Role of gadoxetic acid-enhanced liver magnetic resonance imaging in the evaluation of hepatocellular carcinoma after locoregional treatment
2022-07-30GattiMainoDarvizehSerafiniTricaricoGuarneriInchingoloIppolitoRicardiFonioFaletti
Gatti M, Maino C, Darvizeh F, Serafini A, Tricarico E, Guarneri A, Inchingolo R, Ippolito D, Ricardi U, Fonio P, Faletti R
Abstract Locoregional treatments, as alternatives to surgery, play a key role in the management of hepatocellular carcinoma (HCC). Liver magnetic resonance imaging (MRI) enables a multiparametric assessment, going beyond the traditional dynamic computed tomography approach. Moreover, the use of hepatobiliary agents can improve diagnostic accuracy and are becoming important in the diagnosis and follow-up of HCC. However, the main challenge is to quickly identify classical responses to loco-regional treatments in order to determine the most suitable management strategy for each patient. The aim of this review is to provide a summary of the most common and uncommon liver MRI findings in patients who underwent loco-regional treatments for HCC, with a special focus on ablative therapies (radiofrequency, microwaves and cryoablation), transarterial chemoembolization, trans-arterial radio-embolization and stereotactic ablative radiotherapy techniques, considering the usefulness of gadoxetate disodium (Gd-EOB-DTPA) contrast agent.
Key Words: Carcinoma; Hepatocellular; Magnetic resonance imaging; Gd-EOB-DTPA; Radiofrequency ablation; Catheter ablation; Ablation techniques
lNTRODUCTlON
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and represents a major public health issue worldwide[1]. Treatment of patients with HCC is closely related to Barcelona Clinic Liver Cancer (BCLC) stage. Locoregional treatments, as alternatives to surgery, play a key role in the management of HCC. Percutaneous thermoablation strategies are considered as an alternative to surgery in patients with BCLC 0-A, transcatheter embolization therapies are essential in patients with more advanced disease (i.e., BCLC B) and both together with Stereotactic Body Radiation Therapy(SBRT) are useful in patients who are not a surgical candidate[2].
The evaluation of both short- and long-term treatment outcomes is crucial for patient management.According to the guidelines, this evaluation should be performed using either dynamic computed tomography (CT) or magnetic resonance imaging (MRI)[2].
Liver MRI enables a multiparametric assessment, going beyond the vascularity assessment provided by traditional dynamic CT[3,4]. In recent years, liver MRI has made significant advances in the evaluation of HCC thanks to the introduction of new sequences for non-contrast evaluation (e.g.,diffusion-weighted sequences), the high temporal resolution sequences for optimal contrast enhancement assessment[5,6] and finally, the use of hepatobiliary contrast agents (gadoxetate disodium:Gd-EOB-DTPA, Eovist/Primovist; gadobenate disodium: Gd-BOPTA, Multihance). Gd-EOB-DTPA combines the properties of an extracellular agent, allowing for conventional contrast-enhanced multiphasic imaging, and a hepatocyte agent, allowing for hepatocyte uptake and biliary excretion evaluation, with the added benefit of tumor assessment in the transitional phase (TP) and delayed hepatobiliary phase (HBP) (20-min)[4,7,8]. This contrast agent improves the diagnostic accuracy and is becoming increasingly important in the detection of HCC[9].
The use of liver MRI with hepatobiliary contrast agents has become critical in patients with HCC.Therefore, understanding the typical and atypical features of treatment outcomes and recurrences is of paramount importance for proper treatment strategy.
The purpose of this review is to provide a comprehensive overview of locoregional therapies and to describe how to assess treatment outcomes using contrast-enhanced hepatobiliary MRI.
ABLATlVE THERAPlES
There are several ablation techniques available for the treatment of HCC as reported in a recent literature review by Makaryet al[10], such as radiofrequency ablation (RFA), microwaves (MWA),cryoablation (CA), irreversible electroporation (IRE), laser-induced interstitial thermotherapy (LITT),and high-intensity focused ultrasound (HIFU). The process of choosing among alternative techniques is principally based on institutional expertise and should be fitted to each patient. The main target for the abovementioned treatment methods is an early-stage HCC with a maximum axial diameter of 3 cm with BCLC class 0 or A. Moreover, this approach may lead to better clinical outcomes in patients with larger HCC lesions (axial diameter of 3 cm to 5 cm), when combined with trans-arterial chemoembolization(TACE)[10].
Even though the ablation techniques are widely performed, they are not risk-free: it is important to underline that the treated patients can present post-ablation syndrome, loco-regional bleeding, and adjacent organs injuries. The ablative therapy shows similar outcomes to surgical resection in the treatment of HCC with a maximum diameter of 3 cm, with a remarkable advantage of no pre-and postsurgical complications[10].
Most published studies on electronic medical databases including PubMed and Web of Science are strictly linked to RFA and MWA, while CA, IRE, LITT, and HIFU are not deeply evaluated and consequently data regarding response to therapy are lacking. On this basis, we’ll present the most common MR imaging findings regarding RFA, MWA and CA therapies.
RFA
RFA technique relies on the destruction of tumoral tissue by producing frictional heat using rapidly alternating electrical current thanks to the application of at least one electrode inside the region of treatment. The ablation zone includes a wider region than the tumor itself, of at least 5 mm to 10 mm[8].The use of RFA results in hyperthermia-induced coagulation necrosis and represents a heterogeneous low or mixed signal intensity on T1-weighted (T1w) and homogeneously low signal intensity on T2-weighted (T2w) images.
