Assembling Superfine Bi3TaO7 Particles into 2D Fe2O3 Nanosheets for Enhanced Usability to Aqueous Tetracycline Residues
2022-07-16LiuXiaoqingChengHongjunWangZishaZhangJianLanYanhuaWangXiaojing
Liu Xiaoqing; Cheng Hongjun; Wang Zisha; Zhang Jian; Lan Yanhua; Wang Xiaojing
(1. School of Enνironment and Safety Engineering, North Uniνersity of China, Taiyuan, 030051;2. Inner Mongolia Key Laboratory of Chemistry and Physics of Rare Earth Materials, School of Chemistry and Chemical Engineering, Inner Mongolia Uniνersity, Huhhot, Inner Mongolia, 010021)
Abstract: Composites of 2D/0D Fe2O3-Bi3TaO7 (FO-BTO) prepared by a hydrothermal method in which superfine Bi3TaO7 particles were mounted onto lamellae of Fe2O3 sheets could efficiently remove aqueous tetracycline (TC) residues. The optimal composite FO-3BTO had a TC removal rate of 95% in 120 min under solar light, and its overall properties were better than those of reported photocatalysts. According to XRD, HRTEM, XPS, SEM, PL, EIS, and photocurrent tests,Fe2O3 and Bi3TaO7 composites formed on effective S-scheme heterojunctions, and the tight contact structure contributed to the increase in efficiency of aqueous TC residue removal.
Key words: Bi3TaO7; Fe2O3; S-scheme heterojunction; Tetracycline degradation; Photocatalytic efficiency
1 Introduction
Environmental problems have become major challenges because of economic development and rapid expansion of industrialization. Water, a vital resource, has particularly suffered from environmental pollution. Water pollution seriously affects human health, threatens the environment,and restricts economic progress and sustainable development[1-5]. The tetracycline hydrochloride(TC) series is one example of pollutants. Generally,conventional methods such as chemical coagulation,advanced oxidation, adsorption, membrane separation,and biodegradation are ineffective in decontamination of aqueous TC residuals because of the resistant structure,wide solubility, and high biotoxicity of TC[6-11]. By contrast, photocatalytic technology provides a feasible approach for the degradation of TC, because it is costeffective, environmentally friendly and sustainable, and operationally convenient[12-17].
In photocatalytic degradation of TC, the valence band position of the catalyst needs to be higher than the redox potential (2.40 eV vs NHE) and the H2O/O2ratio (1.24 eV vs NHE) of TC[18]. Therefore, an efficient photocatalyst must have enough high band potential in order to have a force strong enough to drive the photocatalytic reaction.Bismuth tantalate (Bi3TaO7) is a semiconductor with large positive chemical potential, and therefore, oxidation by Bi3TaO7in the photocatalytic degradation of TC is much higher than that of general photocatalytic materials[19-21].Moreover, Bi3TaO7is stable in acidic or alkaline solutions and thus has great potential for the treatment of contaminants in actual complex aqueous environments[22].However, Bi3TaO7also has limitations, and in particular,the solar response range is relatively narrow and thus use of visible light is low[19]. The high recombination of photo-generated carriers is another significant problem that affects the process of TC degradation. In industry,functionality of materials can be improved by controlling crystal sizes to remain at the nanoscale in order to increase surface area[22]. However, super small size may be a challenge for reusability because of high dispersion and agglomeration in aqueous environments. In general,it is important to combine a narrow-gap semiconductor with a wide-gap one in order to better use sunlight[23-25].Simultaneously, such composites can significantly inhibit recombination of photo-generated carriers[26-28].
A narrow band-gap hematite (Fe2O3) is currently widely used in the study of photocatalytic treatment of organic pollutants in the environment because it is environmentally friendly and thermodynamically stable(stable through a wide pH range), has excellent visible light response (band gap 2.2 eV), and is nontoxic and low-cost[29-31]. For example, an Fe2O3/MoO3/AgBr ternary catalytic system showed excellent degradation and cycling stability for the removal of organic dye acid blue[32]. Chen et al.[33]prepared Pt-supported α-Fe2O3nanocatalysts by controlling the exposure of (113) and (100) crystal plane,and the removal rate of formaldehyde on Pt/α-Fe2O3at room temperature was very high and stable. Therefore,Fe2O3would be a good choice as one of the photocatalyst components in the treatment of TC residues because it is effective, nontoxic, cheap, and easy to prepare.
