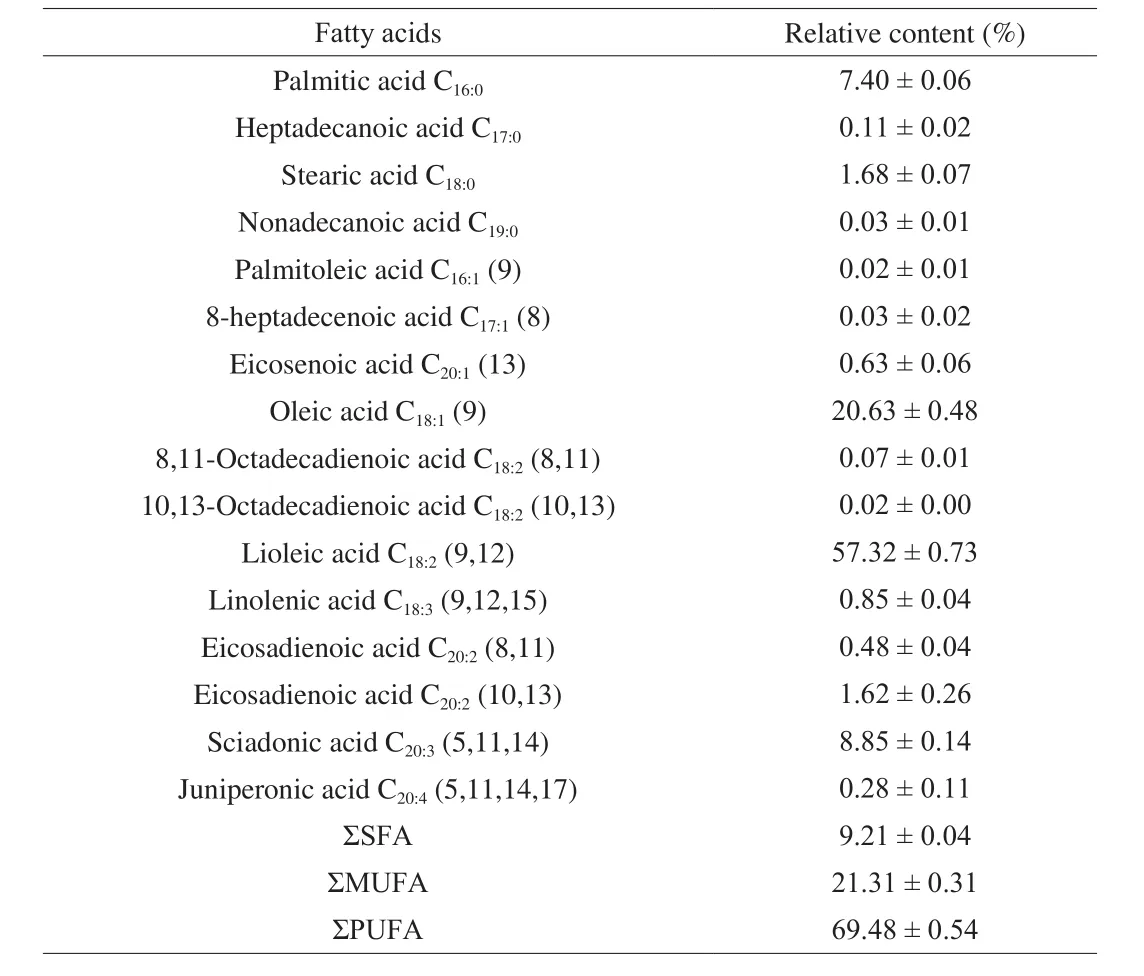
Table 1Fatty acid compositions of the T.grandis seed oil.
