华中五味子根皮中1个新的菖蒲烯类倍半萜
2022-07-21张东东黄仕其刘媛媛李玉泽张化为宋小妹
张东东,黄仕其,刘媛媛,孙 玉,李玉泽,张化为,邓 翀,宋小妹,王 薇
华中五味子根皮中1个新的菖蒲烯类倍半萜
张东东,黄仕其,刘媛媛,孙 玉,李玉泽,张化为,邓 翀,宋小妹,王 薇*
陕西中医药大学药学院,陕西 咸阳 712046
研究华中五味子根皮的化学成分及其细胞毒活性。采用硅胶柱色谱、sephadex LH-20葡聚糖凝胶柱色谱和半制备液相色谱进行分离纯化,结合NMR、MS和ECD等数据进行化合物结构鉴定,采用MTT法测定化合物的细胞毒活性。从华中五味子根皮中分离得到11个化合物,分别鉴定为(7,10,11)-2,11,12-三羟基菖蒲烯(1)、五味子醇丁(2)、12-demethylwuweilignan I(3)、戈米辛M1(4)、异戈米辛O(5)、schisphenlignan C(6)、marlignan F(7)、wilsonilignan A(8)、sphenlignan A(9)、schinegllignan A(10)、schinegllignan B(11),化合物2~5和7对人肺癌A549细胞、人结直肠癌HCT116细胞和SW620细胞具有细胞毒活性,半数抑制浓度(median inhibition concentration,IC50)值为12.1~38.7 μmol/L。化合物1为新的倍半萜类化合物,命名为华中五味子倍半萜A;化合物2为新天然产物,化合物3~5、7、8、10~11首次从华中五味子中分离得到,化合物2~5和7具有细胞毒活性。
华中五味子;华中五味子倍半萜A;五味子醇丁;戈米辛M1;异戈米辛O;木脂素;细胞毒活性
华中五味子根皮,又名长胜七,是陕西秦巴地区著名“太白七药”之一,其来源为五味子科(Schisandraceae)五味子属Michx.华中五味子Rehd. et Wils的干燥根皮,主要分布于秦岭山脉和大巴山区各地,具有健脾化湿、涩精止泻等功效,主要用于消化不良,肺虚咳嗽等病症[1]。华中五味子作为我国传统中药,又称“南五味子”,其化学成分主要为木脂素、三萜、倍半萜以及黄酮等[2-4],现代药理研究表明其提取物和单体化合物具有保肝、抗炎、抗肿瘤、抗氧化、免疫抑制等多种药理作用[3-6],目前对于华中五味子植物化学成分的研究主要集中在果实和藤茎等地上部位,而对于根部位的研究较少,为进一步研究华中五味子根皮的化学成分,本研究在前期实验基础之上[3-4]继续对其乙醇提取物的醋酸乙酯部位进行系统分离,从中共鉴定了11个化合物,分别为(7,10,11)-2,11,12-三羟基菖蒲烯[(7,10,11)- 2,11,12-trihydroxycalamenene,1]、五味子醇丁(schisandrol D,2)、12-demethyl- wuweilignan I(3)、戈米辛M1(gomisin M1,4)、异戈米辛O(isogomisin O,5)、schisphenlignan C(6)、marlignan F(7)、wilsonilignan A(8)、sphenlignan A(9)、schinegllignan A(10)、schinegllignan B(11)。其中化合物1为新的倍半萜类化合物,命名为华中五味子倍半萜A,化合物2为新的天然产物,命名为五味子醇丁,化合物3~5、7、8、10~11为首次从该植物中分离得到。细胞毒活性筛选结果发现,化合物2~5和7具有细胞毒活性。
1 仪器与试药
IS50傅里叶变换红外光谱仪(Thermo Nicolet公司);Chirascan CD圆二色谱仪(英国应用光物理公司);SGW-3自动旋光仪(上海精密仪器仪表有限公司);Burker AVANCE 400核磁共振波谱仪(瑞士Burker公司);6550Q-TOF质谱仪(美国 Agilent Technologies);WFH-203三用紫外分析仪(上海精科实业有限公司);Sephadex LH-20(美国GE公司);岛津LC-6AD型半制备液相色谱仪(检测器SPD-20A);CAPCELL PAK C18MGⅡ半制备色谱柱(250 mm×10 mm,5 µm);薄层色谱用硅胶GF254(青岛海洋化工有限公司);硅胶柱色谱(100~200、200~300目;青岛海洋化工厂产品);色谱甲醇,色谱乙腈(天津市科密欧化学试剂有限公司);其他试剂均为分析纯(天津市天力化学试剂有限公司);人肺癌A549细胞、人结直肠癌HCT116细胞和SW620细胞均购于中科院上海细胞库。顺铂(批号B21M11L110000),源叶生物科技有限公司。