After contrast media administration (extracellular or hepatobiliary agents), the RFA zone manifests as a well-demarcated hypointense area with the absence of contrast enhancement[8]. The presence of slight rim signal intensity on T2w images and contrast enhancement especially in the equilibrium phase should be considered as a physiological response to thermal injury, mainly during early follow-up[11].The typical appearance of HCC recurrence is the hypervascularity on arterial phase [arterial phase hyperenhancement (APHE)], hypointensity on portal-venous and HBPs. However, in clinical practice,the discrimination between hyperemia and residual HCC might be difficult. This aspect was demonstrated by Mikamiet al[12], who reported a typical enhancement pattern in only 17.5% of recurrent HCCs, while 40.6% with APHE without washout in the portal venous phase and 11.9% with portal venous phase washout without APHE.
In this setting, the use of Gd-EOB-DTPA can help to distinguish vascular pseudolesions from hypervascular tumors including recurrent HCC[13]. Since 2013, it has been reported[14] the efficacy of EOB-MRI in the decision-making of curative treatment for HCC: it was demonstrated that the HBP improves the diagnostic accuracy and treatment decision making in the early-stage HCC and these results can perfectly fit candidates to RFA. Imaiet al[15], by enrolling 97 patients who underwent RFA,revealed that EOB-MRI had higher diagnostic accuracy and sensitivity for detection of recurrent hypervascular HCC (44vs24,P< 0.001) in comparison to CT. They also reported a good inter-observer agreement between the 2 readers, underlying the effectiveness of EOB-MRI.
As known, the main advantage of EOB-MRI is the HBP, where the radiologist can face a hypointense nodule without significant hypervascular appearance. On this basis, it is important to determine the risk of non-hypervascular HCC recurrence after loco-regional treatment. The systematic review and metaanalysis by Kimet al[16] demonstrated the post-ablation recurrence of HCC: they showed that hypointense nodules in the HBP represent a high-risk factor for recurrence (hazard ratio = 1.74-3.07),underlying the importance of EOB-MRI in the detection of HCC recurrence post-RFA.
More recently, a retrospective study by Baeet al[17] on 183 patients who underwent different treatments including RFA, showed that satellite nodules and peritumoral hypointensity on HBP images were associated with poor disease-free survival and overall survival (P= 0.018 andP= 0.016,respectively).
On the other hand, the study by Rimolaet al[18], on 49 patients who underwent locoregional treatment, determined that MRI with extracellular agents has a similar specificity in detecting viable HCC in comparison to EOB-MRI (84%vs85%, respectively), with a higher AUROC (0.80vs0.72,respectively). These findings are not in line with most studies, however, should be carefully considered due to the small sample size and the remarkable risk of bias, since different ablation techniques were evaluated.
Finally, EOB-MRI was found efficient not only in the evaluation of treatment response but also in the feasibility of ablative margin grading before RFA. Kodaet al[19] by enrolling 124 HCCs, showed that EOB-MRI enables an early assessment of RFA effectiveness in the majority of HCC nodules with the tumor size as an independent predictor factor for local tumor progression.
To conclude, EOB-MRI can be considered as a useful tool in the detection of HCC recurrence after RFA treatment, however, large prospective studies are needed to determine a more accurate evaluation of diagnostic performance.
Figure 1 depicts a liver MRI after RF treatment with no evidence of disease persistence or relapse, and Figure 2 depicts its evolution. Figure 3 depicts a case of HCC recurrence after RF treatment.
Microwave ablation
MWA technique is based on analogous physical principles of RFA, however, it employs an antenna that produces electromagnetic waves which can interact with water molecules and, consequently, raises the temperature[20].

Figure 1 hree-month follow-up liver magnetic resonance imaging of a 69-yr-old male post radiofrequency ablation of right hepatic lobe hepatocellular carcinoma (segment Vll). A: Out-phase T1-weighted image; B: In-phase T1-weighted image; C: T2-spectral attenuated inversion recovery; D:T2-weighted image; E: High b-value diffusion weighted imaging; F: Apparent diffusion coefficient map; G: Arterial phase magnetic resonance imaging (MRI); H:Arterial phase MRI with image subtraction technique; I and J: Hepatobiliary phase MRI. Follow-up MRI (A-I) after 3 mo post treatment revealed a good outcome characterized by inhomogeneous high signal hyperintensity in T1 sequences due to the presence of coagulative necrosis with associated signal hypointensity in T2 weighted sequences and the absence signal hyperintensity in diffusion weighted imaging. Dynamic study showed no enhancement in arterial phase with inhomogeneous hypointensity during hepatobiliary excretion. Findings are suggestive of complete tumor ablation also when compared to the similar pre-treatment sequences (J).

Figure 2 The evolution of the ablation zone shown in Figure 1. A: Hepatobiliary phase (HBP) phase magnetic resonance imaging (MRI) prior to radiofrequency ablation (RFA); B: HBP MRI 3 mo after RFA; C: HBP MRI 9 mo after RFA; D: HBP MRI 19 mo after RFA. Liver MRIs demonstrate a progressive reduction of ablation zone together with fibrotic changes.
MR findings after MWA are like those reported for RFA. On unenhanced T1w images, the ablated lesion shows a target-like appearance: linear hypointensity in the center of the ablation zone surrounded by hyperintense region due to coagulative necrosis, and outer hypointense areas of signal intensity corresponding to a slight to high signal intensity on T2w images due to inflammation[21]. After injection of contrast media, no APHE should be seen to consider a complete response. However, only a few studies evaluated the usefulness of EOB-MRI in the detection of HCC recurrence after MWA. Imaiet al[15] reported that the diagnostic value of EOB-MRI is better than CT to evaluate HCC after treatment by image-guided tumor procedures, including patients who underwent both ablation and chemoembolization.
