Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: A review
2022-07-30RishaGangulyAshutoshGuptaAbhayPandey
Risha Ganguly, Ashutosh Gupta, Abhay K Pandey
Abstract Baicalin is a natural bioactive compound derived from Scutellaria baicalensis,which is extensively used in traditional Chinese medicine. A literature survey demonstrated the broad spectrum of health benefits of baicalin such as antioxidant, anticancer, anti-inflammatory, antimicrobial, cardio-protective, hepatoprotective, renal protective, and neuroprotective properties. Baicalin is hydrolyzed to its metabolite baicalein by the action of gut microbiota, which is further reconverted to baicalin via phase 2 metabolism in the liver. Many studies have suggested that baicalin exhibits therapeutic potential against several types of hepatic disorders including hepatic fibrosis, xenobiotic-induced liver injury, fatty liver disease, viral hepatitis, cholestasis, ulcerative colitis, hepatocellular and colorectal cancer. During in vitro and in vivo examinations, it has been observed that baicalin showed a protective role against liver and gut-associated abnormalities by modifying several signaling pathways such as nuclear factor-kappa B,transforming growth factor beta 1/SMAD3, sirtuin 1, p38/mitogen-activated protein kinase/Janus kinase, and calcium/calmodulin-dependent protein kinase kinaseβ/adenosine monophosphate-activated protein kinase/acetyl-coenzyme A carboxylase pathways. Furthermore, baicalin also regulates the expression of fibrotic genes such as smooth muscle actin, connective tissue growth factor, βcatenin, and inflammatory cytokines such as interferon gamma, interleukin-6 (IL-6), tumor necrosis factor-alpha, and IL-1β, and attenuates the production of apoptotic proteins such as caspase-3, caspase-9 and B-cell lymphoma 2. However,due to its low solubility and poor bioavailability, widespread therapeutic applications of baicalin still remain a challenge. This review summarized the hepatic and gastrointestinal protective attributes of baicalin with an emphasis on the molecular mechanisms that regulate the interaction of baicalin with the gut microbiota.
Key Words: Baicalin; Biotransformation; Gut microbiota; Hepatobiliary and gastrointestinal disorders; Signaling pathways
lNTRODUCTlON
The liver is the largest and central digestive organ in the body, which plays a vital role in several physiological processes including growth, nutrition, immunity, and metabolism of xenobiotics[1-3]. The hepatobiliary system mainly consists of the liver, and intra-hepatic and extra-hepatic bile ducts including the gall bladder. The liver in association with the intestine plays an essential role in digestion with the help of digestive enzymes, which facilitate the breakdown of larger biomolecules into simpler forms such as monosaccharides, amino acids, fatty acids, and glycerol. The intestinal microbiota also interacts with bile and other digestive juices, aiding the process of digestion. The complex network of molecular pathways and signal molecules that are involved in the functioning of the hepatobiliary system are also part of the immune cascade[4-6]. Therefore, any disruption in the gastrointestinal (GI)tract or gut microbiota results in the generation of an inflammatory response. Hepatic disorders such as fibrosis, viral hepatitis, non-alcoholic fatty liver, cirrhosis, cholestasis, and hepatocellular carcinoma(HCC) can be identified by alteration in the levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-1β, and nuclear factor-kappa B (NF-κB)[7,8]. Researchers in the past two decades have found numerous natural compounds, which have the ability to interact with the gut microbiome and aid in the treatment of diseases of the hepatobiliary system[9,10]. Natural products and their derivatives form a group of compounds known as secondary metabolites produced by the plants. Several such metabolites such as silymarin, ellagic acid, phyllanthin, rutin, and glycyrrhizin have been used to treat hepatic fibrosis, viral hepatitis, fatty liver disease, and cirrhosis[11,12].
Baicalin (5, 6-dihydroxy-7-O-glucuronide) is a flavonoid isolated predominantly from the roots ofScutellaria baicalensis(S. baicalensis), a Chinese medicinal herb that belongs to the family Lamiaceae and is widely known as Chinese skullcap[13]. The roots ofS. baicalensisalso contain several other significant bioactive molecules such as baicalein and wogonin[14]. Numerousin vitroandin vivostudies have indicated different pharmacological properties of baicalin, which include anti-oxidative, antiviral, antiinflammatory, cardioprotective, hepatoprotective, neuroprotective, and pro-apoptotic properties. These biological activities can be attributed to the ability of baicalin to target multiple pathways and bind with several signaling molecules[15-18]. In addition, baicalin possesses anti-obese, antidyslipidemia, and proapoptotic effects, which help to improve hepatic function after injury, alleviate liver diseases due to alcohol abuse, and promote apoptosis of proliferating hepatocytes[19].
In the last two decades, there has been growing interest and research on the hepatoprotective and anticancer properties of baicalin indicated by the increasing number of publications on PubMed. In recent years (2017 to present), there has been a remarkable rise in the number of research and review articles on the biological potential of baicalin as shown in Figure 1. This review gives an account of the therapeutic effects of baicalin exerted on the hepatobiliary system and the mitigation of GI and liverassociated disorders. The use of baicalin alone or in combination with drugs in severalin vitroandin vivoexperiments in the last two decades, impact of baicalin on the gut microbiota, its interaction with molecules and receptors at different molecular pathways, and the range of doses at which baicalin has shown maximum activity have also been discussed.