In this study, a series of composites of Fe2O3and Bi3TaO7were prepared by a hydrothermal method, and then,photocatalytic degradation of TC was investigated under simulated sunlight. Composite structure, morphology,chemical constitution, and optical and electrochemical performances were characterized, and a possible mechanism to explain increases in photocatalytic degradation was proposed. Effects of solution pH and ionic strength on TC adsorption by Fe2O3-Bi3TaO7were also explored.
2 Experimental
2.1 Reagents
Reagents used in this investigation were all analytical grade, and no further purification was required. Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), tantalum pentachloride (TaCl5), potassium hydroxide (KOH), nitric acid (HNO3), tetracycline hydrochloride (C22H24N2O8),sodium carbonate (Na2CO3), and anhydrous ethanol(C2H6O) were purchased from Aladdin (Shanghai, China).
2.2 Preparation of samples
A hydrothermal method was used to prepare Bi3TaO7.First, 2.1830 g of Bi(NO3)3·5H2O and 0.5372 g of TaCl5were dissolved in 20 mL of anhydrous ethanol, with continuous stirring with a magnetic stirrer for 60 min.Then, TaCl5solution was added to this solution and stirred for another 30 min. The pH of the mixed solution was adjusted to 10.0 with a 7 mol/L KOH solution. Stirring was continued for another 60 min, and then, the solution was transferred to a 100-mL autoclave and heated to 220 °C for 24 h. After cooling to room temperature, the material was alternately washed with water and anhydrous ethanol twice. The Bi3TaO7sample was obtained after oven-drying overnight at 60 °C.
The Fe2O3sample was prepared in the following steps.First, 1.09 g of FeCl3·6H2O was dissolved in 40 mL of anhydrous ethanol. After continuous stirring for 30 min on a magnetic stirrer, 3.2 g of NaAc (CH3COONa) was added to the solution. The solution was transferred to a 100-mL hydrothermal reactor and placed in an oven at 180 °C for 12 h. After cooling to room temperature, the Fe2O3sample was centrifuged and alternately washed with distilled water and anhydrous ethanol twice. Last,the sample was dried at 80 °C in a vacuum oven for 24 h.The fabrication route of Fe2O3-Bi3TaO7complexes was consistent with the process used for Bi3TaO7. The pH of a mixed solution of Bi(NO3)3and TaCl5was adjusted to 10.0 with a 7 mol/L KOH solution. Then, different amounts of Fe2O3were added to the mixed solution,and the mixtures were stirred continuously for 30 min.Mixtures were transferred to 100-mL hydrothermal reactors and reacted at 220 °C for 24 h. After naturally cooling to room temperature, solid products were centrifuged and then washed twice with deionized water and absolute ethanol. Products were dried at a constant oven temperature of 60 °C for 10 h. A series of Fe2O3-Bi3TaO7composites were obtained by controlling the molar ratio of Fe2O3to Bi3TaO7. The Fe2O3:Ta ratios were 1:1, 1:2, 1:3, and 1:4, which were named FO-BTO, FO-2BTO, FO-3BTO, and FO-4BTO, respectively.
2.3 Characterization
Crystallinity and structure of the as-prepared catalysts were characterized by powder X-ray diffraction (XRD,Panalytical Empyream Instrument) using Cu kα (λ =1.5418 Å) radiation under the operating conditions of 40 kV and 50 mA. Morphologies and element contents were analyzed using transmission electron microscopy(TEM), high-resolution transmission electron microscopy(HRTEM), and energy dispersive X-ray spectrum(EDX) on a Tecnai G2 F20 S-TWIN apparatus with an acceleration voltage of 200.0 kV. To characterize chemical valence states of prepared samples, X-ray photoelectron spectra analysis (XPS) was performed on an ESCALAB 250 with an Al kα (1486.6 eV) line. Fourier transform infrared spectroscopy (FT-IR, Bruker VERTEX 70v)was used to detect the existence formation on the surface of the samples. The diffuse reflection spectrum of photocatalysts was determined by an UV-visible diffusive reflectance spectrophotometer (UVIKON XL/XS), with BaSO4used as the reference standard.