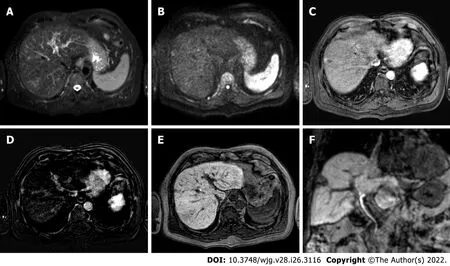
Figure 3 Two-years liver magnetic resonance imaging follow-up of a 71-yr-old male with liver cirrhosis and previous subcapsular hepatocellular carcinoma in segment Vlll underwent radiofrequency ablation. A: T2-spectral attenuated inversion recovery; B: High b-value diffusion weighted imaging; C: Arterial phase magnetic resonance imaging (MRI); D: Arterial phase MRI with image subtraction technique; E and F: Hepatobiliary phase MRI.Liver MRI showed recurrent hepatocellular carcinoma (8 mm) located infero-lateral to the radiofrequency scar, characterized by moderate to high signal hyperintensity in T2, hyperintensity in high b-value diffusion sequences, with arterial “wash in” and hypointensity in hepatobiliary excretion phase.
CA
As reported by Song[22], CA should be included in the family of thermal ablation in HCC patients. The physical bases are like RFA and MWA, however, the employed temperature is not hot but cold, thanks to chemical agents such as liquid nitrogen. According to a recent meta-analysis[23], the effects of MWA and CA appeared to be similar to those of RFA, but there were lower rates of local tumor progression and higher rates of complete thermal ablation in large tumors compared to RFA (P< 0.05). MR imaging findings after the CA procedure are similar to RFA and MWA, as reported above: the ablation zone at 24h appears hypointense and heterogeneously hyperintense on T1w and T2w images, respectively[24].Foci of inhomogeneous signal intensities on T1w and T2w images should be principally due to blood products and can be present in further follow-up examinations[24]. The peripheral zone shows a slight to hyper- signal intensity on T2w images, due to inflammation and granulation tissue formation. The residual tumor presents analogous imaging features to the treated lesion, while the recurrence can be seen as an area of APHE with consequent hypointensity on portal-venous and HBP. Even if not fully reported in the literature, a focal hypointense area of HBP images without APHE might be referred to a suspected recurrence.
TACE AND TRANS-ARTERlAL RADlOEMBOLlZATlON
Treatment options for HCC depend on tumor burden and extent, patient's performance status, liver function, extra-hepatic disease, and co-morbidities, according to the BCLC system[10]. Intra-arterial therapies, including TACE and trans-arterial radio-embolization (TARE), come into play to improve survival and quality of life where curative treatments like ablation, liver resection, or liver transplantation are not applicable[10,25]. TACE and TARE are regarded as a standard of care for a patient with intermediate-stage HCC (BCLC class B, Child-Pugh A-B) not eligible for surgical resection or transplantation and without portal vein thrombosis or extrahepatic spread. Moreover, the application of these techniques range from curative-intent for small tumors, to downstaging or bridging to resection and transplantation for early and intermediate disease, and locoregional control and palliation for advanced disease[26].
The main contraindications to TACE and TARE are decompensated liver cirrhosis, bilobar liver involvement, and technical infeasibility; absolute contraindication specific to TARE is the presence of hepato-pulmonary shunt or hepato-enteric shunts[10].
In the case of portal vein thrombosis, in clinical practice, TACE is contraindicated, even if Patidaret al[27] described a good outcome of patients with segmental-branch or first-border branch portal vein thrombosis treated with TACE. On the contrary, TARE may have applications even if there is portal vein thrombosis because of the reduced embolic effect of the technique[10].
TACE
TACE is performed by cannulation of the arteries that feed the HCC through a catheter or a microcatheter and releasing both the chemotherapeutic agent and embolic particles. The treatment may be performed in two ways, conventional TACE that uses lipiodol, an oily radiopaque material mixed with one or more chemotherapeutics agents, followed by administration of an embolic agent and drugeluting beads TACE (DEB-TACE) with slowing releasing of chemotherapy by particles[26].
Multiphasic MRI is performed to evaluate the response to treatment and to detect new HCCs from 1 mo to 3 mo after treatment for the first imaging study, followed by every 3 mo for 2 years[28]. As previously mentioned, MRI may use gadolinium-based extracellular contrast agents, or gadoliniumbased hepatobiliary agents[28,29].
MRI imaging after TACE is related to tumor necrosis caused by ischemic injury from arterial embolization and chemotoxic injury from the administered chemotherapy[28]. Gd-EOB-DTPA allows similar dynamic acquisition of extracellular agents in arterial and portal phases, essential in detection of a viable tumor. If the treatment is successful the expected findings are: absence of APHE, associated with surrounding perfusion changes related to edema and arterial embolization, a thin APHE phase due to inflammatory, that may persist up to a year, and a volumetric reduction of the treated lesion over time[28]. The HBP contributes to the diagnosis of complete response to treatment, showing a corresponding hypointensity of the lesion if complete necrosis occurs. A thin, continuous, and smooth APHE rim associated with a corresponding isointense area in the HBP defines a benign finding[30].
After treatment, the damage of the liver parenchyma around the treated lesion may cause geographic perfusion changes and corresponding hypointensity on the HBP, a feature that needs careful evaluation and examination of additional sequences to avoid diagnostic mistakes of infiltrative disease[31]. If pseudolesions related to treatment occur (arterial-venous shunt and portal vein obstruction), they usually don’t show hypointensity on the HBP, an element that helps in the diagnosis of benignity,although hypointense pseudolesions on the HBP mimicking malignancy have been described by Motosugiet al[32].
The sign of residual and/or recurrent disease is represented by the presence of thick, peripheral,irregular, eccentric, nodular APHE with or without washout within the treated lesion, or enhancement similar to pre-treatment tumor. Aslamet al[30] showed findings suggestive of the presence of viable tumor after thermoablation, applicable for TACE too: the presence of thick, nodular and eccentric hypointense signal on HBP or a discontinuous hypointense rim. Considering these findings on the HBPlike viable tumor is much more important when there is equivocal APHE, consisting of viable isoenhance or hypoenhance tumor in arterial phase[33].