SOURCES OF BAlCALlN

Figure 1 lncreasing trend of publications on “hepatoprotective and anticancer properties of baicalin” indexed by PubMed.
Baicalin is the most abundant and important bioactive ingredient obtained from the roots of the medicinal plantS. baicalensis[20]. The roots ofS. baicalensispossess baicalin in the range of 8% to 15%.Baicalin is also the main component of other species ofScutellariasuch asS. rivularia,S. galericulata, andS. lateriflora[21,22]. Baicalin, chrysin, and its glucoside derivatives have also been obtained from various other parts of the popular Asian medicinal plantOroxylum indicum, belonging to the family Bignoniaceae[23]. Baicalin, and its aglycone baicalein, are gaining increasing importance in the pharmaceutical, food, and cosmetics industries due to their remarkable biological properties. Baicalin and baicalein, in particular, have shown anti-inflammatory effects and the potential to ameliorate mitochondrial dysfunction[24], and combination strategies with baicalin or baicalein as chemotherapy adjuncts have been shown to be effective in various cancers and associated signaling pathways[25]. Due to growing interest in the properties of baicalin as a potential therapeutic agent, many studies in last few years have focused on developing appropriate techniques for the identification and quantification of baicalin in raw drug formulations including simple thin layer chromatography, and different modifications of the sophisticated technique of high-performance liquid chromatography[26,27].
CHEMlSTRY AND BlOAVAlLABlLlTY
Baicalin is a flavone glycoside (molecular mass = 446.4 g/moL; melting point = 202-205 °C), which is hydrolyzed to its aglycone baicalein in the stomach after ingestion. Baicalin is hydrolyzed to baicalein immediately after administration with the help of β-glucuronidase from gut bacteria. Baicalein is reconverted to baicalin in the systemic circulation by uridine 5'-diphospho(UDP)-glucuronosyltransferase-glucuronosyl transferaseviaphase 2 metabolism[21]. It is noteworthy that the circulating baicalin in the system is not the parent molecule but the conjugated metabolite of baicalein. Circulating baicalin returns to the GI system primarily by bile excretion in the form of glucuronides. The bile excretion of baicalin is mainly mediated by the multidrug resistance (MDR) protein 2 transporter. When baicalin and baicalein are given orally, the conjugated metabolites actually contribute to thein vivoeffect because the glucuronide/sulfate of baicalin circulates predominantly in plasma[25]. Baicalin is moderately absorbed in the stomach and poorly absorbed in the small and large intestines (Figure 2).
PHARMACOKlNETlCS OF BAlCALlN lN THE Gl SYSTEM
The pharmacokinetic profile of baicalin in the GI system involves hydrolysis, enterohepatic recycling,carrier-mediated transport, and complex routes of metabolism with the interaction of gut microbiota.Baicalin administration is safe and endurable, and no evidence of liver or kidney toxicity has been recorded. The main obstacles to the clinical use of baicalin are its low water solubility (approximately 67.0 μg/mL) and bioavailability. Several nano-techniques such as solid nanocrystals, nanoemulsions,and lipid-based solid nanoparticles have been used to improve baicalin lysis, thus improving bioavailability[28-30]. Incomplete absorption in the GI system has emerged as the main barrier to bioavailability[31]. MDR protein 2 is the most important transporter of baicalin, which mediates bile outflow to hepatocytes[32]. In fact, biliary excretion of baicalin in rats with MDR protein 2 deficiency is significantly reduced with a significant increase in plasma baicalin levels[33]. Baicalin is also capable of crossing the blood-brain barrier and may be protective against a variety of neurodegenerative diseases[34,35].