3.2 Effects of T.grandis seed oil on body weight, food intake,and organ index
After one week of normal feed adaptation, the experimental groups were fed a high-fat diet.Fig.1A displays the weight gains in different groups.The body mass of the mice in HFD group were always higher than those in control group ((42.39 ± 0.75) g versus(36.96 ± 0.67) g,P< 0.01), and the differences in body weight between the two groups increased concomitantly with feeding time.The rates of weight gain were 59.08%, 42.27%, and 48.78% in the HFD + TO250, HFD + TO550, and HFD + TO850 groups,respectively, while it was 62.25% in the HFD group.Meanwhile,food intake of mice was measured weekly, the results are shown in Fig.1B.The food intake of the control group was significantly higher than that of the experimental groups (P< 0.001), and no significant difference was observed among the different experimental groups.Similarly, the liver ((55.41 ± 0.77) mg/g) and adiposity index((41.38 ± 3.79) mg/g) of the HFD group were significantly higher than those of the control group ((37.43 ± 2.86), (20.17 ± 1.54) mg/g,P< 0.01).Compared with the HFD group, supplemental oils reduced the liver and adiposity indices in all the groups (Figs.1C and 1D).In addition, the HFD + TO550 group exhibited a significant efficacy in reducing weight gain and lowering liver ((42.81 ± 0.78) mg/g,P< 0.001) and adiposity ((31.78 ± 4.79) mg/g,P< 0.01) indices.

Fig.1 T.grandis seed oil prevented development of obesity in mice.(A) body mass gain; (B) food intake; (C) liver index and (D) adiposity index.Data are expressed as mean ± SD (n = 8).***P < 0.001, **P < 0.01 and *P < 0.05 versus HFD group.
3.3 Effects of T.grandis seed oil supplement on serum biochemical indices
The serum biochemical indicators of mice are depicted in Fig.2.Compared with those of the control group, TC (P< 0.01),TG (P< 0.01) and LDL-C (P< 0.001) levels in HFD group significantly increased, while HDL-C levels decreased significantly(P< 0.01); these results were consistent with the characteristics of hyperlipidemia.Compared with the HFD group, theT.grandisseed oil-treated groups showed a similar trend as that of HFD + S group and also demonstrated an evident dosage effect.Especially,the HFD + TO550 group displayed a significant reduction in LDL-C(P< 0.01), TC (P< 0.01), and TG (P< 0.01) levels, and a significant enhancement in HDL-C (P< 0.05) level.

Fig.2 Effects of T.grandis seed oil on serum biochemical indicators.(A) TC level, (B) TG level, (C) LDL-C level, and (D) HDL-C level.Data are expressed as mean ± SD.***P < 0.001, **P < 0.01 and *P < 0.05 versus HFD group.
3.4 Effects of T.grandis seed oil supplement on fatty acid composition in livers
Fatty acid composition of the liver is presented in Table 2.In the HFD group, the levels of SFA were significantly increased ((42.04 ±0.71)% versus (39.47 ± 0.49)%,P< 0.05), while PUFA levels were significantly decreased ((44.18 ± 0.53)% versus (47.99 ± 0.21)%,P< 0.05) than those in the control group.However, no significant differences were observed in the levels of MUFA.Furthermore, the levels of SFA decreased (P< 0.05) in the HFD + TO250, HFD +TO550, and HFD + TO850 groups, while PUFA levels significantly increased (P< 0.05) in the HFD + TO250 and HFD + TO550 groups than those in the HFD group.Additionally, higher levels of the MUFA were observed in mice fed withT.grandisseed oil, (16.10 ±0.54)% in HFD + TO250 group, (19.20 ± 0.45)% in HFD + TO550 group, and (20.98 ± 0.37)% in HFD + TO850 group compared with(13.78 ± 0.57)% in HFD.Notably, considerable amounts of SCA((4.11 ± 0.26)%, (3.84 ± 0.27)%, (4.29 ± 0.50)%) were accumulated in allT.grandisseed oil-treatment groups.However, the arachidonic acid (AA) was significantly lower (P< 0.05) in the mice fed withT.grandisseed oil than that in HFD group.

Table 2Fatty acid compositions in the liver of mice.
3.5 Effects of T.grandis seed oil supplement on histopathology of livers
Images of liver sections stained with H&E at magnifications 100 × and 200 × are shown in Fig.3.The livers of the control group were clear, showing a normal morphology, and the hepatocytes radiated from the central venous and arranged in a regular and orderly way.In the HFD group, the structure of hepatic cord was disordered,and there were many white neutral lipids between the cytoplasm,forming vacuoles and a few ballooned hepatocytes.The nuclei were squeezed to one side and showed obvious fatty degeneration.In the HFD + TO250 group, some white neutral lipids were also observed; however, the proportion was much lower than that in the HFD group.Furthermore, the tiny neutral lipids were found in the HFD + TO550 group occasionally, and the phenomenon of microvesicular fat accumulation was reduced compared with that in the HFD + TO250 group.

