Upgradation of Heavy Crude Oil Via Hydrodynamic Cavitation Through Variations in Asphaltenes
2022-07-16LiKangHanHaiboLeiJieWangYouheLiDekunMarkRoodFazleSubhanYanZifeng
Li Kang; Han Haibo; Lei Jie; Wang Youhe; Li Dekun;Mark J. Rood; Fazle Subhan; Yan Zifeng
(1. State Key Laboratory of Heaνy Oil Processing, China Uniνersity of Petroleum, Qingdao 266580, China;2. Luoyang R & D Center of Technology of Sinopec Engineering (Group) Co. Ltd., Luoyang 471003, China;3. Shandong Oil Refining Association, Jinan 250013, China;4. Department of Ciνil and Enνironmental Engineering, Uniνersity of Illinois, IL 61801, USA;5. Department of Chemistry, Abdul Wali Khan Uniνersity Mardan, Mardan, KP, Pakistan)
Abstract: In this work, Saudi heavy crude oil (SHCO) was upgraded by the hydrodynamic cavitation technique. The collapse of cavitation bubbles instantly produces extreme conditions such as high temperature, pressure, and jet flow and strong shear forces, which can play a significant role in the upgradation process. The results revealed that the viscosity and Conradson carbon residue of SHCO decreased from 13.61 to 7.22 mm2/s and from 7.16% to 6.48%, respectively.True boiling point distillation findings showed that the vacuum residue (VR) decreased by 1%. Atmospheric-pressure photoionization Fourier-transform ion cyclotron resonance mass spectrometry, X-ray diffraction, dynamic light scattering,Fourier-transform infrared spectroscopy, and scanning electron microscopy were employed to characterize the molecular composition, crystalline structure, asphaltene aggregate particle size distribution, functional groups, and morphology,respectively, to understand the effects of hydrodynamic cavitation on asphaltenes. The obtained results demonstrate that hydrodynamic cavitation upgradation reduced the interaction forces between the asphaltene molecules, weakening the crystalline structure of the asphaltene aggregates, reducing the degree of association of the aromatic compounds in SHCO and asphaltenes, and decreasing the average particle size. The delayed coking properties of the VR were further investigated,and the cavitation treatment was found to decrease the coke yield by 1.85% and increase the liquid and gas yields by 1.52%and 0.33%, respectively. Hence, hydrodynamic cavitation can effectively enhance the processing performance of crude oil by improving the properties and structural characteristics of asphaltenes.
Key words: hydrodynamic cavitation; heavy oil upgradation; asphaltene crystalline structure; particle size distribution;agglomeration force
1 Introduction
The development of heavy oil upgradation technologies is of great interest to the global refining industry[1]. As a unique means of supplying energy to a system, the phenomenon of cavitation has been widely studied in the field of heavy oil upgradation[2-7]. Cavitation is the process of formation, growth, and collapse of gas or vapor bubbles in a liquid because of a reduction in the static pressure of the liquid below the vapor pressure[8].The collapse of cavitation bubbles instantly produces an extreme environment, including high temperature,pressure, and jet flow and strong shear forces, during operation under ambient conditions. Niazi et al.[9]used computational fluid dynamics to simulate the entire cavitation process in crude oil. The results indicated that bubble collapse led to a pressure and temperature of up to 300 MPa and 3200 K at 25 °C. Each individual cavitation bubble can essentially be regarded as a microreactor.Askarian et al.[10-11]examined the effect of the cavitation conditions on the properties of heavy fuel oil from the
Lavan Oil Refining Company in Iran and independently designed hydrodynamic cavitation equipment. At a cavitation temperature of 80 °C, atmospheric pressure,and a circulation time of 10-15 min, the viscosity of the heavy fuel oil was found to decrease by 33%, while the diesel yield and API gravity increased by 6.5% and 2.9,respectively, Moreover, Kaushik et al.[12]studied the effects of ultrasonic treatment parameters, such as ultrasonic frequency, time, and probe diameter, on the properties of the vacuum residue (VR). The asphaltene content in the VR was reduced from 13.5% to 7.0%. Similarly, Price and coworkers[13]investigated the influence of ultrasonic cavitation treatment on the properties of distillates (C8-C26). They reported that the thermal cracking of these distillates can be ascribed to the local energy generation upon cavitation bubble collapse. Lin and Yen[14]described the occurrence of free radical reactions during cavitation, with the light components acting as hydrogen donors. The heavy and light oil molecules underwent thermal cracking to form free radicals. The recombination of these generated free radicals is an intrinsic feature of cavitation upgradation. Dunn and Yen[15]studied the cavitation upgradation of heavy oil in the presence of the hydrogen donor tetrahydronaphthalene,which resulted in a similar hydrogenation reaction and further confirmed the free radical reaction mechanism during cavitation upgradation. However, it is difficult to thoroughly demonstrate the effects of cavitation upgradation on reducing viscosity and improving processing performance[16]. In other words, the influence of cavitation upgradation on asphaltene composition and structural characteristics has not yet been comprehensively elucidated.Therefore, it remains crucial to investigate the effects of hydrodynamic cavitation on the molecular composition and asphaltene structure of heavy oil.