华中五味子根皮(长胜七)于2019年9月采自陕西眉县境内,经陕西中医药大学王薇教授鉴定为五味子科五味子属植物华中五味子Rehd. et Wils的根皮。
2 提取与分离
干燥的华中五味子根皮(15.0 kg),用80%乙醇6倍量回流提取3次,每次1.5 h。减压回收乙醇得浸膏,加水分散,用石油醚,醋酸乙酯依次萃取,得到醋酸乙酯部位450 g。醋酸乙酯部位(450 g)经硅胶柱色谱分离,二氯甲烷-甲醇(1∶0~0∶1)梯度洗脱,得到14个组分(SSE1~14),SSE5(30 g)经硅胶柱色谱,二氯甲烷-甲醇(1∶0~0∶1)梯度洗脱,得到7个组分(SSE5-1~5-7),SSE5-2经葡聚糖凝胶Sephadex LH-20柱色谱,二氯甲烷-甲醇(1∶1)洗脱,合并相同流分后再经半制备液相色谱,甲醇-水(69∶31)洗脱,得到化合物2(R=22.3 min,12.3 mg)、3(R=24.1 min,46.4 mg)、4(R=30.5 min,9.2 mg)和5(R=41.0 min,8.2 mg)。SSE5-4经葡聚糖凝胶Sephadex LH-20柱色谱,二氯甲烷-甲醇(1∶1)洗脱,得到4个组分SSE5-4-1~SSE5-4-4,SSE5-4-3经半制备液相色谱,乙腈-水(65∶35)洗脱得到化合物6(R=13.4 min,10.5 mg)、7(R=16.2 min,9.7 mg)、8(R=20.9 min,11.4 mg)和9(R=31.7 min,10.3 mg)。SSE5-4-4经半制备液相色谱,甲醇-水(62∶38)洗脱,得到化合物1(R=16.4 min,13.7 mg)。SSE6(8 g)经硅胶柱色谱,二氯甲烷-甲醇(1∶0~0∶1)梯度洗脱,合并相同流分后,得到5个组分(SSE6-1~6-5),SSE6-2经葡聚糖凝胶Sephadex LH-20柱色谱,二氯甲烷-甲醇(1∶1)洗脱,合并相同流分后,采用半制备液相色谱,甲醇-水(38∶62)洗脱,得到化合物10(R=16.3 min,13.6 mg)和11(R=21.1 min,8.6 mg)。
3 结构鉴定

图1 化合物1的化学结构及主要的1H-1H COSY、HMBC和NOESY相关信号
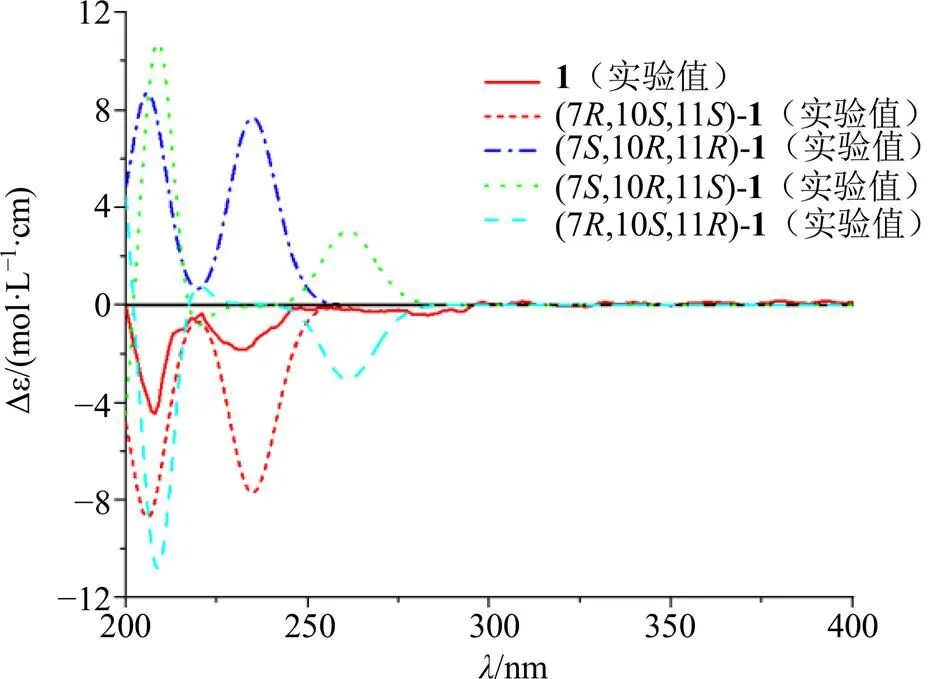
图2 化合物1的实验和计算ECD谱图
化合物2:白色粉末(甲醇),ESI-MS/: 417 [M+H]+;1H-NMR (400 MHz, CD3OD): 6.54 (1H, s, H-11), 6.46 (1H, s, H-4), 5.94 (2H, d,= 0.9 Hz, 12, 13-OCH2O), 5.91 (2H, m, 2, 3-OCH2O), 4.50 (1H, s, H-6), 3.84 (3H, s, -OCH3), 3.75 (3H, s, -OCH3), 2.46 (1H, dd,= 13.8, 9.7 Hz, H-9a), 2.02 (1H, d,= 13.8 Hz, H-9b), 1.81 (1H, m, H-8), 1.33 (3H, s, CH3-17), 1.09 (3H, d,= 7.2 Hz, CH3-18);13C-NMR (100 MHz, CD3OD): 150.4 (C-12), 148.9 (C-3), 142.5 (C-1), 141.8 (C-14), 137.4 (C-13), 137.2 (C-2), 135.9 (C-5), 135.8 (C-10), 122.5 (C-16), 122.4 (C-15), 107.6 (C-4), 103.9 (C-11), 102.4 (12, 13-OCH2O), 102.07 (2, 3-OCH2O), 86.8 (C-6), 74.4 (C-7), 60.0 (-OCH3), 59.8 (-OCH3), 42.8 (C-8), 37.3 (C-9), 29.3 (C-17), 19.2 (C-18)。以上数据通过HSQC和HMBC谱图进行验证,与文献报道基本一致[10],该文献中并没有将此化合物命名,故将其命名为五味子醇丁。