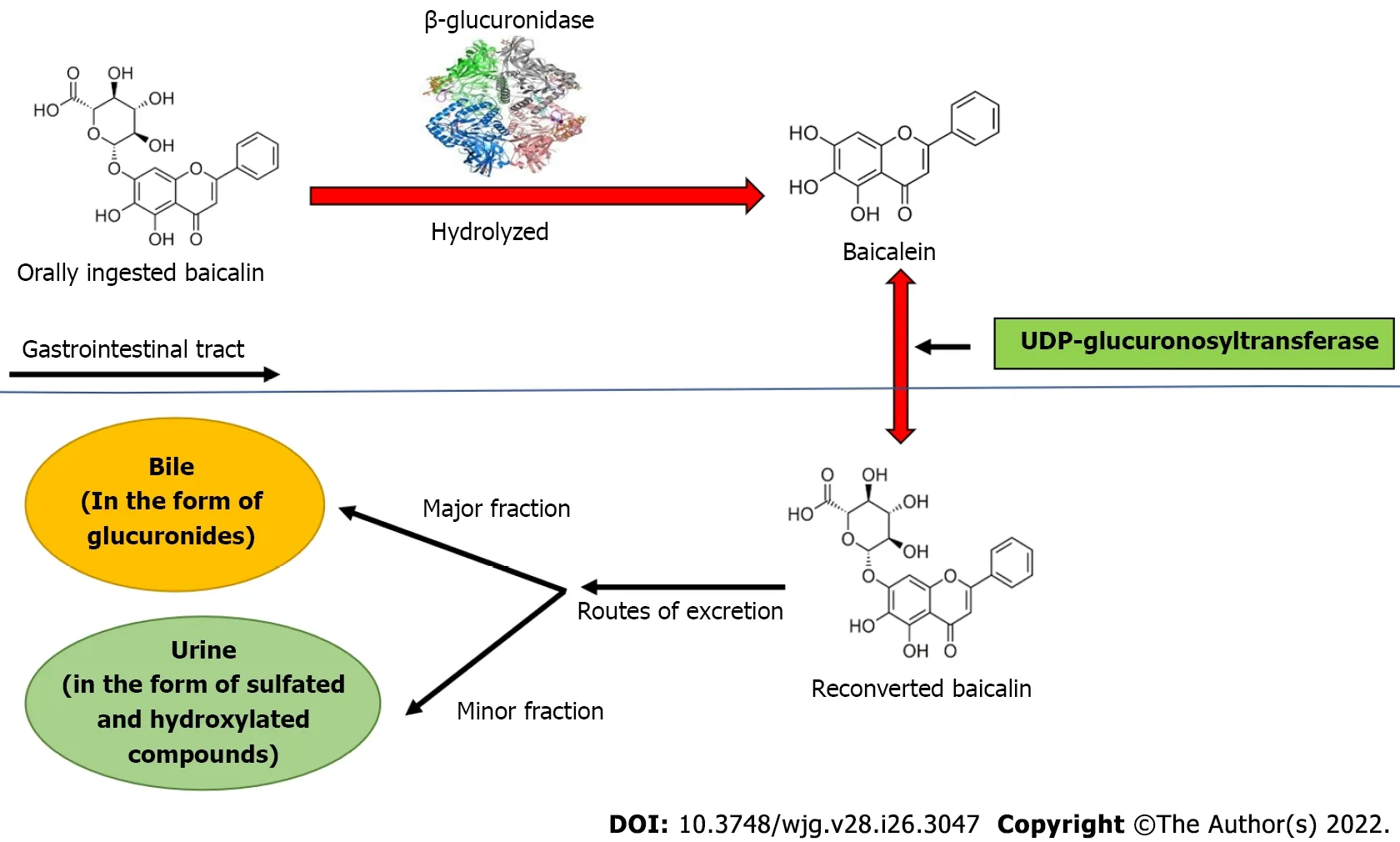
Figure 2 Biotransformation of baicalin after oral ingestion. Orally administered baicalin is hydrolyzed to baicalein by β-glucuronidase. Further, baicalein is reconverted to baicalin by uridine 5'-diphospho-glucuronosyltransferase in intestine. The major part of baicalin excretion takes place via the biliary route in the form of glucuronides, and a small fraction of baicalin is excreted via urine in the form of sulfated and hydroxylated compounds. UDP: Uridine 5'-diphospho.
β-glucuronidase and UDP-glucuronosyltransferase are important metabolic enzymes involved in thein vivotransformation of baicalin. In fact, five bile metabolites have been identified in rat liver after baicalin administration. The main metabolites include baicalein 6,7diβ-glucopyranuloside and 6oβglucopyranuronosyl baicalein-7 sulfate. These conjugated metabolites are hydrolyzed to baicalein in the GI tract by β-glucuronidase/sulfatase. Thirty-two baicalin metabolites have been reported in plasma,urine, and other rat tissues using more efficient approaches[36,37]. In terms of tissue distribution, liver and kidney contain most of the metabolites, indicating that these are the major sites of baicalin metabolism. Baicalin undergoes multiple chemical transformationsin vivoincluding hydrolysis,methylation, hydroxylation, methoxylation, glucuronide conjugates, sulfate conjugates, and their combined reactions[37]. The pharmacokinetics of baicalin may help to understand its therapeutic implications in the liver. As a result of enterohepatic circulation, baicalin remains particularly concentrated in the liver and is thus beneficial in the treatment of hepatic anomalies. Baicalein also plays a crucial role in the treatment of hepatic diseases, indicating that the mechanism of baicalin's emphasis on liver-associated disorders may include the ameliorative effects of the metabolites as well. The major part of baicalin excretion takes placeviathe biliary route in the form of glucuronides, and a small fraction of baicalin is excretedviaurine in the form of sulfated and hydroxylated compounds[38,39].