Asphaltenes have the highest molecular weight and polarity among the components present in crude oil,in addition to possessing a highly complex molecular structure, and are generally considered to be key contributors to the nature of heavy crude oil[17-18].Mullins[19]proposed an improved version of the Yen model[20-21]that is now referred to as the Yen-Mullins asphaltene structure model. In this model, each asphaltene molecule contains 4-10 fused aromatic rings to form a single polycyclic aromatic hydrocarbon plane.
Various analytical techniques, such as X-ray diffraction(XRD)[22-23], Fourier-transform infrared (FT-IR)spectroscopy[24-25], and Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR MS)[26-29], have been utilized to characterize the crystalline structure,functional groups, molecular composition, aggregate particle size distribution, and morphology of asphaltenes to elucidate their effects on the properties and processing performance of heavy oil. AlHumaidan and coworkers[30-31]discussed the influence of thermal cracking on asphaltene structure and agglomeration. The results revealed that the asphaltene structure became weaker,the aggregates became smaller, and the yields of valueadded liquid products increased upon thermal cracking.Consequently, the properties and processing performance of heavy oil can be greatly affected by the structural characteristics of the asphaltenes.
The energy produced by cavitation can be effectively exploited to upgrade the properties of heavy oil. Thus,there is an urgent need to improve our understanding of the influence of hydrodynamic cavitation on the molecular composition and structural characteristics of asphaltenes. The aim of this study was to investigate the changes that occurred both in the physicochemical properties and molecular composition of heavy crude oil and in the crystalline structure, aggregate particle size distribution, functional groups, and morphology of the asphaltenes. In addition, the delayed coking performance of the VR from the heavy crude oil after hydrodynamic cavitation was examined.
2 Experimental
2.1 Materials
Saudi heavy crude oil (SHCO) was obtained from Sinopec Luoyang Company.n-Heptane, toluene, and KBr(all analytical reagent grade) were also used in this work.
2.2 Methods
A custom-built hydrodynamic cavitation experimental setup[32]was used to investigate the effect of hydrodynamic cavitation upgradation on the properties of SHCO. The operating conditions included room temperature and a cavitation pressure of 4 MPa. After hydrodynamic cavitation, the samples were left to stand quiescently at ambient temperature and pressure for 24 h prior to analysis. The upgraded SHCO is referred to as HCSHCO. The aromatic hydrocarbon classes of SHCO and HCSHCO are denoted SHCO-Ar and HCSHCO-Ar,respectively. The asphaltenes in SHCO and HCSHCO were separated according to ASTM D6560-2017 and are referred to as SHCO-As and HCSHCO-As, respectively.The aromatic hydrocarbon classes in SHCO-As and HCSHCO-As are denoted SHCO-As-Ar and HCSHCOAs-Ar, respectively.
2.3 Characterization
A boiling point distillation apparatus (FY-3, GECIL Process) was used to separate the SHCO and HCSHCO into gasoline (boiling point <200 °C), diesel (200-350 °C), vacuum gas oil (VGO; 350-515 °C), and VR(>515 °C).
The molecular weight distributions and molecular compositions were determined using an FT-ICR MS system (15 T SolariX XR, Bruker) equipped with an atmospheric-pressure photoionization (APPI) source. The samples were dissolved in toluene to a concentration of 0.5 mg/mL. The general molecular formula for common organic compounds can be written as CcHhSsNnOo, wherec,h,s,n, andodenote the numbers of carbon, hydrogen,sulfur, nitrogen, and oxygen atoms, respectively. The double bond equivalent (DBE), which refers to the sum of the number of rings and number of double bonds in an organic molecule, was calculated according to the following formula: DBE =c− 0.5h+ 0.5n+ 1.
The crystalline structure of the asphaltenes was evaluated using an X-ray diffractometer (X’Pert Powder, Malvern Panalytical, Netherlands) with Cu Kα as the radiation source (λ= 1.54055 Å), a working voltage of 40 kV, a current of 40 mA, and a 2θscanning range of 5°-80°.