表1 化合物1的1H-NMR (400 MHz, CD3OD) 和13C-NMR (100 MHz, CD3OD) 数据
Table 1 1H-NMR (400 MHz, CD3OD) and 13C-NMR (100 MHz, CD3OD) data of compound 1
碳位δHδC (type) 1—128.6 (C) 2—154.6 (C) 36.59 (1H, s)114.8 (CH) 4—134.0 (C) 57.16 (1H, s)122.2 (CH) 6—143.1 (C) 72.93 (1H, m)44.3 (CH) 81.75 (1H, m, H-8a)30.0 (CH2) 2.03 (1H, m, H-8b) 91.69 (1H, m, H-9a)24.3 (CH2) 1.73 (1H, m, H-9b) 102.73 (1H, m)33.8 (CH) 11—76.8 (C) 123.46 (2H, brs)68.9 (CH2) 131.13 (3H, s)24.3 (CH3) 141.27 (3H, d, J = 7.0 Hz)22.8 (CH3) 152.12 (3H, s)16.0 (CH3)
化合物3:白色粉末(甲醇),ESI-MS/: 417 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.51 (1H, s, H-11), 6.48 (1H, s, H-4), 6.00 (2H, d,= 1.2 Hz, 2, 3-OCH2O), 5.72 (1H, s, 12-OH), 3.92 (3H, s, 13-OCH3), 3.73 (6H, s, 1, 14-OCH3), 3.66 (1H, d,= 8.4 Hz, H-6), 3.02 (3H, s, 6-OCH3), 2.36 (1H, dd,= 13.0, 6.7 Hz, H-9a), 1.95 (1H, d,= 13.0 Hz, H-9b), 1.80 (1H, m, H-8), 1.65 (1H, m, H-7), 0.90 (3H, d,= 6.9 Hz, CH3-18), 0.85 (3H, d,= 7.0 Hz, CH3-17);13C-NMR (100 MHz, CDCl3): 150.9 (C-1), 148.9 (C-12), 147.6 (C-3), 141.8 (C-14), 137.5 (C-10), 137.3 (C-13), 137.2 (C-2), 132.9 (C-5), 123.6 (C-16), 121.7 (C-15), 109.4 (C-11), 107.2 (C-4), 101.3 (2, 3- OCH2O), 90.5 (C-6), 60.9 (14-OCH3), 60.1 (13-OCH3), 59.8 (1-OCH3), 56.0 (6-OCH3), 38.8 (C-9), 36.9 (C-7), 36.8 (C-8), 18.3 (CH3-18), 17.1 (CH3-17)。以上数据与文献报道基本一致[11],故鉴定化合物3为12-demethylwuweilignan I。
化合物4:白色粉末(甲醇),ESI-MS/: 387 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.48 (1H, s, H-11), 6.41 (1H, s, H-4), 5.91 (2H, m, 2, 3-OCH2O), 3.86 (3H, s, 12-OCH3), 3.80 (3H, s, 13-OCH3), 3.79 (3H, s, 1-OCH3), 2.58 (1H, dd,= 13.4, 7.5 Hz, H-9a), 2.44 (1H, dd,= 13.4, 2.0 Hz, H-9b), 2.22 (1H, dd,= 13.2, 9.5 Hz, H-6a), 2.00 (1H, d,= 13.2 Hz, H-6b), 1.88 (1H, m, H-8), 1.78 (1H, m, H-7), 0.98 (3H, d,= 7.2 Hz, CH3-17), 0.74 (3H, d,= 7.1 Hz, CH3-18);13C-NMR (100 MHz, CD3OD): 152.3 (C-12), 150.1 (C-3), 149.0 (C-1), 142.5 (C-14), 139.4 (C-10), 136.2 (C-5), 135.6 (C-13), 135.5 (C-2), 122.6 (C-15), 118.9 (C-16), 107.9 (C-4), 104.1 (C-11), 102.0 (2, 3-OCH2O), 61.1 (13-OCH3), 59.9 (1-OCH3), 56.3 (12-OCH3), 42.3 (C-8), 40.1 (C-6), 36.4 (C-9), 35.0 (C-7), 21.9 (CH3-17), 13.1 (CH3-18)。