Fig.3 The H&E staining of liver sections in control and experimental groups (A, 100 ×; B, 200 ×).
3.6 Effects of T.grandis seed oil supplementation on the diversity and composition of the gut microbiota in HFD-fed mice
The community structures of fecal microbiota in mice from different treatment groups were examined by 16S rRNA sequence analysis.Principal co-ordinates analysis (PCoA) was then used to compare the differences among the fecal microbial community structures, the results are presented in Fig.4.Our results suggested that the gut microbial community structures of the control group clearly deviated from that of the HFD group.A similar microbiota composition was observed among the control, HFD + TO250, as well as HFD + TO550 group.However, the community structure of the HFD + TO850 group diverged considerably, not only from the HFD group, but also from the control group.

Fig.4 Community structures of gut microbiota in different groups.PCoA based on unweighted unifrac metrics.Different colors represent different groups.The higher the similarity among samples, the closer the cluster they form in the graph, and vice versa.
The dominant bacteria of the gut microbiota in different groups are presented in Fig.5.At the level of the phylum (Fig.5A), fecal microbiota mainly included Firmicutes, Bacteroidetes, Actinobacteria,and Proteobacteria; these 4 phyla accounted for more than 98% bacteria in all the groups.After 8 weeks of HFD intervention, the abundance of Bacteroides increased from 42.00% to 51.66%, while that of Firmicutes decreased from 49.27% to 39.83%.Compared with the HFD group, the Firmicutes (P< 0.05) and Actinobacteria(P< 0.05) phyla increased, while Bacteroidetes (P< 0.05) and Proteobacteria decreased both in the HFD + TO550 as well HFD +TO850 groups, which suggests that theT.grandisseed oil treatment groups were able to reverse the changes in gut microbiota caused by the high-fat diet.
At the family level (Fig.5B), Porphyromonadaceae,Erysipelotrichaceae, Lachnospiraceae, Lactobacillaceae,Coriobacteriaceae, Rikenellaceae, Ruminococcaceae, and Prevotellaceae were the dominant families in the control group,which accounted for 85.87% of gut microbiota.The high-fat diet led to a higher abundance of Porphyromonadaceae (43.95% versus 31.78%, HFD group versus control group), but lower abundance of Erysipelotrichaceae (26.58% versus 34.41%), Lactobacillaceae(2.04% versus 4.27%), Coriobacteriaceae (1.61% versus 2.81%)and Prevotellaceae (1.42% versus 2.07%).However, in the groups that supplemented withT.grandisseed oil, an opposite trend in terms of microbiota compositions was observed.The abundance of Porphyromonadaceae was decreased to 15.57%, 10.96%, 6.97%(P< 0.05), while that of Erysipelotrichaceae (20.32%, 36.55%,38.75%,P< 0.05) and Coriobacteriaceae (7.65%, 1.94%, 3.07%,P< 0.05) were significantly increased, compared with HFD group.Additionally, a significantly higher abundance of the beneficial bacteria, Lactobacillaceae (20.52%,P< 0.05) and Bifidobacteriaceae(10.88%,P< 0.01) were found in the HFD + TO850 group.

Fig.5 Changes in the abundance of gut microbiota in mice.(A) phylum levels; (B) family levels.
Through redundancy analysis (RDA), 92 OTUs were determined to be related to diseases induced by high-fat diet (Fig.6).Among them, 54 OTUs were recovered afterT.grandisseed oil treatment, and the effects of restoration of 30 OTUs was consistent with the doses.Sixteen OTUs, which were enriched due to the oil supplementation,belonged toLactobacillus,Faecalibaculum,Bifidobacterium_pseudolongumandAllobaculum.Compared with the HFD group,the groups supplemented with TO250, TO550, TO850 altered the abundance of 55, 61, and 75 OTUs, respectively.