AMELlORATlNG EFFECTS OF BAlCALlN AGAlNST HEPATlC AND COLORECTAL DlSEASES
Fatty liver syndrome
Fatty liver syndrome (FLS) occurs due to excess accumulation of non-esterified fatty acids in the hepatocytes. FLS accounts for approximately one-fourth of all liver-related anomalies in the world[40].FLS causes several hepatic anomalies such as non-alcoholic fatty liver disease, non-alcoholic steatohepatitis (NASH), and advanced FLS may also lead to cirrhosis and ultimately hepatic failure[17,41]. Due to its antioxidant and hepatoprotective potential, baicalin is effective against FLS and associated diseases.Baicalin improves lipid metabolism and suppresses hepatic lipid production by inhibiting the calcium/calmodulin-dependent protein kinase kinase-β/adenosine monophosphate-activated protein kinase (AMPK)/acetyl-coenzyme A (CoA) carboxylase pathway[42,43]. Additionally, baicalin binds directly to carnitine palmitoyl-transferase-1α (CPT-1α) and promotes the influx of lipid into mitochondria, where it is oxidized[44]. Baicalin also downregulates lipid-producing genes such as fatty acid synthase (FASN), peroxisome proliferator-activated receptor-α (PPAR-α), and sterol regulatory element-binding protein-1c (SREBP-1c) to inhibit lipid accumulation in the liver[42,45]. Baicalin (200 mg/kg) has been found to reduce the expression of TNF-α, monocyte chemoattractant protein (MCP-1)and IL-1β, downregulate caspase-3 to alleviate NASH induced by a methionine- and choline-deficient(MCD) diet. In addition, treatment with baicalin can partially lessen the accumulation of lipids induced by the MCD diet in the liver by modulating the expression of SREBP-1c, FASN, PPAR-α, and CPT-1α[46]. Moreover, baicalin (50 mg/kg) also reduces the synthesis of inflammatory cytokines including TNF-α, IL-6, and IL-1β, and suppresses the Toll-like receptor 4 (TLR-4) signaling pathways in MCD diet fed mice to inhibit fat accumulation in liver. Thus, baicalin also acts as an anti-inflammatory compound in the attenuation of non-alcoholic FLS[47]. In a study of high-fat diet induced FLS, it was found that baicalin (25-100 mg/kg) exerted a substantial ameliorating effect on FLS by activating the expression of hepatic PPAR-γ receptors[48]. In addition, baicalin (5 g/kg) also alleviates FLS by reducing the levels of serum hepatic enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT),and inflammatory mediators such as TNF-α and MCP-1. Moreover, baicalin also inhibited the phosphorylation of Janus kinase (JNK), and suppressed the production of inflammatory enzyme cyclooxygenase-2 and pro-oxidative enzyme CYP-2E1 in the liver of a mouse model[49]. In another study of orotic acid-induced FLS, baicalin (12.5-50 mg/kg) downregulated SREBP-1c and upregulated AMPK to reduce the toxic effects of free fatty acids, subsequently inhibiting fat accumulation in the liver[45]. In anin vitrostudy of palmitic acid-induced FLS in AML-12 hepatocytes, baicalin (6.25-25 μM)alleviated FLS by reducing endoplasmic reticulum stress and suppression of the thioredoxin-interacting protein/Nod-like receptor protein 3 pathway[50].
Liver injury
The liver is the most important organ for the metabolism and elimination of toxins from the human body. Normal liver function can be deterred with excessive hepatic injuries, which occur due to a variety of factors including alcohol intake, chemical contaminants, hepatocellular ischemia, and drug damage[50,51]. Liver damage is a complex process that can manifest extensive hepatocellular apoptosis[52]. Baicalin (120 mg/kg) has been shown to alleviate alcohol-induced liver injury in a rat modelviathe reduced expression of inflammatory cytokines such as TNF-α, IL-6 and IL-1β and enhances the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px),which is further regulated by blocking the sonic-hedgehog signaling pathway[19]. Similarly, baicalin (30 mg/kg) has been shown to produce anti-inflammatory effects by downregulating the expression of IL-17 in a rat model with acetaminophen-induced liver injury[53]. Baicalin (80 and 200 mg/kg) activates heme oxygenase-1viathe nuclear respiratory factor (NRF)-2 antioxidant pathway and inhibits the activity of reactive oxygen species (ROS) generating enzymes in a liver injury model. Baicalin plays an important role in blocking the combination of NRF-2 and Kelch-like ECH-associated protein 1 (Keap-1)that causes phosphorylation of NRF-2, thus reversing liver damage[54,55]. Furthermore, baicalin (60 mg/kg) also downregulates extracellular signal-regulated kinase (ERK) in acetaminophen-induced liver injury[56]. In addition, baicalin (100 mg/kg) downregulates caspase-3 and caspase-9 and also increases the expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2). This helps to significantly reduce NG-nitro-L-arginine methyl ester-induced liver injury in rats[57].