以上数据与文献报道基本一致[12],故鉴定化合物4为戈米辛M1。
化合物5:白色粉末(甲醇),ESI-MS/: 417 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.58 (1H, s, H-11), 6.56 (1H, s, H-4), 5.98 (2H, m, 2, 3-OCH2O), 4.22 (1H, d,= 9.2 Hz, H-6), 3.86 (3H, s, 1-OCH3), 3.85 (3H, s, 12-OCH3), 3.72 (3H, s, 13-OCH3), 3.69 (3H, s, 14-OCH3), 2.48 (1H, dd,= 12.8, 7.2 Hz, H-9a), 2.03 (1H, m, H-9b), 1.82 (1H, m, H-8), 1.50 (1H, m, H-7), 0.96 (3H, d,= 6.8 Hz, CH3-17), 0.88 (3H, d,= 7.0 Hz, CH3-18);13C-NMR (100 MHz, CD3OD): 154.5 (C-12), 153.2 (C-14), 149.6 (C-3), 142.6 (C-1), 141.1 (C-13), 138.5 (C-10), 138.0 (C-2), 135.4 (C-5), 124.0 (C-16), 123.9 (C-15), 107.9 (C-11), 107.3 (C-4), 102.6 (2, 3-OCH2O), 81.5 (C-6), 61.3 (1-OCH3), 61.3 (14-OCH3), 59.9 (13-OCH3), 56.5 (12-OCH3), 40.2 (C-7), 38.9 (C-9), 34.7 (C-8), 18.5 (CH3-17), 16.9 (CH3-18)。以上数据与文献报道基本一致[13],故鉴定化合物5为isogomisin O。
化合物6:白色粉末(甲醇),ESI-MS/: 509 [M+H]+。1H-NMR (400 MHz, CD3OD): 7.60~7.18 (5H, overlapped, -OBz), 6.75 (1H, s, H-4), 6.68 (1H, s, H-11), 5.98 (2H, m, 2, 3-OCH2O), 5.97 (1H, brs, H-6), 3.71 (3H, s, 1-OCH3), 2.80 (3H, s, 14-OCH3), 2.58 (1H, dd,= 13.9, 9.8 Hz, H-9a), 2.13 (1H, d,= 13.9 Hz, H-9b), 2.06 (1H, m, H-8), 1.25 (3H, s, CH3-17), 1.15 (3H, d,= 7.1 Hz, CH3-18);13C-NMR (100 MHz, CD3OD): 166.7 (C-1′), 149.0 (C-3), 147.1 (C-13), 146.1 (C-14), 142.6 (C-1), 138.6 (C-2), 137.4 (C-12), 134.4 (C-2′), 134.1 (C-5′), 131.4 (C-10), 130.7 (C-5), 130.6 (C-4′, 6′), 129.4 (C-3′, 7′), 123.7 (C-16), 122.3 (C-15), 111.9 (C-11), 108.4 (C-4), 102.7 (2, 3-OCH2O), 85.7 (C-6), 73.7 (C-7), 60.2 (1-OCH3), 59.2 (14-OCH3), 43.9 (C-8), 37.0 (C-9), 28.9 (CH3-17), 19.2 (CH3-18)。以上数据与文献报道基本一致[14],故鉴定化合物6为schisphenlignan C。