Bragg’s law was used to calculate the crystalline parameters of the asphaltenes. The 002 band at 2θ≈ 26°reflects the layer distance between two aromatic sheets(dm), which corresponds toλ/(2sinθ002-band). The average height of the clusters of aromatic sheets perpendicular to the sheet plane (Lc) can be calculated as 0.9λ/(ωcosθ002).Theγband (2θ≈ 20°) indicates the distance between the saturated portions of the molecules or interchain layer (dγ),which corresponds toλ/(2sinθγ-band). The 10 band (2θ≈40°) reflects the average diameter of the aromatic sheets(La), which can be calculated as 1.84λ/(ωcosθ10). The average number of associated aromatic sheets in a stacked cluster (M) corresponds toLc/dm+1. In these equations,θdenotes the Bragg angle,λis the wavelength of the Cu Kα radiation, andωis the full peak width at half-maximum.The size distributions of the asphaltene aggregates were determined using a dynamic light scattering particle size analyzer (NANO-flex 180°, Colloid Metrix, Germany)over the range of 0.3 nm to 10 µm. These measurements were performed in toluene with an asphaltene concentration of 2000 μg/g at room temperature and pressure.
7. I have a daughter: Fairy tales are filled with mothers--both witches and regular mothers--trying to marry off their daughters in favorable circumstances. They include the mother in Cinderella and the troll-hag in East of the Sun and West of the Moon.Return to place in story.
The chemical structures of the asphaltenes were analyzed using an FT-IR spectrometer (Nicolet 6700, Thermo Corporation). The KBr pellet method was employed with a scanning range of 4000-500 cm−1and a spectral resolution of 4.0 cm−1.
The microstructures of the asphaltenes were characterized by thermal field emission scanning electron microscopy(SEM; JSM-7900F, JEOL, Japan). A cotton swab was used to deposit a small amount of the asphaltene sample onto a conductive adhesive. The sample was then dispersed on the sample stage by high-speed airflow. The sample stage was placed in a blast drying box at 80 °C for 10 min then removed and allowed to cool to room temperature before finally being transferred into the SEM system.
2.4 Delayed coking performance of VR
A custom-built 500 mL kettle delayed coking evaluation device was used to investigate the delayed coking reaction performance of the VR samples. Delayed coking reactions were conducted under a temperature of 495 °C and a pressure of 0.185 MPa for 3.5 h.
3 Results and Discussion
3.1 Effect of hydrodynamic cavitation on the properties of SHCO
The properties of SHCO and HCSHCO are summarized in Table 1. SHCO is a typical intermediate base crude oil. When the hydrodynamic cavitation pressure was maintained at 4 MPa, the density, viscosity, freezing point,and relative molecular mass decreased from 0.8967 g/cm3,13.61 mm2/s, −18 °C, and 467 for SHCO to 0.8710 g/cm3,7.22 mm2/s, −26 °C, and 443 for HCSHCO, respectively.From these results, it can be seen that the cavitation treatment reduced the viscosity of SHCO by 47%, while the Conradson carbon residue (CCR) decreased from 7.16% to 6.48%. Analysis of the composition revealed that the asphaltene content decreased from 3.9% to 3.4%,while the resin content increased from 39.1% to 43.0%.The distillation tests indicated that the distillation point temperatures at recoveries of 5%, 30%, 50%, 70%, and 90% decreased from 107.4, 237.3, 320.6, 410.7, and 508.7°C for SHCO to 65.4, 195.5, 294.8, 391.0, and 496.8 °C for HCSHCO, respectively. These results thus provide strong evidence that hydrodynamic cavitation upgradation is an effective method to improve the properties of SHCO.

Table 1 Properties of SHCO and HCSHCO
The yields of the four liquid products during the true boiling point distillation of SHCO and HCSHCO are plotted in Figure 1. Hydrodynamic cavitation upgradation caused the yields of diesel and VGO to increase by 0.23%and 1.19%, respectively, while the yields of gasoline and VR decreased by 0.42% and 1.01%, respectively. This implies that the true boiling point products distillation were in accordance with the characteristics of thermal cracking. During the cavitation process, the light hydrocarbons in crude oil such as gasoline generate corresponding free radicals through thermal cracking,acting as hydrogen donors. Recombination is an intrinsic characteristic of the hydrodynamic cavitation upgradation of heavy oil. Free radicals from heavy-component VR and light-component gasoline recombine to form diesel or VGO, which are lighter than VR. These reactions reduce the yields of gasoline and VR while increasing the yields of diesel and VGO. Hence, hydrodynamic cavitation breaks various molecular bonds in the SHCO and is considered one of the most effective and efficient methods for upgrading SHCO. Increasing the yields of the diesel and VGO fractions can lead to a considerable improvement in the properties of SHCO based on the dilution mechanism. Nevertheless, according to the kinetic viscosity equation for an Arrhenius mixture[33](lnµ12=x1lnµ1+x2lnµ2, wherex1andx2are the mole fractions of the mixture components andµ1andµ2are their dynamic viscosities), the increases in the yields of diesel and VGO would be expected to reduce the viscosity by only 3%, which is substantially less than the aforementioned viscosity reduction of 47% observed for the hydrodynamic cavitation process. Therefore, it is necessary to further investigate the influence of the variations of the molecular composition and structure of the asphaltenes on the SHCO properties after hydrodynamic cavitation upgradation.