2.4 Assessment of TC photocatalytic degradation activity
Activity of prepared samples was evaluated by degradation of a TC aqueous solution (TC = 20 mg/L) in a photocatalytic reactor. A 25-mg sample was weighed and dispersed directly in 50 mL of TC aqueous solution.The light source was a 500 W xenon lamp (without filter). A constant reaction temperature was maintained by cooling circulating water. The reaction occurred in the dark for 100 min before turning on the light, and the adsorption-desorption equilibrium was determined under constant stirring. After completion of the photochemical reaction, the sample was centrifuged, and TC contents in the centrifugate were detected with a UV-difference spectrophotometer. Detection wavelength was set to 200 -800 nm, and the baseline was calibrated with the deionized water.
Effect of TC concentration on degradation performance was estimated by setting the initial concentration to 10,20, 30, or 40 to 50 mg/L under the same experimental process. Effect of solution pH on degradation was studied by placing 25 mg of catalyst in a 20 mg/L TC solution with pH was adjusted to 3, 5, 7, 9, or 11 with 0.1 mol/L HCl or NaOH.
2.5 Photo-electrochemical measurement
Electrochemical impendence spectroscopy (EIS) was examined on an FTO conductive glass with a thin film,which was prepared via a manual deposition method in a sodium sulfate electrolyte solution (0.2 mol/L).Regulation frequency was from 100 kHz to 0.1 Hz. The photocurrent was investigated on an Autolab model AUT302N-FRA32M.V electrochemical workstation by using a standard system of three electrodes. A standard Ag/AgCl electrode, platinum wire electrode, and the asobtained samples were the reference electrode, auxiliary electrode, and working electrode, respectively. An LED light (LDCNW, neutral white, 4100K, 700 mA, 690 lm) was used to provide the visible light, and the time interval was 20 s between illumination and darkness.To prepare the working electrode, 5 mg of catalyst was dispersed into a mixed solution that consisted of 90 µL of anhydrous ethanol, 60 µL of deionized water, and 10µL of perfluorosulfonic acid-PTFE copolymer, and after stirring for 12 h, the prepared solution was dip-coated by a manual coating method.
3 Results and Discussion
For pure phase Bi3TaO7, all diffraction peaks corresponded to the standard cards of Bi3TaO7(JCPDS 00-044-0202), and there were no impurity peaks (Figure 1). Diffraction peaks of pristine Fe2O3matched well with the standard card of Fe2O3(JCPDS 98-005-6372) (Figure 1), suggesting it was pure and highly crystalline. For FO-BTO composite phases, characteristic diffraction peaks were observed for both Bi3TaO7and Fe2O3, and no impurity appeared in the observed peak regions (Figure 1). In addition, the intensity of Bi3TaO7increased with the increase in molar ratio of Bi3TaO7to Fe2O3.

Figure 1 The XRD patterns of Fe2O3, Bi3TaO7, and Fe2O3–Bi3TaO7 composites with different molar proportions

Figure 2 The XPS survey spectrum of (a) FO-3BTO composite and XPS spectra of (b) Fe 2p orbitals, (c) Bi 4f orbitals, (d)Ta 4f orbitals, and (e) and O 1s orbitals
An XPS analysis was conducted to further examine the chemical valence states. In the XPS survey spectrum of FO-3BTO (Figure 2(a)), Bi, Ta, Fe, O, and C elements all appeared without other impurity elements. The binding energy at 284.75 eV ascribed to C elements was the calibration value of C 1 s. The Fe 2p spectra was resolved into two peaks at 710.7 and 724.4 eV with a spacing of 13.7 eV (Figure 2(b)), indicating an Fe3+(2p3/2and 2p1/2)oxidation state[34]. In addition, a peak was identified at~718.9 eV between Fe 2p1/2and Fe 2p3/2peaks. The peak was considered to be an associate satellite peak of the Fe 2p3/2main peak, which is a characteristic feature ofBimodal peaks at 158.8 and 164.2 eV were attributed to Bi3+4f (Figure 2(c)), whereas bimodal binding energies at 25.6 and 28.2 eV corresponded to Ta5+4f7/2and Ta5+4f5/2(Figure 2(d)), respectively[36-39]. In Figure 2e, O 1s orbitals were mainly attributed to 529.6,530.9, and 532.3 eV, which were assigned to lattice oxygen, hydroxyl oxygen adsorbed on the surface, and O2adsorbed on the surface, respectively[40-43]. The binding energy of lattice oxygen also shifted to a value 0.2 eV higher for FO-3BTO than for both Fe2O3and Bi3TaO7,indicating a strong interaction between Fe2O3and Bi3TaO7during formation of the complex.