Figure 4 depicts a pre-treatment CT images and subsequent TACE treatment of an HCC nodule, the results of which are illustrated in Figure 5.
TARE
TARE is a trans-arterial catheter treatment based on delivering microspheres (glass or resin) coated with90Y.90Y is an unstable isotope that releases a beta particle during its decay into a stable element,zirconium 90, inducing destruction of the target tumor with a limited depth of penetration to minimize radiation exposure of surrounding parenchyma[33].
90Y microspheres have a main radiant effect and a small microembolic effect due to the little diameter of microspheres; therefore, tumor necrosis related to TARE is mostly radiation-induced and the arterial flow to adjacent parenchyma is largely conserved, which is an important advantage in the case of portal venous thrombosis (i.e., contraindication for TACE)[33].
Multiphasic MRI after TARE is performed at 3-mo intervals even for the first examination, because imaging performed earlier may show exuberant arterial phase involving the treated lesion and the surrounding parenchyma with consequently difficult interpretation[29]. MRI imaging findings in TACE and TARE are not similar because of the different mechanisms of action: TARE induces modifications that evolve because of the impact of radiation on the liver parenchyma surrounding the lesion[28,30].
The expected Gd-EOB-DTPA imaging findings of TARE-treated HCC are persistent APHE or portal washout that can persist for at least one year, geographic peri-tumoral APHE, complete loss of APHE,thin rim of peripheral APHE, transitory growth in the size of the tumor due to edema, and cytostatic effect of radiation, a delay in tumor decreasing size. In TARE, in the immediate post-treatment setting,the penetration of beta particles in the surrounding liver parenchyma causing inflammation, edema,hemorrhage, leads to a heterogeneous enhancement, more accentuated than TACE. Even in this case,Gd-EOB-DTPA may show a related hypointensity on the HBP, an important element to be assessed since it can be misdiagnosed with an infiltrative disease. This finding may usually resolve after 6 mo, so in the early phase is very important to consider other sequences to make the differential diagnosis,especially T2w and DWI images. The changes in the lesion diameter should be characterized with caution based on the persistent APHE and typical washout of the lesion after TARE. Viable tumor manifests itself with an increase in the size of the treated nodule with new or growing nodular enhancement within or beyond the margin of the post-radiation zone with hypointense signal on the HBP[28,30].

Figure 4 A 62-yr-old male with typical hepatocellular carcinoma in segment lVa who underwent trans-arterial chemoembolization with microparticles (Lifepearl 100 +/- 25 microns) preloaded with 50 mg of Farmorubicin and later with non-loadable Hydropearl 400 +/- 75 microns microparticles. A: Pre-treatment computed tomography (CT) images: arterial phase; B: Pre-treatment CT images: portal phase; C: Pre-treatment CT images: delayed phase; D: Pre-trans-arterial chemoembolization (TACE) angiographic image; E: Post-TACE angiographic image; F: Post-TACE cone beam CT.

Figure 5 Post-trans-arterial chemoembolization follow-up magnetic resonance imaging with hepatospecific contrast agent in the same patient described in Figure 4. A: In-phase T1-weighted image; B: T2-weighted image C: High b-value diffusion weighted imaging (DWI); D: Apparent diffusion coefficient map; E: Arterial phase magnetic resonance imaging (MRI); F: Arterial phase MRI with image subtraction technique; G: Portal venous phase MRI; H:Hepatobiliary phase MRI; I: Ten-month computed tomography (CT) follow-up: arterial phase; J: Ten-month CT follow-up: delayed phase. Liver MRI shows an inhomogeneously hyperintense nodule in T1WI in its right posterior portion, coexisting with a more hyperintense area in T2WI with high signal intensity in high b-value DWI in the left anterior portion. Dynamic study showed no arterial enhancement confirmed with the subtraction technique image, absence of vascularization in the portal phase and inhomogeneous hypointensity in the hepatobiliary phase. The findings are suggestive of good treatment outcome with presence of both coagulative and colliquative necrosis and no residual disease and CT scans control after 10 mo confirmed the outcome.
For both TARE and TACE techniques the use of Gd-EOB-DTPA implies knowledge of some technical concerns. An optimal arterial phase may be more difficult to obtain compared to extracellular-contrast agents because of acute transient severe motion artifacts affecting EOB-MRI. Several approaches have been applied to reduce motion artifacts: (1) Lowering thecontrast injection rate; (2) Using multiple arterial phasetechnique; (3) Shortening the scanning time; and (4) Using a modified breathing command, and dilutingcontrast medium[5,34]. Moreover, "washout" evaluation of the nodule has to be assessed in the portal phase because hypointensity during the TP could be related to true washout or pseudo-washout resulting from the hyperintensity of the surrounding parenchyma[34]. Another element to be considered is the adequacy of the HBP, strictly linked to the assessment of the degree of contrast uptake in the liver parenchyma relative to the hepatic vessels and of the presence of contrast excretion into the biliary system. A signal intensity ratio of 1.8 between liver and vein, with the maximal enhancement of liver parenchyma and hypointensity of the veins, is the best predictor of the ideal phase. In a functional liver, this ideal condition is achieved at 15-20 min after contrast injection[35]. In this setting, the main limitation of Gd-EOB-DTPA is the necessity of a functional liver for an optimal HBP; the cirrhotic liver may have diminished or delayed parenchymal enhancement leading to failure of the mechanism of contrast uptake and excretion. Therefore, in a situation with deeply compromised liver function (Child-Pugh B-C) acquisition of delayed HBP beyond the 20 min is necessary.