化合物7:白色粉末(甲醇),ESI-MS/: 419 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.57 (1H, s, H-4), 6.41 (1H, s, H-11), 3.87 (3H, s, 2-OCH3), 3.84 (3H, s, 13-OCH3), 3.71 (3H, s, 14-OCH3), 3.42 (3H, s, 1-OCH3), 3.00 (3H, s, 6-OCH3), 3.66 (1H, m, H-6), 2.33 (1H, m, H-9a), 1.90 (1H, dd,= 12.9, 9.9 Hz, H-9b), 1.83 (1H, m, H-8), 1.62 (1H, m, H-7), 0.91 (3H, d,= 6.8 Hz, CH3-18), 0.86 (3H, d,= 6.9 Hz, CH3-17);13C-NMR (100 MHz, CD3OD): 152.9 (C-1), 152.8 (C-3), 151.3 (C-14), 150.0 (C-12), 141.8 (C-2), 139.8 (C-13), 138.2 (C-10), 135.2 (C-5), 124.0 (C-16), 122.6 (C-15), 117.0 (C-4), 111.2 (C-11), 91.5 (C-6), 61.1 (1-OCH3), 61.0 (2-OCH3), 60.6 (13-OCH3), 60.3 (14-OCH3), 56.0 (6-OCH3), 39.7 (C-7), 38.5 (C-9), 38.3 (C-8), 18.3 (CH3-17), 16.8 (CH3-18)。以上数据与文献报道基本一致[15],故鉴定化合物7为marlignan F。
化合物8:白色粉末(甲醇),ESI-MS/: 403 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.93 (1H, s, H-4), 6.48 (1H, s, H-11), 5.93 (2H, brs, 12, 13-OCH2O), 4.42 (1H, d,= 1.5 Hz, H-6), 3.86 (3H, s, 1-OCH3), 3.75 (3H, s, 2-OCH3), 3.46 (3H, s, 3-OCH3), 2.15 (1H, m, H-7), 1.97 (2H, m, CH2-9), 1.87 (1H, m, H-8), 1.02 (3H, d,= 7.1 Hz, CH3-17), 0.66 (3H, d,= 7.0 Hz, CH3-18);13C-NMR (100 MHz, CD3OD): 152.1 (C-1), 150.6 (C-3), 150.5 (C-12), 142.1 (C-2), 140.5 (C-14), 139.3 (C-5), 138.2 (C-10), 136.3 (C-13), 121.5 (C-16), 121.1 (C-15), 111.9 (C-4), 103.9 (C-11), 102.2 (12, 13-OCH2O), 74.0 (C-6), 61.2 (2-OCH3), 60.7 (1-OCH3), 59.9 (3-OCH3), 44.2 (C-7), 40.8 (C-8), 35.5 (C-9), 22.4 (CH3-18), 8.1 (CH3-17)。以上数据与文献报道基本一致[16],故鉴定化合物8为wilsonilignan A。
化合物9:淡绿色粉末(甲醇),ESI-MS/: 403 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.55 (1H, s, H-4), 6.43 (1H, s, H-11), 5.97 (2H, brs, OCH2O), 4.20 (1H, d,= 9.3 Hz, H-6), 3.86 (3H, s, 1-OCH3), 3.72 (3H, s, 2-OCH3), 3.69 (3H, s, 3-OCH3), 2.38 (1H, m, H-9a), 1.90 (1H, m, H-9b), 1.77 (1H, m, H-8), 1.54 (1H, m, H-7), 0.96 (3H, d,= 6.8 Hz, CH3-17), 0.86 (3H, d,= 6.9 Hz, CH3-18);13C-NMR (100 MHz, CD3OD): 153.1 (C-1), 151.5 (C-3), 149.5 (C-12), 142.6 (C-2), 139.9 (C-14), 138.3 (C-10), 137.9 (C-13), 124.2 (C-16), 122.4 (C-5), 122.3 (C-15), 111.3 (C-4), 107.3 (C-11), 102.5 (12, 13-OCH2O), 81.5 (C-6), 61.1 (2-OCH3), 60.9 (1-OCH3), 59.9 (3-OCH3), 40.1 (C-7), 39.9 (C-9), 39.1 (C-8), 18.6 (CH3-18), 16.2 (CH3-17)。以上数据与文献中化合物数据基本一致[17],故鉴定化合物9为sphenlignan A。
化合物10:白色粉末(甲醇),ESI-MS/: 389 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.53 (1H, s, H-4), 6.43 (1H, s, H-11), 3.86 (3H, s, 12-OCH3), 3.82 (3H, s, 2-OCH3), 3.79 (3H, s, 14-OCH3), 3.54 (3H, s, 1-OCH3), 2.60 (1H, dd,= 13.5, 7.6 Hz, H-6a), 2.45 (1H, dd,= 13.5, 1.9 Hz, H-6b), 2.21 (1H, dd,= 13.0, 9.4 Hz, H-9a), 1.97 (1H, d,= 13.0 Hz, H-9b), 1.74 (1H, m, H-8), 1.88 (1H, m, H-7), 0.98 (3H, d,= 7.1 Hz, CH3-18), 0.74 (3H, d,= 7.1 Hz, CH3-17);13C-NMR (100 MHz, CD3OD): 152.4 (C-12), 152.3 (C-14), 151.4 (C-1), 148.9 (C-3), 141.3 (C-2), 139.7 (C-13), 135.7 (C-5), 135.6 (C-10), 122.0 (C-16), 119.1 (C-15), 112.4 (C-4), 108.0 (C-11), 61.1 (14-OCH3), 61.0 (2-OCH3), 60.8 (1-OCH3), 56.2 (12-OCH3), 42.6 (C-8), 40.1 (C-6), 36.2 (C-9), 35.2 (C-7), 22.1 (C-17), 13.0 (C-18)。以上数据与文献报道基本一致[18],故鉴定化合物10为schinegllignan A。
化合物11:白色粉末(甲醇),ESI-MS/: 389 [M+H]+。1H-NMR (400 MHz, CD3OD): 6.55 (1H, s, H-4), 6.41 (1H, s, H-11), 3.87 (3H, s, 12-OCH3), 3.84 (3H, s, 13-OCH3), 3.80 (3H, s, 2-OCH3), 3.55 (3H, s, 14-OCH3), 2.52 (1H, dd,= 13.4, 7.5 Hz, H-6a), 2.41 (1H, dd,= 13.4, 2.0 Hz, H-6b), 2.24 (1H, dd,= 13.1, 9.4 Hz, H-9a), 2.04 (1H, d,= 13.1 Hz, H-9b), 1.86 (1H, m, H-7), 1.43 (1H, m, H-8), 1.00 (3H, d,= 7.1 Hz, CH3-18), 0.73 (3H, d,= 7.1 Hz, CH3-17);13C-NMR (100 MHz, CD3OD): 153.6 (C-14), 152.7 (C-12), 150.1 (C-3), 148.7 (C-1), 140.9 (C-13), 140.0 (C-2), 135.9 (C-10), 135.3 (C-5), 123.1 (C-16), 118.1 (C-15), 115.7 (C-4), 104.4 (C-11), 61.2 (13-OCH3), 61.1 (14-OCH3), 60.8 (2-OCH3), 56.2 (12-OCH3), 42.4 (C-8), 39.8 (C-6), 36.5 (C-9), 35.3 (C-7), 22.1 (C-17), 13.0 (C-18)。以上数据与文献报道基本一致[18],故鉴定化合物11为schinegllignan B。