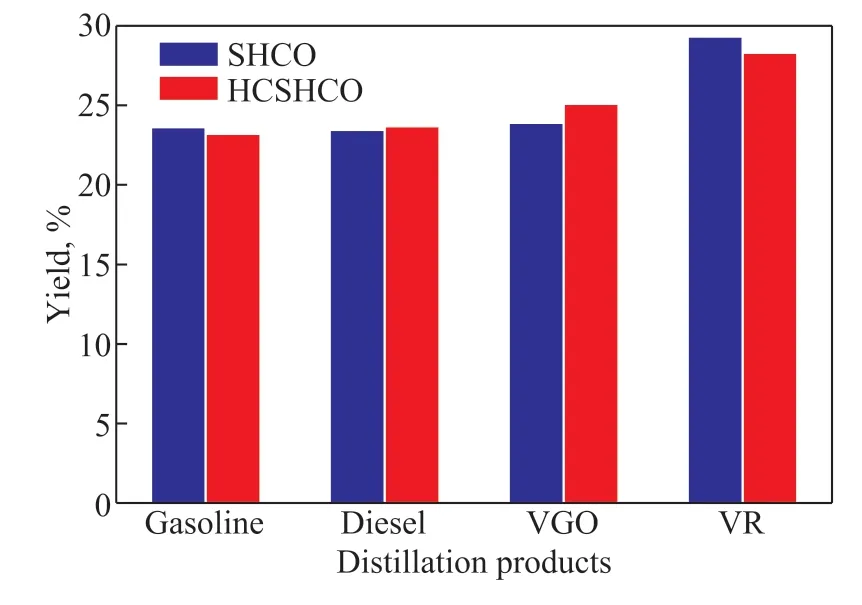
Figure 1 Yields of the four products obtained from the distillation of SHCO and HCSHCO
3.2 APPI FT-ICR MS analysis
The APPI FT-ICR mass spectra of SHCO and HCSHCO are presented in Figure 2. The peak height is positively correlated with the relative content of the corresponding compounds. Figure 2 also reveals that the chemical compositions of SHCO and HCSHCO were remarkably similar despite the somewhat different molecular weight distributions. The relative content of compounds withm/zvalues of 480-525 increased significantly, whereas that of compounds withm/zvalues of 525-550 decreased.It was further verified that the VGO yield was higher whereas the VR yield was lower for HCSHCO. The highenergy microenvironment generated by the hydrodynamic cavitation process altered the molecular distribution of SHCO and effectively improved the distribution performance of heavy oil products.