At present, the treatment response algorithms used in the clinical practice, such as Modified Response Evaluation Criteria in Solid Tumors (mRECIST), European Association for the Study of the Liver Disease Criteria, and Liver Imaging and Reporting Data System Treatment Response Algorithm are enhancement-based classification systems that don’t include transitional or HBP obtained by Gd-EOBDTPA[28-30]. However, many authors have analyzed the contribution of EOB-MRI in follow-up after TACE and TARE with promising results. To the best of our knowledge the actual studies published in the literature, have analyzed the impact of Gd-EOB-DTPA in the follow-up of loco-regional therapy group (for example both ablation and TACE) without examination of the single procedure, therefore next considerations are almost effective for both TACE and TARE.
In a recent study, Kimet al[33] showed an increased sensitivity in the detection of viable tumor considering all ancillary features (HBP hypointensity, restricted diffusion, and intermediate signal on T2w) with growing sensitivity into the LI-RADS-TR viable category of 57%-87%vs39%-65% without changing in specificity. Parket al[36] obtained similar results for TACE and ablation, with increased sensitivity of 84%vs76%.
Kimet al[37] first, in a recent retrospective study, analyzed the impact of the incorporation of the individual ancillary feature instead of multiple ancillary features on the diagnostic performance of the LI-RADS-TR for viable tumor. They found that hypointense signal during the transition and/or HBP can improve the sensitivity than contrast dynamic assessment alone.
Conversely, Rimolaet al[18] show a small contribution of HBP in the evaluation of tumor response after loco-regional therapy, with greater accuracy, sensitivity, and inter-reader agreement using extracellular agents in comparison with Gd-EOB-DTPA[37]. These results may depend on better conspicuity of the APHE with extracellular agents and to the misdiagnosis of viable tumor for hypointense areas on the HBP related to damaged liver parenchyma, an avoidable mistake considering additional sequences[18,30].
Another important fact is that EOB-MRI in follow-up after TACE and TARE significantly contributes not only to determination of viable tumors on the treated cavity but also for detection of new HCC lesions, that may be disguised by the post-treatment changes of the surrounding hepatic parenchyma[30].
The use of EOB-MRI after TACE and TARE is widespread in the clinical practice and its contribution with transitional and HBP to identify the viable tumor, necrotic cavity, damaged liver tissue, and new HCC has been shown in the literature, although it still has not been included in treatment response algorithms.
Figure 6 depicts the pre-treatment liver MRI and preoperative planning for TARE of a patient with voluminous infiltrating HCC. Figure 7 illustrates the outcome of treatment.
STEREOTACTlC ABLATlVE RADlOTHERAPY
Recent improvements in image guidance and conformal radiation techniques have made it possible to administer high doses of radiation to liver tumors with minimal damage to adjacent parenchymal tissue[38]. Although SBRT is not part of the current BCLC system, it is included as a treatment option in the most recent version of the National Comprehensive Cancer Network (NCCN) for primary liver cancer for unresectable disease or medically inoperable patients[38,39].
Indications for SBRT are applicable in the following cases: (1) Early-stage HCC, not eligible for surgical resection, liver transplantation, or local ablation; (2) Intermediate or advance stage HCC not eligible or not responsive to TACE; (3) Palliative purpose for symptomatic end-stage HCC; and (4)Bridging or downstaging therapy for liver transplantation in patients not eligible for other locoregional therapies[40].
Factors that generally hinder percutaneous ablative therapies, such as proximity of the tumor to vascular structures, bile ducts or the diaphragmatic surface are not contraindication to treatment[41].SBRT is most appropriate in patients with cirrhotic disease in Child-Pugh class A. Treatment is available for patients up to Child-Pugh B7, for whom close clinical monitoring and dose reduction are recommended due to the increased risk of radiation-induced liver disease (RILD). From a technical point of view, the number of target lesions, together with their intraparenchymal location, are potential limitations during treatment planning and dose delivery.

Figure 6 A 62-yr-old patient with infiltrative hepatocellular carcinoma with associated neoplastic portal vein thrombosis. A: High-b-value diffusion-weighted image; B: Arterial phase magnetic resonance imaging (MRI); C: Arterial phase MRI with image subtraction technique; D: Portal venous phase MRI;E and F: Hepatobiliary phase MRI; G: Pre-treatment arteriography; H: Pre-treatment cone beam computed tomography (CT); I and J: Post trans-arterial radioembolization single photon emission computed tomography (SPECT) images. Left panel shows pre-treatment liver MRI, illustrating the cranial portion of the lesion with portal branch infiltration; whereas the right panel shows pre-treatment arteriography, the cone beam CT of the cranial portion of the lesion with the portal vein infiltration, and preoperative planning with SPECT images post administration of Tc-99m-labeled albumin macroaggregates with hyperfixation at both lesion site and portal thrombosis.

Figure 7 Post-trans-arterial radio-embolization images in the patient mentioned in Figure 6. A: T2-spectral attenuated inversion recovery; B: High b-value diffusion weighted imaging; C: Apparent diffusion coefficient (ADC) map; D: Arterial phase magnetic resonance imaging (MRI); E: Arterial phase MRI with image subtraction technique; F: Hepatobiliary phase (HBP) MRI; G: T2-spectral attenuated inversion recovery; H: High b-value diffusion weighted imaging; I: ADC map; J: Arterial phase MRI; K: Arterial phase MRI with image subtraction technique; L: HBP MRI. The liver MRIs reveal almost no signal restriction in DWI and no enhancement in dynamic study in the caudal portion of the lesion (left panel) which are suggestive of a good outcome. Whereas in the cranial infiltrating part of the lesion (right panel) at portal branch level, the high signal in DWI with arterial enhancement is suspected for residual disease.
Ionizing radiations directly impairs the DNA replication process by causing breaks and thus preventing cancer cells from replicating, most powerfully on tissues with a high cell turnover. After radiotherapy, centrilobular hepatocytes die by a hypoxic process secondary to vascular congestion,which does not recover until several months later[42]. Furthermore, proinflammatory mechanisms will be set on the treated region. These histological changes initially lead to an acute reduction in cellular vascular supply, with peripheral hypervascularization induced by the proinflammatory mechanisms,while over time, fibrosis leads to a reduction of tumor size and vascular supply[43]. Damage also occurs in the surrounding liver parenchyma with the evolution of the chronic inflammatory context, characterized initially by edema and subsequently by fibrosis, leading to capsular retraction[42].