4 细胞毒活性测试
采用MTT法测定化合物1~11对A549细胞、HCT116细胞和SW620细胞的细胞毒活性,顺铂为阳性对照,实验所用仪器材料以及实验过程均与本课题组前期报道一致[19-20]。实验结果(表2)表明化合物2~5和7对A549、HCT116、SW620细胞株具有细胞毒活性,IC50值为12.1~38.7 μmol/L,其余化合物的IC50值均大于50 μmol/L。
5 讨论
从华中五味子根皮中分离鉴定了11个化合物,包括1个新的倍半萜(1),10个联苯环辛烯型木脂素(2~11),其中化合物2为新的天然产物,化合物3~5、7、8、10和11为首次从该植物中分离得到,进一步丰富了华中五味子的化学成分。细胞毒活性实验显示化合物2~5和7具有细胞毒活性,但本文仅测定了11个化合物的体外细胞毒活性,关于这些化合物的其他活性有待于进一步研究。
表2 化合物1~11对3种肿瘤细胞的细胞毒活性
Table 2 Cytotoxic activities of 1-11 in three cancer cell lines
化合物IC50/(μmol·L−1)A549HCT116SW620 1>50>50>50 231.7±2.4>50>50 317.4±1.523.6±0.625.2±1.2 413.3±1.412.1±0.518.8±0.7 516.9±0.214.4±0.618.4±0.4 6>50>50>50 738.7±0.5>50>50 8>50>50>50 9>50>50>50 10>50>50>50 11>50>50>50 顺铂32.1±1.343.5±3.332.5±3.4
利益冲突 所有作者均声明不存在利益冲突
[1] 毛水龙. 秦岭七药 [M]. 西安: 西安交通大学出版社, 2011: 68-69.
[2] 刘媛媛, 黄仕其, 李玉泽, 等. 五味子属植物木脂素类化学成分及其药理作用研究进展[J]. 中草药, 2022, 53(6): 1903-1918.
[3] Huang S Q, Zhang D D, Li Y Z,.: A comprehensive review of its botany, phytochemistry, pharmacology, and clinical applications [J]., 2021, 49(7): 1577-1622.
[4] Huang S Q, Liu Y Y, Li Y Z,. Dibenzocyclooctadiene lignans from the root bark of[J]., 2021, 45: 137-141.
[5] 黄泽豪, 秦路平. 华中五味子藤茎的化学成分研究 [J]. 中草药, 2016, 47(19): 3374-3378.
[6] 金银萍, 焉石, 刘俊霞, 等. 五味子科植物中降三萜类成分及其药理作用研究进展 [J]. 中草药, 2014, 45(11): 1643-1650.
[7] Salmoun M, Braekman J C, Ranarivelo Y,. New calamenene sesquiterpenes from[J]., 2007, 21(2): 111-120.
[8] Zhang D D, Shi Y H, Shi S S,. Isatisindigoticanine A, a novel indole alkaloid with an unpresented carbon skeleton from the roots of[J]., 2021, 35(8): 1249-1255.
[9] Zhang D D, Sun Y, Chen Z Q,. Bisindole alkaloids with nitric oxide inhibitory activities from an alcohol extract of theindigotica roots [J]., 2020, 146:104654.