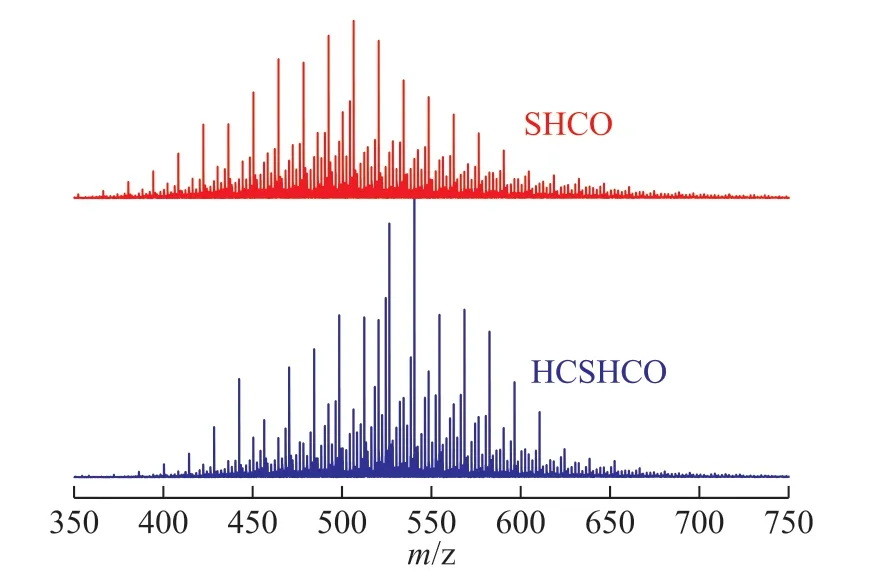
Figure 2 APPI FT-ICR mass spectra of SHCO and HCSHCO
The distributions of DBE versus carbon number for SHCO-Ar and HCSHCO-Ar are plotted in Figure 3a and 3b, respectively. After hydrodynamic cavitation,the aromatic hydrocarbon species with carbon numbers greater than 54 and DBE values less than 12 and those with carbon numbers less than 43 and DBE values greater than 21 were not detected. Meanwhile, new aromatic hydrocarbon classes were detected in the low carbon number and DBE regions. The main reason is that the force of the self-associated intermolecular or interactions of aromatic hydrocarbon classes was reduced after the hydrodynamic cavitation. Overall, the distribution of aromatic hydrocarbon classes after hydrodynamic cavitation tended to be more dispersed, as indicated by the red circles in Figure 3. This analysis indicates that the association of aromatic hydrocarbons in SHCO was reduced after hydrodynamic cavitation, which is one of the reasons for the upgradation and reduced viscosity.
Asphaltenes possess the highest molecular weights and most complex chemical structures among the components present in crude oil. These structural characteristics are expected to significantly influence the processing properties of crude oil. Therefore, the APPI FT-ICR mass spectra of SHCO-As and HCSHCO-As were recorded as shown in Figure 4. The chemical compositions of SHCOAr and HCSHCO-Ar were similar, with a slight difference in the molecular weight distribution. The relative content of compounds withm/zof 550-580 decreased significantly after hydrodynamic cavitation.

Figure 3 DBE versus carbon number distributions for (a) SHCO-Ar and (b) HCSHCO-Ar

Figure 4 APPI FT-ICR mass spectra of SHCO-As and HCSHCO-As
The distributions of DBE versus carbon number for SHCO-As-Ar and HCSHCO-As-Ar are presented in Figure 5a and 5b, respectively. Apparent changes in the distribution can be observed for SHCO-As-Ar and HCSHCO-As-Ar, especially in the region of low DBE values, where the carbon number distribution was significantly reduced. In other words, the association performance of the aromatic hydrocarbons in asphaltenes with low DBE values was reduced. This indicates that hydrodynamic cavitation is beneficial for reducing the association between asphaltene molecules, which is one of the reasons for subjecting heavy oil to hydrodynamic cavitation upgradation. Therefore, it is essential to understand the structural characteristics of asphaltenes to clarify the effect of hydrodynamic cavitation upgradation.
3.3 XRD analysis
Yen et al.[34]were the first to utilize the XRD technique to determine the spacing between the aromatic and aliphatic layers in petroleum asphaltenes. The crystalline parameters of the asphaltene clusters were calculated based on the XRD peak intensities and positions. As shown in Figure 6, the XRD patterns of the asphaltenes exhibited three characteristic diffraction peaks corresponding to the γ band, 002 band, and 10 band.Significant changes in the positions and intensities of these three peaks between SHCO-As and HCSHCOAs were observed, indicating that the hydrodynamic cavitation process greatly affected the crystalline structure of the asphaltenes in SHCO. The crystalline structure parameters for SHCO-As and HCSHCO-As were calculated according to Bragg’s law as summarized in Table 2. The distance between the aromatic sheets (dm)of the asphaltene clusters increased from 3.49 to 3.58 Å, which indicates a reduction in the π-π interactions between the sheets and a concomitant weakening of the asphaltene clusters. Meanwhile, the distance between the saturated portions of the molecules or interchain layer (dγ) increased from 4.16 to 4.56 Å, implying that the asphaltene clusters slackened. Furthermore,the average number of associated aromatic sheets in a stacked cluster (M) reduced from 3.75 to 3.53, indicating that weakly interacting aromatic sheets were separated from the original asphaltene clusters. Such separation reduced the average height of the clusters of aromatic sheets perpendicular to the sheet plane (Lc) from 9.62 to 9.05 Å. Overall, the XRD analysis revealed that the hydrodynamic cavitation made the asphaltene clusters weaker and smaller and disrupted their layer structure,which is vital for their properties.