The morphologies of prepared samples were investigated by TEM. The Bi3TaO7had superfine particles from 3 to 5 nm in size (Figure 3(a)), whereas the Fe2O3was in regular quadrilateral nanosheets from 70 to 100 nm in size (Figure 3(b)). To assemble FO-3BTO, 0D superfine particles were stacked on the lamellae of Fe2O3(Figure 3(c)). As shown by HRTEM (Figure 3(d)), there were two distinct lattice fringes of 0.321 nm and 0.219 nm, corresponding to Bi3TaO7(1 1 1) and Fe2O3(1 1 3) planes, respectively,which indicated that the two pure phases in the FO-3BTO complex were in closely contact[44]. To further investigate the distribution and mass ratio of each element in FO-3BTO, an SEM-EDX test was conducted (Figure 4). Figure 4a shows a sample test selection chart, and Figure 4b shows the signal strength of each element. The uniform distribution of Bi3TaO7on Fe2O3was confirmed by the locations of Bi, Ta, Fe, and O elements (Figure 4cf). Mass ratios of Bi, O, Ta, and Fe were 60.43%, 18.30%,16.48%, and 4.79%, respectively, which were similar to the theoretical values of 64.43%, 13.15%, 18.59%, and 3.83%, respectively (Figure 4g).

Figure 5 (a) UV-visible diffuse reflectance spectrum and (b)band gap of as-prepared samples
To better study the light-harvesting capability of the prepared catalysts, the UV-vis diffuse reflectance spectra were determined for Bi3TaO7, Fe2O3, and FO-3BTO.The Bi3TaO7mainly absorbed ultraviolet light in the range from 250 to 420 nm; whereas Fe2O3had good light absorption ability for visible light at wavelengths from 400 to 650 nm (Figure 5(a)). In the FO-3BTO composite of Bi3TaO7and Fe2O3, the region of enhanced absorption ability was extended from 250 nm to 600 nm. Therefore,the composite effect increased the range of the solar light spectrum for potential excitement. The band gap of the samples was calculated using the formulaahν =A(hν-Eg)n/2, wherea,h, ν,A, andEgrepresent the optical absorption coefficient, Planck constant, frequency of incident light, proportional constant, and band gaps,respectively. Thenvalue is related to the transition type of the semiconductor. When the transition occurs in a direct transition,n= 1, whereasn= 4 for an indirect transition. According to the literature[45-46], Fe2O3and Bi3TaO7are all indirect transitions. Therefore, mapping(ahν)2with photon energy (hν) indicated the band gap values of pure phase Bi3TaO7and Fe2O3were 2.92 eV and 2.08 eV, respectively, whereas for FO-3BTO, the value was 2.15 eV (Figure 5(b)).
To further evaluate transfer ability of photo-generated carriers of the samples, Bi3TaO7, Fe2O3, and FO-3BTO were tested for photocurrent and impedance spectra.The three samples gave timely responses without delay, suggesting fast photoelectric response ability.The composite FO-3BTO had the strongest current intensity and quickly achieved stable attenuation (Figure 6(a)). Therefore, heterojunctions that formed between components of the complex effectively inhibited recombination of photo-excited electrons and holes and thus improved the photoelectric conversion efficiency. In addition, the prepared samples showed a typical circular Nyquist response (Figure 6(b)). The composite FO-3BTO had the smallest arc radius, suggesting the lowest total average resistance. Thus, heterojunctions formed between Bi3TaO7and Fe2O3greatly improved the separation efficiency of photo-generated carriers. The fluorescence spectra of the samples were also tested (Figure 6(c)).Compared with the two pure phases, FO-3BTO had the lowest fluorescence intensity and therefore the lowest recombination rate of photo-generated carriers. Thus, the tight heterojunction structure pushed photo-generated carriers into fast migration, and then, the composite phase effectively suppressed the recombination of photogenerated carriers.