The therapeutic effect of baicalin on reducing liver damageviathe apoptotic pathway has also been demonstratedvia in vitroexperiments (Table 1). Baicalin at 100 μmol/L regulates apoptotic proteins such as caspase-3, caspase-9, and Bcl-2 associated X (Bax), and has a considerable therapeutic impact on the hypoxic model of L02 human hepatocytes[58]. Anotherin vitrostudy with L02 hepatocytes and acetaldehyde-treated HepG2 cells were used to identify the adverse effects of baicalin (20-100 mmol/L)on progression of the epithelial-mesenchymal transition (EMT) in the liver, which is an indicator of liver fibrosis and inflammation. This study demonstrated that baicalin considerably suppressed the progression of EMT by downregulating the transforming growth factor-β (TGF-β)/Smad signaling pathway, thus ameliorating liver damage[59]. Studies have shown that baicalin (60 μM) can repair liver injury in nanosecond pulse electric field (ns-PEF)-induced damage to L02 hepatocytes by stabilizing mitochondrial transmembrane potential and prevent excess ROS production[60]. Furthermore, baicalin(5 and 25 μM) can suppress the oxidation and nitrification of the hemin/nitrite/hydrogen peroxide system and protect HepG2 cells by inhibiting lipid peroxidation and GSH depletion[61]. Another study emphasized that baicalin administration (74 mg/kg) can restore the metabolism of amino acids to normal, improve the tricarboxylic acid cycle and ameliorate acute lipopolysaccharide (LPS)-induced sepsis in mice model[62].
Liver fibrosis
Liver fibrosis occurs due to excess deposition of extracellular matrix proteins and collagen. It is characterized by hepatic tissue degeneration, inflammatory cell infiltration and hepatic cell necrosis[63,64]. It has also been confirmed that activation of hepatic stellate cells (HSCs) plays a central role in liver fibrosis[65]. In the absence of appropriate treatment, advanced liver fibrosis causes chronic hepatitis subsequently leading to liver cancer or cirrhosis[66,67]. In a recent study, it was shown that baicalin (200 mg/kg) decreased the expression of fibrotic genes such as α-smooth muscle actin (SMA) and connective tissue growth factor and inflammatory cytokines such as TNF-α, macrophage inflammatory protein-1α(MIP-1α), IL-1β, MIP-2, thereby effectively suppressing liver fibrosis induced by bile duct ligation (BDL)in mice. In addition, anin vitrostudy also showed the efficacy of baicalin in reducing the activation of HSCs and downregulating the expression of SMA, fibronectin, tissue inhibitor of metalloproteinase-1(TIMP1) protein andcollagen-1[68]. Similarly, baicalin (150 μM) reduced microRNA (miR)-3595 expression and increased the activity of the enzyme long-chain fatty acid CoA ligase 4, significantly inhibiting the activity of HSCs leading to a reduction in fibrosis in HSCT6 hepatocyte cell lines causedby platelet-derived growth factor BB[69]. Moreover, baicalin (67.5-270 μM) helps in the reduction of BDL-induced activity of HSCs by inhibiting PPAR-γviaWnt signaling, leading to alleviation of liver fibrosis[70]. Baicalin (100 mg/kg) attenuated carbon tetrachloride-induced hepatic fibrosis in mice by regulating the rise in TGF-β1, hydroxyproline, type III collagen, and hyaluronic acid laminin (Table 2).In addition, baicalin also attenuates liver fibrosis by suppressing the activity of antioxidant enzymes SOD and GSH-Px[71]. Similarly, baicalin (25-100 mg/kg) evidently reduces the level of PPAR-γ,suppresses the activity of HSCs, and downregulates the expression of TGF-β1, causing inhibition of hepatic fibrosis[72].

Table 1 In vitro hepatoprotective effects of baicalin on different cell lines
Cholestasis
Cholestasis is a condition that occurs due to the obstruction or complete blockage of bile secretion through the intrahepatic or extrahepatic bile ducts[73]. Consequently, there is excess accumulation of conjugated bilirubin, bile salts, and cholesterol in the liver, which leads to hepatic injury and damage to the human body[74]. Baicalin plays an important role at several stages to mitigate cholestasis and related hepatic damage. Baicalin specifically targets nuclear factor-erythroid factor 2-related factor 2(NRF-2) in reversing cholestasis. Recent studies in a mouse model indicate that the interaction of baicalin (50 mg/kg) with NRF-2, inflammatory cytokines, and oxidative stress regulatory elements forms the central pathway of reducing cholestasis induced hepatic damage. Baicalin is capable of activating SMA, TIMP1, and collagen, resulting in amelioration of liver fibrosis due to BDL-induced cholestasis[68]. Another pharmacokinetic study in a rat model indicated that administration of baicalin(50-200 mg/kg) has therapeutic potential for cholestasis. It significantly increases bile excretion rates,which lead to a decline in serum levels of total bile acids as well as hepatic enzymes such as AST, ALT,and alkaline phosphatase in 17α-ethinyl estradiol-induced cholestasis[75,76]. In estrogen-induced cholestasis, it has been shown that baicalin targets NF-κB and inhibits the expression of inflammatory markers such as TNF-α, IL-6, and IL-1β, thereby increasing the activity of hepatic bile acid-metabolizing enzymes. Reports have also shown that baicalin alleviates 17α-ethinyl estradiol-induced cholestasis in mice by suppressing the expression of multidrug resistance protein 2 and bile salt export pump genesviathe sirtuin 1/nuclear hepatic receptor 1α (HNF-1α)/farnesoid X receptor (FXR) pathway[77].