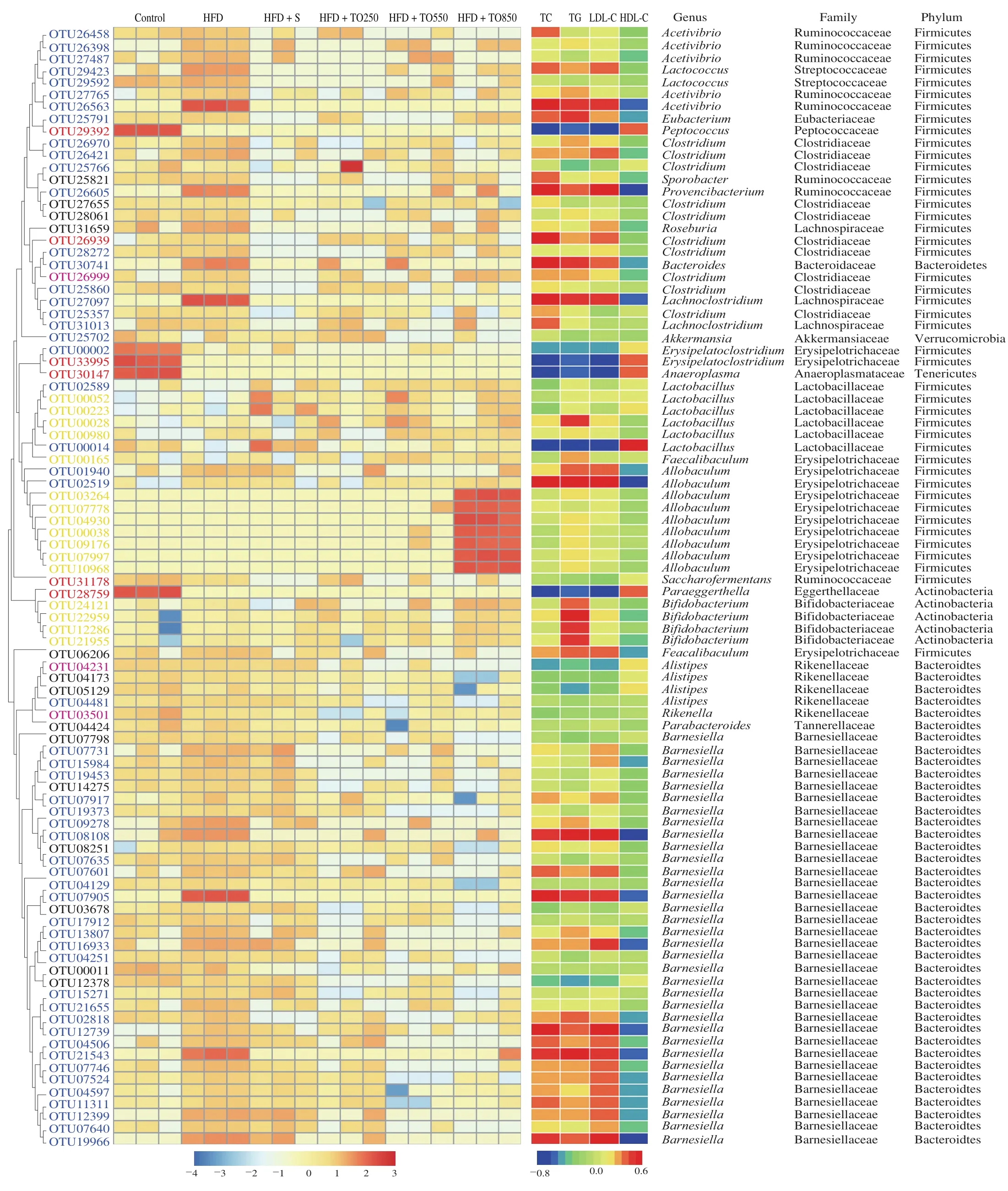
Fig.6 Heat map of the RDA-identified key OTUs in the feces responding to different T.grandis seed oil doses, and Spearman’s correlations between OTU abundance and hyperlipidemia symptoms.The colored squares on the left indicate the normalized and log-transformed relative OTU abundance in each group.The OTUs are ordered via phylogenetic positions.Blue, OTUs where T.grandis seed oil treatment restored HFD-induced increase/decrease; purple, OTUs where T.grandis seed oil treatment aggravated HFD-induced increase/decrease; yellow, OTUs where T.grandis seed oil treatment significantly increased their abundance compared with those in HFD group; black, OTUs where T.grandis seed oil treatment significantly decreased their abundance compared with those in HFD group; red, OTUs where T.grandis seed oil treatment did not restore changes in their abundance due to a high-fat diet.The square color on the right panel represents Spearman’s correlation R-value between OTU abundance and hyperlipidemia symptoms using SPSS software.The phyla, families, and genera are listed on the right.*P < 0.05 **P < 0.01.