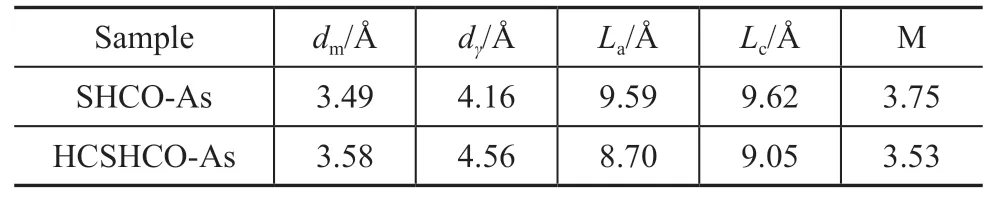
Table 2 Crystalline parameters for SHCO-As and HCSHCO-As

Figure 5 DBE versus carbon number distributions for (a) SHCO-As-Ar and (b) HCSHCO-As-Ar

Figure 6 XRD patterns of (a) SHCO-As and (b) HCSHCO-As
3.4 Particle size analysis
The particle size distributions of the aggregates in SHCOAs and HCSHCO-As are presented in Figure 7. To ensure the accuracy of the data, each sample was analyzed in triplicate and average distributions were calculated. The particle size distributions of the aggregates in SHCOAs and HCSHCO-As ranged from 9.85 to 43.00 nm and from 5.56 to 39.40 nm, respectively. The peak of the particle size distribution decreased from 15.19 to 11.71 nm. In addition, asphaltene aggregates with smaller particle sizes ranging between 5.56 and 9.85 nm appeared. The maximum particle size of the asphaltene aggregates decreased from 43.00 to 39.40 nm. Overall, the hydrodynamic cavitation process broke down the asphaltene aggregates to afford smaller aggregates. It is generally accepted that the particle size distribution of asphaltene aggregates is highly dependent on the crystalline structure and molecular composition. These results thus indicate that the hydrodynamic cavitation process diminished the interactions between the asphaltene aggregates and led to their dissociation and dispersion.
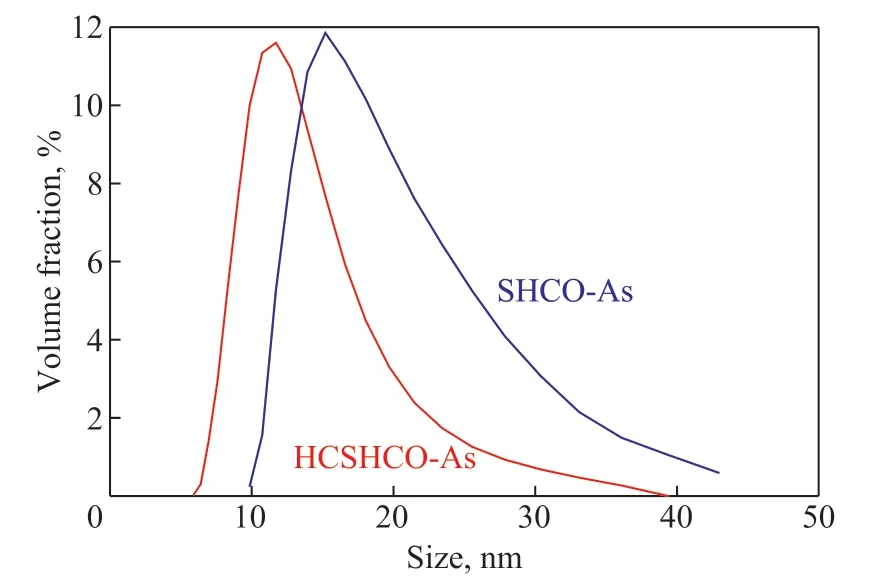
Figure 7 Particle size distributions of the aggregates in SHCO-As and HCSHCO-As
3.5 FT-IR spectroscopy analysis
The FT-IR spectra of SHCO-As and HCSHCO-As are shown in Figure 8 and the corresponding peak areas are summarized in Table 3[24-25]. The IR band at 3434 cm−1was ascribed to intramolecular or intermolecular hydrogenbond stretching vibrations. The peak area of this band decreased from 39.10 to 31.50 because of the decrease in hydrogen bonding between the functional groups and other charge interactions in the asphaltenes. The characteristic IR bands at 3050 and 1600 cm−1correspond to C-H and C=C skeleton stretching vibrations associated with the aromatic rings of the asphaltene molecules.The peak areas of these two bands decreased from 1.30 and 8.86 to 0.55 and 7.76, respectively, indicating weaker C-H and C=C bond interactions in the aromatic rings. In addition, the characteristic IR band at 1440 cm−1corresponds to the C=C bending vibrations in the aromatic rings of the asphaltene molecules, for which the peak area decreased from 5.78 to 1.51. The characteristic IR bands at 2920, 2855, and 1360 cm−1were attributable to -CH2- and -CH3vibrations on the aromatic rings of the asphaltene molecules. The areas of these three bands decreased from 26.66, 14.36, and 0.96 to 12.94, 6.24, and 0.57, respectively. The characteristic IR band at 855 cm−1was ascribed to the bending vibrations of hydrogen atoms on the asphaltene benzene rings. The bending vibration of 2-3 adjacent hydrogen atoms on the benzene rings led to the characteristic IR peak at 805 cm−1. Likewise, the bending vibration of four adjacent hydrogen atoms on the benzene rings accounted for the IR peak at 742 cm−1. The variations of the characteristic peaks at 805 and 742 cm−1indicate that the internal force of the asphaltene aromatic rings was enhanced[35]. The IR results revealed that hydrodynamic cavitation weakened the interaction forces between the asphaltene molecules in SHCO.