Hepatitis
Hepatitis caused by hepatitis A virus (HAV), hepatitis B virus (HBV), and other viral hepatitis are prevalent infectious diseases worldwide[78,79]. Liver-specific proteins and immune complex hypersensitivity have multiple roles in hepatitis[80]. Subsequent studies have proven that baicalin plays a key role in the attenuation of hepatitis by lowering the levels of hepatitis B surface antigen (HBsAg), viral antigen protein hepatitis B e-antigen (HBeAg), and hepatitis B virus (HBV)-DNA, and regulating oxidative stress, inflammation, and apoptosis in hepatic cells[80,81]. It has been found that baicalin (25-100 μM) significantly increasestaurine upregulated 1gene expression to suppress inflammation and apoptosis by downregulating the p38-mitogen activated protein kinase (MAPK) and JNK signaling pathways in L20 and transformed human liver epithelial cell lines to thwart LPS-induced hepatitis[82].Another recent study showed that baicalin (75 μg/mL) significantly suppressed HBV replication and inflammation by downregulating the NF-κB signaling pathway in HepG2 and HuH7 cells[83].Furthermore, it has also been confirmed that baicalin (10 μM) strongly suppresses the transcription of HBV by downregulating the liver-specific HNF-4α/HNF-1α axis in pHBV1.2 HepG2 cells[84]. In another experiment, baicalin (10 μg/kg) significantly reduced HBsAg and HBeAg levels in HepG2 cells,wild-type HBV cells, and young duck models infected with HBV by downregulating the HNF-4α/HNF-1α axis[85]. In addition, baicalin pre-treatment (100-200 mg/kg) attenuated elevated levels of plasma cytokines such as TNF-α, IL-6, and interferon-γ (IFN-γ) in male BALB/c mouse models, resulting in alleviation of hepatocyte necrosis and apoptosis[86]. Similarly in anin vivostudy, pre-treatment with baicalin (0.5-5.0 mg/kg) considerably reduced serum levels of ALT and AST and lowered hepatic oxidative stress in a male Sprague-Dawley rat model[86]. Another study demonstrated the pro-oxidant properties of baicalin (50-200 mg/mL) by upregulating the mitochondrial signaling pathway in human peripheral blood mononuclear cells, thereby inducing the activation of caspase-3 and apoptosis[87].Furthermore, baicalin has often been co-administered with other bioactive flavonoids in combination studies, to yield better results at prevention of hepatitis. For instance, combination of the alkaloid oxymatrine (1 g/L) with baicalin showed more efficacy against HBV than oxymatrine alone[88]. Below 31.50 μg/mL, the baicalin-phospholipid complex exhibits direct anti-duck HAV-1 activity by preventing the adsorption, replication, and release of duck HAV-1 and indirectly by promoting immunity in ducklings[89]. Baicalin (20 μg/mL) regulates the immunomodulatory effects and anti-HAV-1 reproduction by reducing the adsorption and release of HAV-1 in duck-suppressed embryonic hepatocytes[90].
HCC
HCC ranks sixth amongst the most common malignant tumors worldwide, and is the fourth highest cause of mortality due to malignancies[91]. The present modes of treatment for HCC include radiation therapy, local resection therapy, surgery, and liver transplantation. However, these treatments typically cause several side effects and have adverse consequences[91,92]. Several studies have reported that baicalin can effectively ameliorate HCC by indirectly inducing autophagy in liver tumor cells. Studies have revealed that baicalin (50 mg/kg) promotes the polarization of tumor-related macrophages into M1-like macrophages, subsequently increasing autophagy in cancerous cells to make them non-proliferating. Additionally, baicalin mediated anti-cancer effects may also be closely associated with activation of the RelB/p52 signaling pathway[93]. Similarly, baicalin (100 μmol/L) promotes apoptosis in HepG2-HCC cells by activating the ER-mediated TF-6 signaling cascade combined with S-2P protein[94]. Moreover, baicalin (160 μM) suppresses cluster of differentiation 47 and activates apoptosis and autophagy in SMMC7721-HCC cells[95]. Although nsPEFs have been developed as a new mode of treatment for cancer, they also result in the elimination of normal hepatocytes. Therefore, a study was designed combining nsPEF and baicalin for the treatment of HCC, which revealed that baicalin suppresses the proliferation of HCC cells, and protects normal liver cells by increasing mitochondrial membrane potential and reducing ROS production[59]. In recent times, cancer immunotherapy has emerged as a significant line of treatment for HCC. Baicalin (40 μM) is capable of reducing the activity of signal transducer and activator of transcription 3 protein and IFN-γ, thereby blocking the programmed death-ligand 1/programmed cell death protein 1 pathway. This increases the sensitivity of the immune system to hepatic cancerous cells and thus further activates T cells against hepatic cancer cells[96,97]. Based on severalin vitroandin vivostudies, the protective effect of baicalin on many hepatic disorders is summarized in Tables 1 and 2.