Spearman’s correlation analysis was used to explore the correlation between intestinal microbial changes and serum biochemical indicators.Among them, 29 OTUs were significantly correlated with at least one biochemical indicator, and 10 OTUs(OTU26563, OTU30741, OTU27097, OTU00014, OTU02519,OTU08108, OTU07905, OTU12739, OTU21543, and OTU19966),belonging toAcetivibrio,Bacteroides,Lachnoclostridium,LactobacillusandAllobaculumwere significantly correlated with 4 serum biochemical indicators (TC, TG, HDL-C, and LDL-C levels).Twenty-two OTUs were positively correlated with indicators of hyperlipidemia, as they were associated with increased TC, TG, and LDL-C and decreased HDL-C levels, while 7 OTUs were negatively correlated with hyperlipidemia.
Notably, 9 of the 22 OTUs (includingAcetivibrio,Provencibacterium,LachnoclostridiumandBarnesiella) were positively correlated with indicators of hyperlipidemia, demonstrated a decreased abundance in all the oil-treated groups.Similarly,among the 7 OTUs having a negative correlation with indicators of hyperlipidemia, 2 OTUs were found in groups supplemented with oil, and at least one group showed a recovery of intestinal microbial abundance belonging toErysipelatoclostridiumandLactobacillus.
4.Discussion
Excessive intake of high-fat diet has undoubtedly contributed to the obesity epidemic, and obesity is associated with a series of chronic diseases, such as diabetes, hyperlipidemia, and cardiovascular disease.Recent studies provide evidence to support the role of gut microbiome in driving HFD-induced chronic diseases [31].In this study, ICR male mice were fedT.grandisseed oil for 8 weeks and the changes in body mass, adiposity index, liver index, fatty acids, and gut microbiota in both the HFD and oil-treated mice were estimated.It was apparent that the high-fat diet caused obesity in mice, their body mass increased by 62.25% ((26.13 ± 0.34) g to (42.39 ± 0.75) g)after 8 weeks.The gain in body mass (P< 0.01), adiposity index(P< 0.001) and liver index (P< 0.01) were significantly reduced in HFD + TO550 group.These results suggest that theT.grandisseed oil supplement effectively prevented the development of obesity in mice.It is well-known that restraining appetite is one of the factors for managing obesity.In this study,T.grandisseed oil supplementation did not affect food intake in mice.Appetite-suppressing drugs increase tolerance and dependency as indicated in long-term clinical trials [32].Additionally, the fecal samples were also observed and collected and no side effects like diarrhea were found in all the experimental groups.Therefore, we have proposed that dietary supplementation ofT.grandisseed oil would be a much safer way to alleviate obesity.
Hyperlipidemia is characterized by increased TC, TG, and LDL-C levels, and decreased HDL-C level in serum [33,34].In this study,decreased serum TC (–24.8%), TG (–30.7%), and LDL-C (–26.9%),and increased serum HDL-C (42.2%) were observed in the mice withT.grandisseed oil supplementation.
Meanwhile, H&E staining of liver sections indicated that this oil supplement significantly reduced lipid droplets induced by a high-fat diet.Especially, improvement of hepatic steatosis was more obvious in the HFD + TO550 group than in HFD + TO250 group.These results demonstrated that the dietary supplement ofT.grandisseed oil attenuated lipid metabolism disorder in liver cells and displayed evident inhibition of HFD-induced morphologic changes.The fatty acids composition of the liver was further estimated.It was observed that the levels of SCA in theT.grandisseed oil-treated groups were significantly increased (3.24%, 3.84%, and 4.29% in HFD + TO250,HFD + TO550, and HFD + TO850 groups, respectively) accompanied with a decrease in the levels of AA (10.75%, 10.34%, and 6.53% in HFD + TO250, HFD + TO550, and HFD + TO850 groups) as compared to the HFD group.Our results are consistent with those research results of the Endo et al.[19,35].The effects of SCA-containing seed oils, as well as pure SCA, on rat lipid metabolism have been investigated by them.Their results demonstrated that the serum and liver triacylglycerol levels were lower in the rats fed with whether SCA-containing seed oil or pure SCA.These findings indicated thatT.grandisseed oil could modify the lipid metabolism and SCA is one of the important mediators.