[10] Ikeya Y, Taguchi H, Yosioka I. The constituents ofBaill. XII. Isolation and structure of a new lignan, gomisin R, the absolute structure of wuweizisu C and isolation of schisantherin D [J]., 1982, 30(9): 3207-3211.
[11] Luo X, Pu J X, Fan P,. Four new dibenzocyclooctadiene lignans from[J]., 2011, 9(3): 167-172.
[12] Ikeya Y, Taguchi H, Yosioka I. The Constituents ofBaill. X. The Structures of.GAMMA.-schizandrin and four new lignans, (−)-gomisins L1 and L2, (+)-gomisin M1 and (+)-gomisin M2 [J]., 1982, 30(1): 132-139.
[13] Ikeya Y, Ookawa N, Taguchi H,. The constituents ofBaill. XI. The structures of three new lignans, angeloylgomisin O, and angeloyl- and benzoylisogomisin O [J]., 1982, 30(9): 3202-3206.
[14] Liang C Q, Hu J, Shi Y M,. Schisphenlignans A-E: Five new dibenzocyclooctadiene lignans from[J]., 2013, 61(1): 96-100.
[15] Yang G Y, Li Y K, Wang R R,. Dibenzocyclooctadiene lignans fromand their anti-HIV-1 activities [J]., 2010, 73(5): 915-919.
[16] Yang G Y, Li Y K, Wang R R,. Dibenzocyclooctadiene lignans from the fruits ofand their anti-HIV-1 activities [J]., 2010, 12(6): 470-476.
[17] 贺飞. 三种药用植物的化学成分和生物活性研究 [D]. 北京: 中国科学院昆明植物研究所, 2009.
[18] Duan Y X, Cao J L, Wen R R,. Dibenzocyclooctadiene lignans fromand their anti-HIV-1 activities [J]., 2011, 13(7): 592-598.
[19] Sun Y, Ding C, Wang F R,. Pregnane alkaloids with BRD4 inhibitory and cytotoxic activities from[J]., 2021, 45: 63-67.
[20] 张东东, 樊浩, 孙玉, 等. 缬草中1个新的单环氧木脂素 [J]. 中草药, 2022, 53(1): 25-30.
A new calamenene sesquiterpenoid from root bark of
ZHANG Dong-dong, HUANG Shi-qi, LIU Yuan-yuan, SUN Yu, LI Yu-ze, ZHANG Hua-wei, DENG Chong, SONG Xiao-mei, WANG Wei
School of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang 712046, China
To study the chemical constituents from the root bark ofand their cytotoxic activities.The compounds were isolated and purified by silica, sephadexLH-20 and semi preparative-HPLC and the chemical structures were identified by NMR, MS and ECD data analysis.Eleven compounds were isolated and deduced as: (7,10,11)-2,11,12-trihydroxycalamenene (1), schisandrol D (2), 12-demethylwuweilignan I (3), gomisin M1 (4), isogomisin O (5), schisphenlignan C (6), marlignan F (7), wilsonilignan A (8), sphenlignan A (9), schinegllignan A (10), schinegllignan B (11). Compounds 2—5 and 7 showed cytotoxic activity against A549, HCT116 and SW620 cell lines with IC50values ranging from 12.1 to 38.7 μmol/L.Compound 1 is a new sesquiterpene, named as schisphensesquiterpene A, and compound 2 is a new natural product. Compounds 3—5, 7, 8, 10—11 were isolated from theplant for the first time, and compounds 2—5 and 7 exhibited cytotoxic activities.
Rehd. et Wils; schisphensesquiterpene A; schisandrol D;gomisin M1; isogomisin O; lignans; cytotoxity
R284.1
A
0253 - 2670(2022)14 - 4270 - 06
10.7501/j.issn.0253-2670.2022.14.006
2022-01-21
国家自然科学基金项目(82174111);国家自然科学基金项目(82104368);陕西中医药大学学科创新团队项目(2019-YL12)
张东东,男,博士,讲师,主要从事中药药效物质基础研究。E-mail: zhangnatprod@163.com
王 薇,女,博士,教授,主要从事中药药效物质基础及中药炮制研究。E-mail: 2051003@sntcm.edu.cn
[责任编辑 王文倩]