To evaluate photocatalytic activity of the prepared samples, TC degradation was determined under a full light spectrum (i.e., simulated solar light). Before light irradiation, samples were agitated for 100 min in the dark to achieve adsorption-desorption equilibrium. Adsorption of Fe2O3was poor, whereas approximately 25% to 40% of TC was adsorbed by composite FO-BTOs (Figure 7(a)).
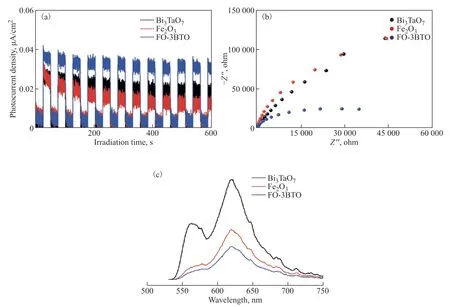
Figure 6 (a) Photocurrent responses, (b) EIS Nyquist plots, and (c) photoluminescence spectra of Bi3TaO7, Fe2O3, and FO-3BTO

Figure 7 (a) Photo-degradation of TC, (b) linear transformation (−ln(C/C0) = kt) of TC degradation, (c) kinetic constants (k)for the TC reaction, and (d) cycling test for TC degradation over FO-3BTO under simulated solar light irradiation
When a 500 W xenon lamp was used without filters, TC degradation was negligible in the absence of catalysts, but all samples accelerated TC removal. The composite FO-3BTO had the best TC removal efficiency, reaching 95%.The TC concentration changed exponentially as light time increased, and therefore, the degradation process was well described by the first-order kinetic equation of −ln(C/C0) =kt, whereC,C0, andkrepresent the initiation concentration, the concentration at timet, and the rate constant, respectively (Figure 7(b)). The FO-3BTO photodegradation rate constant of 1.445 h−1was much higher than that of Bi3TaO7(0.434 h−1) and Fe2O3(0.186 h−1)(Figure 7(c)). A cycle experiment was also conducted over FO-3BTO to determine TC removal in four successive runs. After each test, the photocatalyst was centrifuged,washed, and used in next the run. Degradation activity of FO-3BTO was almost unchanged after the first two cycles, but 30% deterioration was observed after four runs (Figure 7(d)).
The effect of pH value on TC removal was also investigated (Figure 8a). The pH value of initial solutions was adjusted to 3.0, 5.0, 7.0, 9.0, or 11.0 by adding appropriate amounts of HCl or NaOH aqueous solution. With the FO-3BTO composite, adsorption and degradation were excellent under a neutral reactive condition, whereas they decreased when the reactive solution was strongly alkaline (pH 11). Notably, the charge-charge adsorption interaction between FO-3BTO and TC clearly weakened in strongly acidic or alkaline solutions, resulting in reduced degradation of TC. Effects of initial TC concentrations on removal rate were also explored. When the initial concentration increased from 10 mg/L to 50 mg/L, TC removal efficiency decreased from 96% to 79.5% (Figure 8b), indicating that this type of photocatalyst may not be very effective at TC removal at high concentrations. Nevertheless, AMX concentration is approximately 10 to 20 mg/L in effluent of a typical factory, and concentrations are even lower in domestic wastewater. Thus, the samples prepared in this study would generally be effective at removing amoxicillin(AMX) residuals. The photocatalytic performance for removal of TC in this study was compared with that in previous studies (Table 1), and the comparison confirmed the high performance of the as-prepared composite in this work.