SCA is a rare NMIFA of all-cis-5,11,14-eicosatrienoic acid which lacks the Δ8 double bond of arachidonic acid [36].SCA is widely distributed in seeds of most Podocarpaceae and Pinaceae plants [15].The metabolic behavior of SCA has been investigatedin vivoandin vitroby Tanaka [36]and Endo [19]et al.Their results suggested SCA might be effective substitutes for arachidonic acid in phosphatidylinositol.Our results support their hypothesis.
In their study, the effects of SCA on lipid metabolism in rats were analyzed, and the results demonstrated that SCA might have inhibited the synthesis of AA.Tanaka et al.[36]found that SCA reduced the proportion of AA by one half, from 15.9% to 8.7%, in cultured hepatoma HepG2 cells.This result is probably due to the fact that the arachidonyl moiety of AA-containing phosphatidylinositol molecules was significantly substituted by SCA.Consequently, the properties of the membrane, and fatty acid synthesis and catabolism may have altered.
Furthermore, the effects of theT.grandisseed oil on the composition and diversity of gut microbiota were studied.Among the 92 key OTUs identified by RDA, 16 OTUs belonging toLactobacillus,Faecalibaculum,BifidobacteriumandAllobaculumwere enriched due to the oil supplement.Studies have shown that diet can modify intestinal microbiome, which in turn has a profound impact on overall health [37].Current literature also supports the influence of gut microbiota on cardiovascular disease; this is thought to occurviathe sensing of gut microbial-derived products by the host receptor system [38].Among these OTUs, the increase of beneficial bacteria, such asLactobacillusandBifidobacteriumhave been proved to have a positive impact on diseases, such as obesity.A study by Cani et al.[39]suggested that oral administration of inulin-type fructans significantly increased the abundance ofBifidobacteriumspp., which essentially prevented HFD-induced obesity in mice.Moreover, the genera ofLactobacillus,BifidobacteriumandAllobaculumare shown to be short-chain fatty acid (SCFA) producers in the intestinal tract.Various studies indicated that the gut microbiota benefits humansviaSCFA production and that the deficiency in SCFA production is associated with diseases, such as obesity [40,41].SCFA is a subset of saturated aliphatic fatty acids possessing up to 6 molecules of carbon.A systematic review using the PubMed/Medline database was performed by Gabriel and Fantuzzi [42]to uncover the relationship between SCFA and leptin levels and production.In 8in vitrostudies, SCFA was found to increase leptin expression in adipocytes.Furthermore, in 24 animal studies, interventions to modulate highfat diet outcomes predominantly caused an increase in SCFA levels and suppressed weight gain.SCFA is also known to be secreted into the bloodstream and travel to the liver, where it plays a role in energy metabolism [43].These data indicate that SCFA-producing bacteria may contribute to obesity, insulin resistance, and alleviation of inflammation by reducing secretion of intestinal endotoxins into the blood [44,45].
5.Conclusion
Taken together, the results of this study demonstrate thatT.grandisseed oil may affect obesity and alleviate hyperlipidemia through gut microbiota, especially by altering the abundance of bacterial at the genus level, such asLactobacillus,Faecalibaculum,BifidobacteriumandAllobaculum.Obesity development and alleviation presented differences in the gut microbiota, and further studies are needed to address the causality between them.
Acknowledgements
The authors are thankful to HeWang Torreya Company Inc.(Zhejiang, China) for providing ChineseTorreya grandiscv.Merrilliiseed oil.This work was supported by the Regional Demonstration Project of Marine Economic Innovation and Development (2013 and 2016), and the K.C.Wong Magna Fund offered by the Ningbo University.
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
Animal experiments were performed in accordance with the National Guideline for Experimental Animal Welfare and approved by the Institutional Review Board of Ningbo University Laboratory Animal Center.
杂志排行
食品科学与人类健康(英文)的其它文章
- Effects of dietary fiber on human health
- Tea polyphenol - gut microbiota interactions: hints on improving the metabolic syndrome in a multi-element and multi-target manner
- Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways
- Milled flaxseed-added diets ameliorated hepatic inflammation by reducing gene expression of TLR4/NF-κB pathway and altered gut microbiota in STZ-induced type 1 diabetic mice
- Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice
- Effects of soy hull polysaccharide on dyslipidemia and pathoglycemia in rats induced by a high-fat-high-sucrose diet