Figure 8 FT-IR spectra of SHCO-As and HCSHCO-As
3.6 SEM analysis
SEM images of SHCO-As and HCSHCO-As are presented in Figure 9. Although isolated asphaltenes may not reflect the actual colloidal states of asphaltenes in heavy oil, the SEM images still provided clear evidence for the influence of hydrodynamic cavitation upgradation on the structural properties of the asphaltene aggregates.The asphaltene aggregates were stacked to create a layered structure with a thickness of 150-200 nm (Figure 9a and 9b). In addition, the solid particles in SHCOAs possessed smooth surfaces and a dense structure,further indicating that the asphaltenes could not exist with a single-layered structure in SHCO. In the case of HCSHCO-As, the weakening of the interaction forces between the layers made the layered structure unstable.Moreover, the surface of the asphaltene particles became rough and scattered, resulting in the formation of tiny and disordered particles (Figure 9c and 9d). Thus, these SEM images demonstrate that the hydrodynamic cavitation process altered the asphaltene structure within SHCO,which further supports the above results.
3.7 Delayed coking properties
Finally, the distributions of the delayed coking products of VR were analyzed, as plotted in Figure 10. After hydrodynamic cavitation, the gas and liquid product yields increased by 0.33% and 1.52%, respectively,while the coke yield decreased by 1.85%. These results show that the cavitation treatment reduced the coking properties. In addition, these results further supportthat the asphaltene structure, volume of asphaltene aggregates, and degree of asphaltene association all affected the coking properties. The crystalline structure of the asphaltene aggregates became unstable, causing the saturated and aromatic components to be released in the larger-scale resins during the delayed coking process.The suppression of coke formation has a similar principle as the “siphon effect”[36], resulting in an increase in liquid yield. The detailed reaction mechanism is depicted in Figure 11. Hydrodynamic cavitation upgradation reduced the interaction forces between asphaltene molecules,thus weakening the crystalline structure of the asphaltene aggregates. Therefore, the degree of association of aromatic compounds in the asphaltenes and SHCO was lowered, decreasing the particle size distribution of the asphaltene aggregates. These results indicate that the reported structural changes in asphaltenes could provide a potential strategy for improving gas and liquid products by reducing coke formation.

Figure 9 SEM images of (a, b) SHCO-As and (c, d) HCSHCO-As

Figure 10 Distribution of delayed coking products of VR
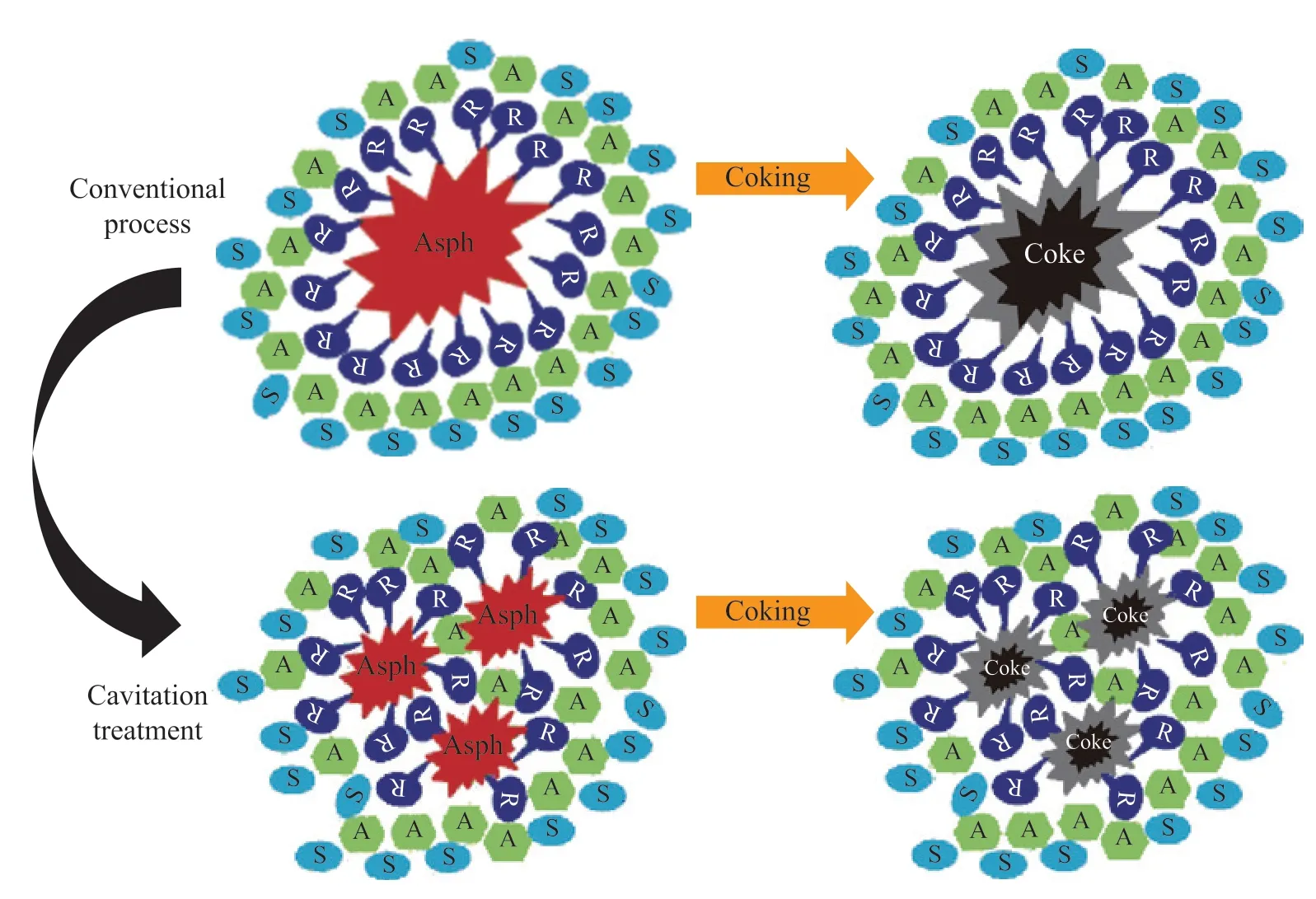
Figure 11 Reaction mechanism of the coking of the VR samples from SHCO and HCSHCO
4 Conclusions
The aim of the present work was to investigate the effects of hydrodynamic cavitation upgradation on heavy crude oil. Comparison of the physicochemical properties and product distribution of SHCO before and after hydrodynamic cavitation indicated that hydrodynamic cavitation upgradation reduced the interaction forces between the asphaltene molecules, decreasing the stability of the crystalline structure of the asphaltene aggregates. This further reduced the association of the aromatic compounds in SHCO and asphaltenes and decreased the average particle size. Evaluation of the delayed coking properties of the VR revealed that hydrodynamic cavitation decreased the coke yield by 1.85% and increased the liquid and gas yields by 1.52% and 0.33%, respectively. More importantly, the influence of hydrodynamic cavitation upgradation on the processing performance of heavy oil was investigated.After upgradation, more value-added liquid products were obtained. Therefore, we believe that hydrodynamic cavitation is a simple, efficient, and feasible heavy oil upgradation technology.
Acknowledgments:This work was financially supported by the Research Program of China Petrochemical Corporation(SINOPEC 117017-8 and 119022-2).
杂志排行
中国炼油与石油化工的其它文章
- Amorphous Catalysts for Electrochemical Water Splitting
- Tetralin Hydrocracking Reaction Network to Single-Ring Aromatics on Bifunctional Catalysts
- Wet Desulfurization of High-Sulfur Petroleum Coke Improved via Pre-calcination, H2O2 Treatment, and Ultrasound
- Removal of Heteroaromatic Sulfur Compounds by a Non-noble Metal Fe Single-atom Adsorbent
- A High-Performance Composite Epoxy Coating Based on the Cooperative Enhancement of 2D Co2(OH)2BDC and Electrospun PAN Nanofiber Membrane
- Influence of Ethanol Addition on the Spray Auto-ignition Properties of Gasoline and Its Relationship with Octane Number