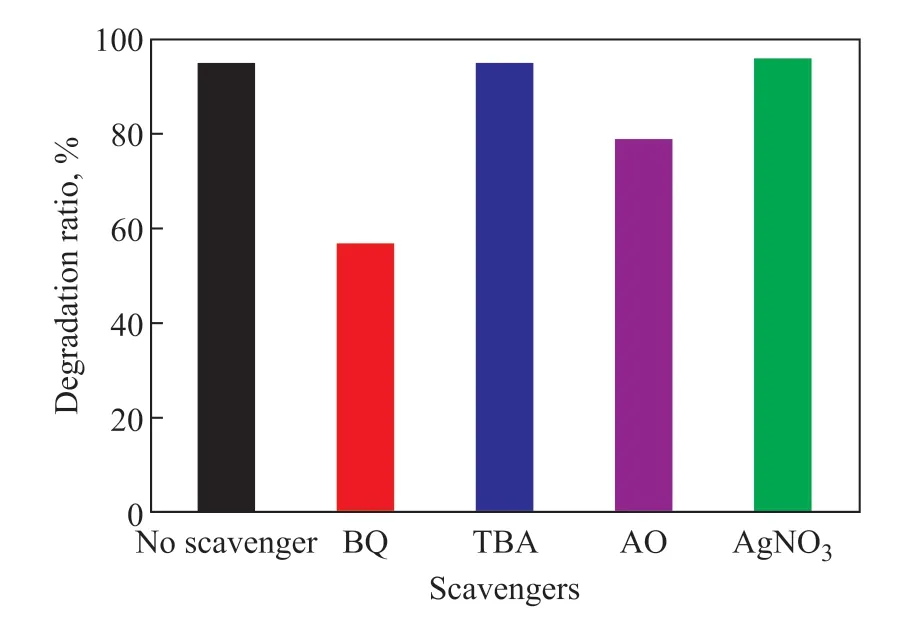
Figure 9 Capturing experiment of radical scavengers for the degradation of TC with FO-3BTO
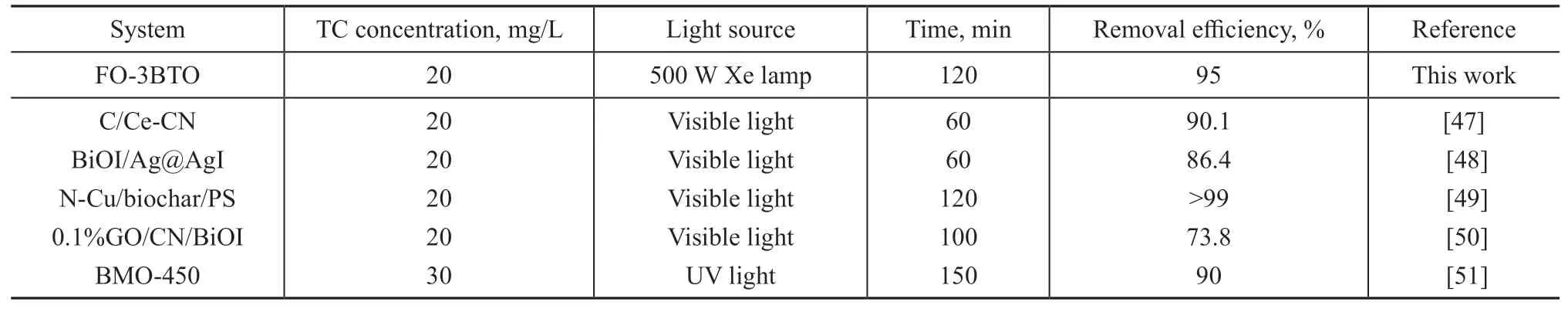
Table 1 Comparison of photo-degradation efficiency of TC between this study and others.

Figure 8 Effects of (a) pH and (b) TC initial concentration on TC photo-degradation under simulated solar light irradiation over the FO-3BTO composite
4 Photocatalytic Mechanism
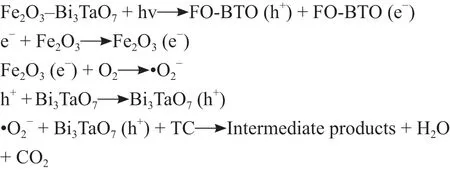
A free radical trapping experiment was implemented with FO-3BTO to estimate the main active species. Different trapping agents were added to the reaction system, with additions of p-benzoquinone (BQ) to capture superoxide radicals (•O2−), tert-butanol (TBA) to trap hydroxyl radicals (•OH), AgNO3to capture electrons (e−), and ammonium oxalate (AO) to trap holes (h+). Additions of BQ and AO greatly decreased photocatalytic activity(Figure 9). However, additions of TBA and AgNO3had little effect on TC removal, indicating that •OH and e−did not participate in the photo-degradation process.Therefore, •O2−and h+are proposed to have major roles in photocatalytic degradation of TC under simulated sunlight, donated as the two main active species.