Acute, subacute and chronic stages can be distinguished by assessing the evolution of histological changes. In the acute stage (1-3 mo) typical peripheral hyperarterialization, defined as a ring-like enhancement during the arterial and portal venous phases, can be observed. In the subacute stage (3-6 mo), the involved parenchyma shows relative hypointensity before and after contrast media injection especially on the portal-venous phase, with progressive enhancement in the late phases, concerning the occlusion of the centrilobular veins and to the reduced clearance of intravenous contrast. In the chronic stage (> 6 mo), imaging will reveal changes caused by radio-induced fibrosis. The subcapsular location of the target lesions also correlates with the progressive occurrence of capsular retraction. This reaction typically appears as a band-like finding, a typical appearance that can help the differential diagnosis with residual or recurrent tumor[44]. Finally, the classic RILD generally occurs within 4 mo of total or subtotal hepatic irradiation, and manifests with hepatomegaly, ascites, and increased alkaline phosphatase levels, without a radiological intrahepatic progression disease. Nowadays, this complication is effectively prevented by sparing a sufficient portion of the parenchyma during therapy.
Patients with pre-existing chronic liver disease undergoing this type of treatment tend to experience a different spectrum of toxicity than with classic RILD, including a general decline in liver function, a marked increase in transaminases or jaundice within 3 mo of the end of therapy, collectively referred as"non-classic" RILD[44].
The radiological findings of SBRT treated lesions differ from the imaging of other locoregional therapies. Indeed, while the latter results in immediate tumor devascularization, SBRT leads to gradual histological changes in the lesion and surrounding parenchyma over time, which may lead to the incorrect assumption of a lack of efficacy.
As previously mentioned, different algorithms are employed to determine the response evaluation,such as mRECIST, applied to CT and MRI[45]. The intensity of arterial enhancement may appear quite early (15-45 d after SBRT) and may persist over time. Therefore, lesions dimensionally stable over time with a reduced arterial hypervascularization should be considered as a responder. The decrease in size occurs more slowly, over 22-24 mo, due to progressive necrosis up to 12 mo after treatment, which results in a slower evolution of structural changes.
In post-SBRT MRI examinations it is common to find a hyperintense perilesional halo on T2w due to the inflammatory reaction and venous congestion induced by veno-occlusive disease or, in subsequent follow-ups, a slight signal hyperintensity on T2w in the whole irradiated field, a direct sign of parenchymal fibrosis. Moreover, the application of DWI sequences can provide information on tissue cellularity and the integrity of cell membranes. In fact, during the early phase after treatment, hepatic edema occurs due to decreased venous outflow secondary to centrilobular obstruction, and there may be mild restricted diffusion and a slight increase in apparent diffusion coefficient (ADC).
Finally, tumor response after SBRT can be challenging, as described above: tumor shrinkage may not happen in the immediate months after SBRT. Even in the absence of volumetric reduction, progressive reduction in tumor enhancement is consistent with treatment response. This is typically accompanied by a corresponding increase in ADC. An increment in ADC of 20%-25% was found to be an indicator of SBRT response in a few small retrospective studies[46,47]. Thus, in stereotactic radiotherapy, a statistically significant increase in ADC values measured in HCC undergoing radiofrequency was found to be the most important independent factor in recurrence and survival rates.
The use of hepatobiliary contrast agents allows the assessment of both lesion and the adjacent hepatocytes: The presence of a hypointense nodular lesion during HBP nearby an SBRT-treated HCC may signify either the presence of neoplastic cells or a scar. The picture is further complicated by the manifestation in such sequences of the focal hepatic reaction induced by radiotherapy. This alteration can be demarcated as a band-like area of signal hypointensity, such as hyperintensity on T2w, related to altered hepatocyte function in the irradiated field[48].
Figure 8 depicts the radiotherapy treatment plan; Figure 9 shows the SBRT treatment outcomes;Figure 10 depicts the treated zone's evolution; and Figure 11 illustrates the outcome 20 mo after treatment.
LlVER MRl ACCURACY AND LlMlTATlONS
Although MRI is notably known as a highly sensitive and accurate imaging technique in detection and characterization of focal liver lesions, there is still no consensus regarding the best imaging follow-up modality for patients affected with HCC after locoregional treatments. Moreover, to date, only few studies have conducted a comparison among imaging techniques after locoregional treatments, and this raises the scientific interest in further investigations.
Kubotaet al[49], by evaluating 84 HCCs underwent TACE treatment, showed the superiority of MRI to CT for early detection of lesion. Authors reported 76.0% sensitivity, 67.6% specificity and 72.6%accuracy for lipiodol-CT in comparison to 100% sensitivity, specificity and accuracy for dynamic MRI.Moreover, DWI and ADC values can help identify the early HCC recurrence: the presence of pathological tissues results in a higher signal intensity on DWI relative to normal liver parenchyma and a low signal on ADC maps, differentiating it from treated lesion (necrotic/edematous area) which manifests as high ADC values[50]. In these settings, the use of Gd-EOB-DTPA can maintain the highest diagnostic values, as mentioned above.
MRI was demonstrated to be useful also prior to treatment, especially by evaluating the HBP. Kimet al[16] found that HBP hypointense nodules without APHE are risk factors for intrahepatic distant recurrence in HCC patients treated with RFA or hepatectomy in a meta-analysis of 842 patients. In patients with HCC who have these nodules on pretreatment gadoxetic acid-enhanced MRI, stratification of patient management in terms of performing additional tests or treatment for these nodules, as well as modification of proper follow-up strategies, may be required.