Colorectal cancer
Colorectal cancer (CRC) is also a common malignant tumor worldwide, and is primarily due to genetic inheritance, colon polyps, and ulcerative colitis (UC)[98,99]. Baicalin, due to its pro-apoptotic properties,results in the killing of CRC cells. Researchers have used the human colon cancer cell line (HCT-166)and transplanted colon tumors into mice to conduct simultaneousin vivoandin vitroexperiments to examine the antitumor mechanism of baicalin (100 and 200 mg/kg). Baicalin induces apoptosis in colon tumors by inhibiting the cells at the G1 stage and arresting EMT protein expression by blocking the TGF-β/Smad pathway[100]. Baicalin (40 mmol/L) promotes Dickkopf protein expression, suppresses the expression of proteins β-catenin and c-Myc, and inhibits miR-217 expression, thereby leading to the apoptosis of HCT-166 cancer cells by inhibition of the Wnt signaling pathway[101]. Another similar study demonstrated that apoptosis of HT-29 colon cancer cells was induced by baicalin (50-200 μM)viainhibition of c-Myc expression and regulation of the miR-10a, miR-23a, miR-31, miR-151a, and miR-205 mechanism[14]. Similarly, it has been reported that the antioxidant properties of baicalin (40 μM)increase progesterone expression in the intestine and leads to the apoptosis of HCT-116 colon cancer cells by activating the Ras/Raf/MEK/ERK pathways[102]. Reports have also shown that the antitumor activity of baicalin can be further enhanced by glycosidase pre-treatment[103]. Several studies have revealed that the development of CRC is closely associated with genetic mutations. In a study on colon cancer cells SW-480, baicalin (50-400 μg/mL) inhibited the expression of the transcription factor SP-1,leading to the apoptosis of cancer cells[104].
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a collective term that refers to the chronic inflammation of GI tract.The two major types of IBD are Crohn’s disease (CD) and UC. CD and UC are nonspecific chronic IBDs that cause inflammation and ulcers on the inner lining of the large intestine[105-107]. Baicalin plays an important role in the treatment of IBD, as it is capable of suppressing oxidative stress, immune regulation, and its anti-inflammatory properties. In addition, baicalin is capable of regulating NF-κB activation, which modulates both autophagic and inflammatory processes in intestinal epithelial cells,subsequently leading to enhancement in paracellular permeability. Baicalin alleviates dextran sulfate sodium (DSS)-induced UC by modulating the polarization of M1 macrophages to the M2 phenotype[107]. Dose-dependent administration of baicalin (30-90 mg/kg) has been found to largely downregulate the inflammatory cytokines IL-1β and TNF-α, and apoptotic genes Bcl-2 and caspase-9 in the colon tissue of rats affected by 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced UC. In addition, baicalin also inhibits the inhibitory κB (IKB) kinase (IKK)/IKB/NF-κB signaling pathway, leading to the alleviation of IBD[107,108]. Likewise, in another study, rats with TNBS-induced UC were given baicalin(5-20 mg) which resulted in the downregulation of inflammatory factors TNF-α, IL-6 and IL-1β in rat intestine and inhibition of the TLR4/NF-κB signaling pathway, leading to alleviation of UC[109].Furthermore, baicalin (30-120 mg/kg) has also been effective in the treatment of TNBS-induced UC by promoting antioxidant enzymes such as catalase, SOD and GSH-PX, and reducing malondialdehyde(MDA). Baicalin also suppresses the regulation of apoptosis by upregulating Bcl-2 and downregulating TGF-β and Bax[110]. In a UC model generated by high temperature and humid environment, baicalin(100 mg/kg) significantly reduced the serum levels of IL-6, IL-1β, and IL-17, and inhibited SOD, GSHPX, and MDA. That study attributed the anti-inflammatory effect of baicalin to suppression of the NFκB and MAPK pathways[111]. In anin vivostudy, baicalin (50-150 mg/kg) reduced myeloperoxidase activity, nitric oxide content, and elevated IL-1β, TNF-α and IL-6 levels in the colon of DSS-induced UC rats[112]. Another study revealed that baicalin (100 mg/kg) attenuated DSS-induced UC by blocking the TLR-4/NF-κB-p65/IL-6 signaling pathway and suppressing TNF-α, IL-6, and IL-13 mRNA expression[113]. Furthermore, baicalin (10 mg/kg) downregulated the expression of macrophage migration inhibitory factor, MCP-1 and MIP-3a in the colon tissue of TNBS-induced UC rat model[114]. Several reports have suggested the association of T helper 16 cell (Th17)/regulatory T cell (Treg) equilibrium with UC. Baicalin (20-100 mg/kg) regulates the Th17/Treg balance by inhibiting the rise in ROS and MDA, whereas simultaneously reducing GSH and SOD levels and regulating the expression of Th17-related factors IL-6 and IL-17 in TNBS-induced UC rats[115,116]. In a clinical study of UC patients,baicalin promoted the production of immune cells like CD4+ and CD29+ and induced immunomodulation to alleviate UC[117] (Figure 3).