Figure 10 Proposed mechanism for TC degradation over Fe2O3–Bi3TaO7 composite under simulated solar light irradiation
Transport and separation of photo-generated carriers during photocatalytic reactions are crucially related to photocatalytic activity. To illustrate the electron and hole separation process of Fe2O3-Bi3TaO7composites, band potentials of Fe2O3and Bi3TaO7were calculated using the following formulas:

whereEVB,ECB, χ,Ee, andEgare the valence band potential (VB), the conductor potential (CB), the absolute electronegativity, the free electron energy (~4.5 eV vs NHE), and the band gap energy under the hydrogen scale.The absolute electronegativity of Fe2O3and Bi3TaO7is 5.18 eV and 6.27 eV, respectively, and the band gap values are 2.08 eV and 2.92 eV, respectively. Therefore,calculated VB potentials of Fe2O3and Bi3TaO7were 1.72 eV and 3.23 eV, respectively., and CB potentials were−0.36 eV and 0.31 eV, respectively. When Fe2O3was combined with Bi3TaO7, the light response range was extended from the ultraviolet to the wide spectrum region of both ultraviolet and visible light. Thus, with such an architecture, both α-Fe2O3and Bi3TaO7semiconductors were excited to generate photo-generated electrons and holes under simulated sunlight. As shown in Figure 10,VB electrons in Bi3TaO7and Fe2O3can be excited to the CB and leave holes in the VB positions. Electrons from the CB of Bi3TaO7can then move through the interface link to the VB of Fe2O3to construct S-step heterojunctions[52]. Thus, the carriers can be effectively separated in space with the strong redox property of the semiconductor retained. In addition, the CB of Fe2O3was more negative than the potential of O2/•O2−, and therefore,electrons produced by photo-excitement in the CB of Fe2O3could bind the oxygen adsorbed on to its surface to form •O2−. The results were combined with those of the free radical capture experiment to propose a possible degradation mechanism for the series of Fe2O3-Bi3TaO7composites (Figure 10), with the following reactive equations:
Remarkably, wide solar light harvesting, excellent carrier separation, and high redox ability characterize Fe2O3-Bi3TaO7composite samples with tight 2D/0D heterojunction structure.
5 Conclusion
To summarize, Fe2O3-Bi3TaO7composites prepared by a simple hydrothermal method efficiently removed TC residues in water. Under simulated sunlight, the composite FO-3BTO had the best photocatalytic activity,with the TC removal rate reaching 95% in 120 min, which is better than that reported for photocatalysts in previous studies. The composite was most effective when solution pH was neutral. According to XRD, HRTEM, XPS, and SEM tests, effective S-scheme heterojunctions formed in the Fe2O3and Bi3TaO7composite. The constructed 2D/0D heterojunction structure promoted efficient charge separation, as demonstrated by EIS spectroscopy and photocurrent response. According to capture experiments,both photo-induced •O2−and h+had major roles in photocatalytic degradation of TC. Therefore, on the basis of the results in this study, a new type of broad spectralresponse photocatalyst with excellent performance and convenient operation can be developed for environmental remediation.
Acknowledgments:Financial support was provided by the National Natural Science Foundation of China (Grants Nos.51901209, 21777078, and 22062016), the Major Project of Inner Mongolia Natural Science Foundation (Grant 2020ZD02),and the Project of Research and Development of the Applied Technology for Inner Mongolia (Grant 2020SGG0065).
杂志排行
中国炼油与石油化工的其它文章
- Amorphous Catalysts for Electrochemical Water Splitting
- Tetralin Hydrocracking Reaction Network to Single-Ring Aromatics on Bifunctional Catalysts
- Upgradation of Heavy Crude Oil Via Hydrodynamic Cavitation Through Variations in Asphaltenes
- Wet Desulfurization of High-Sulfur Petroleum Coke Improved via Pre-calcination, H2O2 Treatment, and Ultrasound
- Removal of Heteroaromatic Sulfur Compounds by a Non-noble Metal Fe Single-atom Adsorbent
- A High-Performance Composite Epoxy Coating Based on the Cooperative Enhancement of 2D Co2(OH)2BDC and Electrospun PAN Nanofiber Membrane