Figure 8 Radiotherapy treatment planning of a 70-yr-old male patient with two hypovascular hepatocellular carcinomas in segment Vlll(4.3 cm) and segment Vl (1.3 cm). A-C: Hepatobiliary phase magnetic resonance imaging (MRI); D-F: Computed tomography (CT) simulation for radiation therapy planning. In the upper panel from A to C are reported hepatobiliary phase MRI images prior to stereotactic ablative radiotherapy treatment. In the bottom panel from D to F are reported CT images from radiotherapy treatment plan with isodose curve distributions.
Considering the therapeutic response to RFA, literature may not be much of a help since the two most important studies in this field were published in the early 2000s[51]. Both studies reported that MRI may have an edge over CT in the early detection of local regrowth in particular after 4 mo of treatment[51]. This aspect should be carefully considered according to readers' experience, MR image quality, and the scattered use of hepatobiliary contrast agents, which was not widely used 20 years ago.
Regarding therapeutic response to TACE, it is important to underline that beam hardening artifacts can lead to the underestimation of hyperenhancement during the CT arterial phase and thus reducing the sensitivity and accuracy dramatically[49,52]. On the other hand, the wider use of DEB-TACE can partially solve this issue due to the lack of beam hardening artifacts[53]. The abovementioned issues can be partially left out when using MR to detect recurrence. On the other hand, the use of dual-energy CT can help increase diagnostic accuracy, even if not widely available[54]. CT and MR diagnostic accuracy can be considered completely superimposable after TARE[26], although the added value of HBP imaging was not deeply evaluated.
In the recent years, perfusion techniques, both with CT and MR, were used to diagnose HCC and determine different treatment responses, especially after medical therapy: in this setting CT perfusion can be considered a reliable tool in the early detection of recurrence, as reported by Choiet al[55]. More recently the effectiveness of CT perfusion was evaluated by Ruffet al[56], demonstrating that residual tumor perfusion parameters are linked to HCC recurrence. However, both CT and MR perfusion imaging need an extra time, added costs and are not widely available to be considered convenient tool in daily clinical practice[57].
In the recent years, radiomics models based especially on texture analysis are showing promising results[58]. Zhanget al[59], by enrolling 132 patients who underwent locoregional therapies,demonstrated that radiomic features extracted from Gd-EOB-DTPA MR imaging associated with clinical data can predict the early tumor recurrence. Even if pioneering, radiomics analyses should be carefully evaluated, especially due to different types of software and MR techniques used which vary between centers. However, further studies should focus on these aspects to validate its potential values[60].
Overall, it is important to underline the presence of artifacts during MR acquisition, especially in the arterial phase when using Gd-EOB-DTPA studies. As previously reported and demonstrated[61], flow rate and acquisition protocol[5] can lead to the degradation of the arterial phase and should be carefully managed to avoid the leak of important data, linked to the risk of early recurrence.
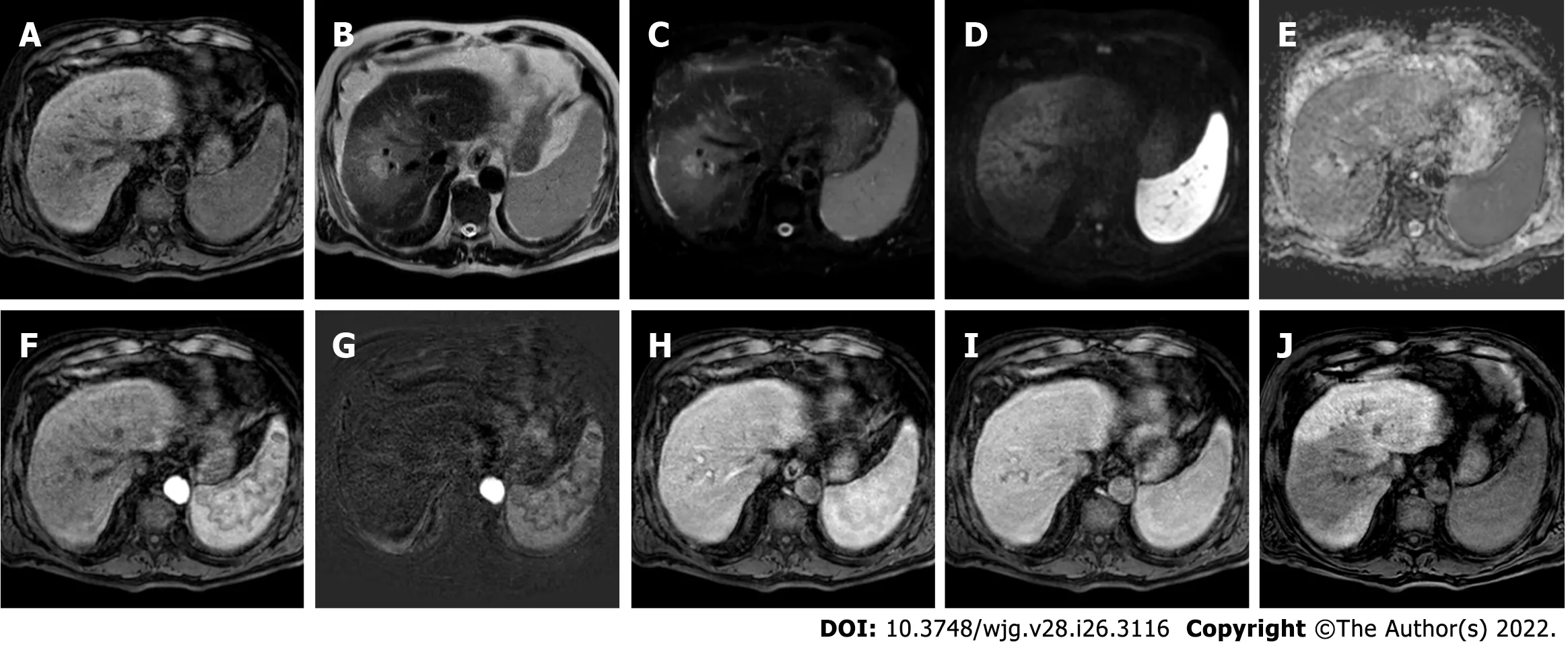
Figure 9 Four-months follow-up post stereotactic body radiation therapy liver magnetic resonance imaging of the hepatocellular carcinoma in segment Vlll shown in the Figure 8. A: Non contrast T1-weighted high resolution isotropic volume examination; B: T2-weighted image; C: T2-spectral attenuated inversion recovery; D: High b-value diffusion weighted imaging (DWI); E: Apparent diffusion coefficient (ADC) map; F: Arterial phase magnetic resonance imaging (MRI); G: Arterial phase MRI with image subtraction technique; H: Portal venous phase MRI; I: Delayed phase MRI; J: Hepatobiliary phase MRI.The liver MRI shows an ill-defined area of signal hypointensity in T1 in the treatment zone with a blurred and inhomogeneous signal hyperintensity with evidence of increased signal hyperintensity in the residual nodule. A shaded signal hyperintensity on DWI without signal hyperintensity of the previous lesion, with higher ADC values relative to the surrounding liver parenchyma. No areas of enhancement are shown, and a coarse hypointense signal in the hepatobiliary phase due to the initial fibrotic evolution of the treated zone is evident.

Figure 10 The evolution of the hepatocellular carcinoma stereotactic body radiation therapy treatment zone in patient shown in Figures 8 and 9. A: Hepatobiliary phase (HBP) magnetic resonance imaging (MRI) prior to stereotactic body radiation therapy (SBRT); B: SBRT plan with isodose curve distributions; C: HBP MRI 4 mo after treatment; D: HBP MRI 8 mo after treatment; E: HBP MRI 20 mo after treatment. The MRIs demonstrated the progressive fibrotic evolution of the treatment area.
Finally, the use of CT or MRI is strictly linked to each institution's flow charts: it is important to underline the low CT associated costs, the need for less patient cooperation, and its usefulness when ascites is present[62]. Also in these settings, a direct comparison with MR with HBP imaging is lacking and further studies are needed to validate the abovementioned data.
CONCLUSlON
Locoregional therapy is critical in the treatment of patients with HCC. The accurate evaluation of tumor response using imaging modalities is essential for optimal management. Gadoxetic acid-enhanced liver MRI is an important radiological tool in these patients because it allows for optimal non-invasive tissue characterization using a multiparametric approach. For HCC patients to get proper therapeutic management, radiologists must know the usual post-treatment imaging findings associated with locoregional therapy, as well as a thorough comparison of imaging before and after therapy. Overall,gadoxetic acid-enhanced liver MRI is a critical modality in the assessment of results following locoregional HCC therapy, having a significant impact on patient management.

Figure 11 Follow-up liver magnetic resonance imaging of abovementioned patient (Figures 8-10), 20 mo after stereotactic body radiation therapy. A: Out-phase T1-weighted image; B: Non contrast T1-weighted high resolution isotropic volume examination; C: T2-spectral attenuated inversion recovery;D: High b-value diffusion weighted imaging; E: Apparent diffusion coefficient map; F: T2-weighted image; G: Arterial phase magnetic resonance imaging (MRI); H:Arterial phase MRI with image subtraction technique; I: Portal venous phase MRI; J: Portal venous phase MRI with image subtraction technique; K and L:Hepatobiliary phase MRI. MRI revealed parenchymal retraction with signal hypointensity on T1-weighted images corresponding to inhomogeneous hyperintensity in axial and coronal T2 sequences, with no significant increase in diffusion signal. Dynamic perfusion study after contrast medium administration: no enhancement in arterial phase and a progressive enhancement in delayed phase with corresponding hypointense signal in the hepatobiliary excretion phase testifying the fibrotic evolution of the treated liver and the absence of locoregional recurrence.
FOOTNOTES
Author contributions:Gatti M was involved in conception and design of the study; Gatti M, Maino C, Serafini A and Tricarico E were involved in literature review, analysis and writing of the original draft; Darvizeh F was involved in writing of the original draft; Guarneri A, Inchingolo R, Ippolito D, Ricardi U and Fonio P took part in supervision of the study; Faletti R took part in supervision of the study and is the guarantor of the study; all the authors worked together to editing, reviewing and final approval of article.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Marco Gatti 0000-0001-8168-5280; Cesare Maino 0000-0002-5742-802X; Fatemeh Darvizeh 0000-0002-3735-0472; Alessandro Serafini 0000-0002-4992-4824; Eleonora Tricarico 0000-0001-9805-2551; Alessia Guarneri 0000-0003-1711-3645; Riccardo Inchingolo 0000-0002-0253-5936; Davide Ippolito 0000-0002-2696-7047; Umberto Ricardi 0000-0003-4406-7621; Paolo Fonio 0000-0002-1106-2990; Riccardo Faletti 0000-0002-8865-8637.
S-Editor:Gao CC
L-Editor:A
P-Editor:Chen YX
杂志排行
World Journal of Gastroenterology的其它文章
- Involvement of Met receptor pathway in aggressive behavior of colorectal cancer cells induced by parathyroid hormone-related peptide
- Clinical implications and mechanism of histopathological growth pattern in colorectal cancer liver metastases
- Bifidobacterium infantis regulates the programmed cell death 1 pathway and immune response in mice with inflammatory bowel disease
- Tumor-feeding artery diameter reduction is associated with improved short-term effect of hepatic arterial infusion chemotherapy plus lenvatinib treatment
- Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease
- Endoscopic techniques for diagnosis and treatment of gastro-entero-pancreatic neuroendocrine neoplasms:Where we are