lNTERACTlON OF BAlCALlN WlTH THE lNTESTlNAL MlCROBlOTA
In the past decade, the intestinal microbiota has become an emerging aspect of research for the evaluation of several diseases. Besides playing an important role in the metabolism and breakdown of biomolecules into simpler molecules like fatty acids, amino acids, vitamins and bile salts, the gut microbiota is also capable of interacting with the host and affect the functioning of various organs including the liver and kidney to regulate homeostasis and disease development[5,118]. Baicalin, a flavonoid, exerts many therapeutic effects by modulating gut microbiota homeostasis. Intake of high fat diet causes imbalance of the gut microbiota, leading to several metabolic syndromes. Baicalin administered (200 mg/kg/d) to mice with high fat diet induced metabolic syndrome led to an increase in short-chain fatty acid (SCFA)-producing gut bacteria, thereby effectively reducing the metabolic syndrome in mice[119]. Baicalin also reduces damage to the intestinal barrier caused due to hypertension. A study reported that baicalin (100 mg/kg) significantly increased the number of SCFAproducing bacteria and altered the intestinal microflora, leading to a reduction in damage of the intestinal barrier in rats caused by hypertension[120]. Another study revealed that baicalin (25-100 mg/kg) helped increase SCFA-producing bacteria such asEubacteriumspp,Subdoligranulumspp, andButyricimonasspp, thereby ameliorating TNBS-induced UC[114]. In some cases, the gut microbiome can also downregulate the therapeutic efficacy of baicalin[121]. The gut microbiota regulates hepatobiliary homeostasisviathe gut-hepatic axis, and although it can regulate baicalin activity, baicalin can also modulate the gut microbiota[122]. Consequently, baicalin has the potential to exert a therapeutic role in liver and gut diseases by modulating FXR and TGR5-mediated crosstalk involving bile acids associated with the gut microbiome.
DRUG DELlVERY, CLlNlCAL TRlAL, AND FUTURE PROSPECTS
The clinical application of baicalin in pharmacology has been challenging, due to its low solubility and bioavailability. In the last decade, many researchers have designed novel delivery strategies for baicalin that include phospholipid complex, liposomes, solid baicalin nanocrystals, and micelle formation[123].The dissolution and solubility of baicalin is considerably enhanced when administered in combination with other molecules in complex form. For instance, baicalin has exhibited improved oral bioavailability, distribution, targeting, and therapeutic efficacy when combined with polyethylene glycol and folic acid in the form of liposomes. β-Cyclodextrin complex has also been used as an effective formulation to facilitate the effective delivery of baicalin with wide range of therapeutic outcomes[123,124]. Moreover, baicalin is commonly used as adjuvant therapy for hepatitis. In a clinical study, single dose baicalin (500 mg/kg) in combination with cyclosporin A was found to be safe and well tolerated in adult human subjects without any severe adverse effects[125]. However, the co-administration of baicalin with other herbal formulations or drugs might impede baicalin’sin vivoactions and consequently its efficacy. Therefore, it is important to thoroughly study the clinically approved doses of baicalin which can be administered in combination with other compounds that help to improve the absorption and effectiveness of baicalin.
CONCLUSlON
GI disorders have emerged as the leading cause of mortality in the recent years across the world.Baicalin, a major flavone obtained fromS. baicalensis, exerts protective effect against hepatobiliary and colorectal disorders by modulating signaling pathways. Further, novel delivery strategies that are used in the transport of baicalin for ready absorption including phospholipid complex, liposomes, solid baicalin nanocrystals, and β-cyclodextrin complex have paved way for its widespread use as a pharmacological alternative in hepatic and GI disorders.
ACKNOWLEDGEMENTS
All of the authors acknowledge DST-FIST and UGC-SAP facilities of the Department of Biochemistry,University of Allahabad, Prayagraj, India.
FOOTNOTES
Author contributions:Ganguly R performed the literature review and wrote the manuscript; Ganguly R and Gupta A made the tables and figures; Pandey AK conceptualized the idea, critically reviewed and revised the manuscript; All authors have read and approved the final manuscript.
Supported byUniversity Grants Commission, New Delhi, India in The Form of UGC-Junior and Senior Research Fellowships.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORClD number:Risha Ganguly 0000-0002-6190-8923; Ashutosh Gupta 0000-0002-6547-6312; Abhay K Pandey 0000-0002-4774-3085.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Involvement of Met receptor pathway in aggressive behavior of colorectal cancer cells induced by parathyroid hormone-related peptide
- Role of gadoxetic acid-enhanced liver magnetic resonance imaging in the evaluation of hepatocellular carcinoma after locoregional treatment
- Clinical implications and mechanism of histopathological growth pattern in colorectal cancer liver metastases
- Bifidobacterium infantis regulates the programmed cell death 1 pathway and immune response in mice with inflammatory bowel disease
- Tumor-feeding artery diameter reduction is associated with improved short-term effect of hepatic arterial infusion chemotherapy plus lenvatinib treatment
- Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease
