Kinetic Study of Homogeneous Rhodium-Catalyzed Ethanol Carbonylation Using Bis(diphenylphosphino)methane Ligands
2022-07-16LongHaoyuXuLinSongJianhuaWangXiaopingWangWeichongLiuDianhua
Long Haoyu; Xu Lin; Song Jianhua; Wang Xiaoping; Wang Weichong; Liu Dianhua
(Stake Key Laboratory of Chemical Engineering, East China Uniνersity of Science and Technologu, Shanghai 200237)
Abstract: Homogeneous rhodium-catalyzed carbonylation of ethanol is a potential route for the preparation of propionic acid, although its commercialization has been hindered by a low reaction rate. In this work, various bis(diphenylphosphine)ligands were evaluated in the rhodium-catalyzed reaction system in an effort to improve the reaction rate. By comparing the space-time yield and selectivity of the catalytic system in a Hastelloy autoclave reactor, the effects of the choice of ligand,ligand:rhodium ratio, carbon monoxide pressure, temperature, rhodium concentration, ethyl iodide content, and lithium iodide content were investigated. In the presence of the ligand bis(diphenylphosphino)methane disulfide (dppmS2), the activity of the catalyst was improved significantly. The results revealed that the optimal conditions included a dppmS2:Rh molar ratio of 0.5:1, a rhodium concentration of 1000 μg/g, an ethyl iodide content of 10%, and a lithium iodide content of 10%. The most suitable reaction temperature and carbon monoxide pressure were 463 K and 3.0 MPa, respectively, which resulted in a maximum space-time yield of 4.30 mol/(h·L). A kinetic model was established in the temperature range of 442- 463 K, and the model data were regressed and validated, revealing an activation energy of 28.01 kJ/mol. Residual analysis and statistical tests demonstrated the reliability of the kinetic experiments.Key words: ethanol; propionic acid; carbonylation; kinetic model; catalyst
1 Introduction
Propionic acid is an important short-chain fatty acid with numerous industrial applications, including as a food preservative, an ingredient in cosmetics, and a chemical intermediate for the modification of synthetic cellulose and the production of herbicides[1-3]. The calcium and sodium salts of propionic acid can also be used as food additives to mitigate the growth of microorganisms[4].Common industrial preparation methods of propionic acid include propionaldehyde oxidation, ethylene carbonylation, ethanol carbonylation, and light hydrocarbon oxidation[5-6]. At present, most commercial synthesis of propionic acid is conducted through the petrochemical route. However, with the fluctuation of oil prices, the production of propionic acid by direct biological approaches or the carbonylation of bioethanol has also attracted increasing research attention[1,3].
Monsanto began developing its rhodium- and iodidecatalyzed carbonylation of methanol to acetic acid in 1966, which significantly reduced production costs[7].
However, the carbonylation rate of alcohols decreases in the order of methanol > ethanol > 1-propanol, with relative rates of 21:1:0.47[8]. It is therefore necessary to improve the reaction rate of ethanol carbonylation and reduce the catalyst cost. A variety of catalysts can be applied to ethanol carbonylation, such as rhodium,cobalt, iridium, and palladium compounds, among which rhodium catalysts typically afford the highest reaction rate under milder reaction conditions[8-12].
In the homogeneous rhodium-catalyzed carbonylation reaction system, kinetic studies indicate that oxidative addition is the rate-determining step[13]. The inclusion of ligands in the reaction system can increase the electron density on the metal center, thereby increasing the carbonylation rate. A wide variety of rhodium carbonyl complexes have been used in methanol and ethanol carbonylation reactions. Ligands containing nitrogen and phosphorus are believed to effectively improve the reaction rate and selectivity under mild conditions[14-18].However, further screening of phosphine ligands is necessary to improve the ethanol carbonylation activity.The mechanistic and kinetic aspects of rhodiumcatalyzed methanol carbonylation have been studied extensively[19-21]. The mechanism of ethanol carbonylation is considered to be similar to that of methanol carbonylation[13,17,22], although there have been few reports concerning this mechanism for ethanol carbonylation promoted by phosphine ligands. It is therefore necessary to further elucidate the mechanism and kinetics of this reaction to facilitate the development and commercialization of improved catalysts.
In this study, a series of bis(diphenylphosphine) ligands were applied to the homogeneous rhodium-catalyzed carbonylation of ethanol to prepare propionic acid, which revealed that the bis(diphenylphosphino)methane disulfide(dppmS2) ligand afforded the best reactivity. In addition,a reaction mechanism is proposed for the rhodiumcatalyzed carbonylation of ethanol in the presence of dppmS2, and the reaction kinetics are established at 439 -463 K.
2 Experimental
2.1 Materials
Rhodium iodide (RhI3, Rh content > 21%), ethanol(EtOH, 99.5%), propionic acid (EtCOOH 99.5%),bis(diphenylphosphino)methane (dppm, 99%),1,2-bis(diphenylphosphino)ethane (dppe, 99%),1-propanol and sulfur (S, 99.5%) were purchased from Shanghai Macklin Biochemical Co. Ltd. Selenium was purchased from Adamas Beta (Shanghai) Chemical Reagent Co. Ltd. Hydroiodic acid (HI, 45%) was purchased from Beijing Innochem Science & Technology Co. Ltd. Ethyl iodide (EtI, 99.5%) and lithium iodide(LiI, 99.5%) were purchased from Carbon monoxide(CO, 99.99%) was purchased from Shanghai Air Liquide Compressed Gas Co.
2.2 Synthetic methods
All of the bis(diphenylphosphine) ligands reported in this paper were synthesized on a Schlenk line under oxygen-free conditions with nitrogen as the inert gas.
Bis(diphenylphosphino)methane disulfide (dppmS2) and bis(diphenylphosphino) methane monoselenide (dppmSe)were prepared according to the methods provided in the study published by Suranna[22]. Bis(diphenylphosphino)methane monoxide (dppmO) and bis(diphenylphosphino)ethane monoxide (dppeO) were prepared according to the methods reported by Grushin[23]. Bis(diphenylphosphino)ethane monosulfide (dppeS) was prepared according to the method provided in the literature published by Grim[24]. The obtained bis(diphenylphosphine) ligands were all characterized by31P NMR spectroscopy on a 500 MHz Bruker NMR instrument at room temperature(162 MHz, CDCl3). The results revealed that the prepared ligands were of high purity and the peaks were consistent with those described in the literature[17,23,25-27].
The homogeneous carbonylation of ethanol was conducted in a 50 mL Hastelloy autoclave reactor equipped with a pressure gauge and magnetic stirrer.The temperature of the reactor was controlled using a thermocouple. The flow of CO gas entering the reactor was monitored using a flow totalizer.
In a typical experiment, a total of 25 g of reagents,including ethyl iodide, ethanol, lithium iodide, hydroiodic acid, water, rhodium iodide bis(diphenylphosphine)ligand and propionic acid solvent was added to the reactor. The reactor headspace was then purged with CO gas to remove air. Next, the reactor was heated to 413 K under a CO pressure of 1 MPa and a stirring speed of 960 r/min, and the catalyst was activated for 2 hours before the reaction to convert the catalyst from rhodium triiodide to a complex of rhodium and bisdiphenylphosphine. The temperature was then adjusted to the desired temperature for the reaction. When the temperature had stabilized at the 463 K, the CO pressure was maintained at the 3.0 MPa for 40 min by adjusting the pressure regulator.Finally, the heater and magnetic stirrer were turned off and the reactor was placed in cold water for rapid cooling.The reactor was carefully reduced to atmospheric pressure and the liquid reaction mixture was sampled for analysis.
2.3 Analytical methods
All samples were analyzed on a gas chromatography system (Agilent 7890A) equipped with an Elite-WAX capillary column (30 m × 0.25 mm × 0.25 μm) and a flame ionization detector using high-purity nitrogen as the carrier gas. 1-propanol was used as an internal standard.The water contents of the product were determined by Karl Fischer titration. All samples were analyzed in triplicate and the results are reported as average values.
In industrial production, in order to reduce the cost of product separation, propionic acid is often used as a reaction solvent. The rate-controlling step of the ethanol carbonylation reaction is the oxidative addition reaction of iodoethane[22], and the use of propionic acid as a solvent does not substantially affect the rate of the reaction. Therefore, propionic acid was chosen as the solvent in this paper. The propionic acid produced by the carbonylation reaction should be the difference between the sum of propionic acid and ethyl propionate substances in the solution after the reaction and the propionic acid substance added before the reaction. For the accuracy of the kinetic data, the carbonylation selectivity (S) was defined and calculated according to equation (1):

npropionicacid(produced)refers to the amount of substances added to propionic acid after the end of the reaction.nethylpropionate(produced)refers to the amount of substances added to ethyl propionate after the end of the reaction.nethanol(total)refers to the amount of ethanol species added before the reaction.nethanol(unreacted)refers to the amount of propionic acid remaining after the reaction. The carbonylation spacetime yield (STY) is the ratio of the stoichiometric sum of ethyl propionate and propionic acid to the volume of the reaction solution, as expressed in equation (2):
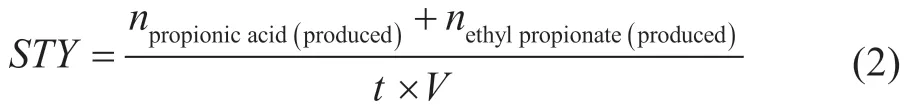
wheret(h) refers to the time for the reaction to proceed,V(L) refers to the total volume of the reaction solution.
3 Reaction Optimization
3.1 Influence of ligand
As shown in Figure 1, the addition of the bis(diphenylphosphine) ligands profoundly affected the space-time yield and selectivity of the Rh-catalyzed carbonylation reaction. Although the presence of most of the ligands reduced the space-time yield relative to the ligand-free reaction, the addition of dppmS2afforded an excellent reaction rate, with the space-time yield and selectivity increasing from 3.55 to 3.93 mol/(h·L) and from 77.6% to 85.4%, respectively.

Figure 1 Influence of different ligands
In the ligand-free reaction, the catalytically active species is [Rh(CO)2I2]-, which is generated at a certain temperature and CO pressure. The oxidative addition reaction between this species and ethyl iodide is the rate-determining step[8]. Consequently, the catalytic reaction rate can be enhanced by introducing strongly electron-donating ligands[28]. The addition of bis(diphenylphosphine)ligands may lead to the replacement of the carbonyl or iodide ligands originally coordinated to rhodium, thereby affecting the rate of oxidative addition of alkyl iodides to the complexes.
The dppeO and dppeS ligands can form six-membered chelate rings with rhodium. The stability of these rings is generally believed to be poor, such that catalyst dissociation may account for the decreased catalytic performance with these ligands. The dppmO ligand provided higher catalytic activity for methanol carbonylation only at low temperature and low pressure than without the addition of the ligand[18]. The dppmO ligand is hemilabile and a change in the coordination number occurs during the catalytic process, which reduces the activity. Single-crystal XRD analysis of the complex between rhodium and dppmSe revealed a large Rh-Se bond length, which may be cleaved at high temperature and reduce the reactivity[27].
Among the ligands tested, dppmS2exhibited the greatest ability to accelerate the rate of ethanol carbonylation.Triphenylphosphine sulfide (Ph3P=S), which is similar to dppmS2in terms of coordinative bond formation with rhodium, has been shown to promote the rate of methanol carbonylation[27]. This may be explained by using hard/soft acid/base theory to consider the interaction between the metal and the element forming the complex bond. In the complex Rh2(CO)4(μ-dppmS2)I2,the sulfur donor is soft and can strongly interact with the soft rhodium center, thereby increasing the electron density on Rh, thereby promoting the complex interaction with iodoethane complex oxidative addition reactions.Therefore, we selected dppmS2as the optimal ligand for kinetic experiments.
3.2 Influence of dppmS2 to Rh ratio
Next, the influence of the dppmS2:Rh ratio on the carbonylation reaction was investigated. As shown in Figure 2, the space-time yield reached a maximum when the dppmS2:Rh ratio was 0.5:1, and further ligand addition caused the space-time yield to decrease significantly.This indicates that each dppmS2ligand simultaneously coordinated with two rhodium atoms.
However, when rhodium is not coordinated with the bis(diphenylphosphine) ligand, it will coordinate with more CO molecules, which would be expected to lead to decreased complex solubility and stability. As the ligand dppmS2is added, the [Rh(CO)2I2]−will gradually be converted into Rh2(CO)4(μ-dppmS2)I2(equations (3) and (4)):
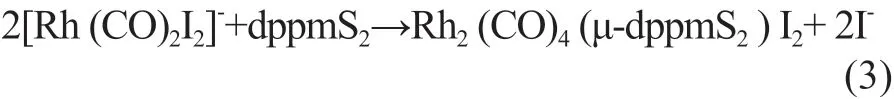
When the dppmS2:Rh ratio reaches 0.5:1, [Rh(CO)2I2]−is completely converted to Rh2(CO)4(μ-dppmS2)I2. It can be seen from Figure 2 that both the space-time yield and selectivity were the highest at this point, which indicates that Rh2(CO)4(μ-dppmS2)I2is the active species and its formation process is facile. Therefore, the optimal dppmS2:Rh ratio was determined to be 0.5:1.
3.3 Influence of CO pressure
The influence of CO pressure on the reaction was also examined, and the results are presented in Figure 3. The space-time yield increased with increasing CO pressure in the range of 2.1 - 3.0 MPa and thereafter remained constant.
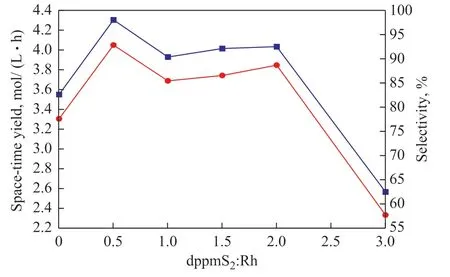
Figure 2 Influence of dppmS2:Rh ratio

Figure 3 Influence of CO pressure
This behavior is similar to that reported in previous studies for CO pressures in excess of 3.0 MPa[22]. Because the solubility of CO reaches saturation at this point, the reaction rate is no longer affected by the mass transfer of CO.
3.4 Influence of temperature
The effect of temperature on the reaction was next studied from 442 to 470 K. As shown in Figure 4, the reaction rate increased with increasing temperature in the range of 442- 463 K, which is in accordance with a kinetic reaction model13. However, when the temperature was further increased to 470 K, the reaction rate sharply decreased.This may be due to the dissociation and inactivation of Rh2(CO)4(μ-dppmS2)I2above 463 K. Thus, the most suitable reaction temperature was determined to be 463 K.

Figure 4 Influence of temperature
3.5 Influence of catalyst concentration
The effect of rhodium content on the reaction was investigated in the range of 500 -1500 μg/g, and the results are presented in Figure 5. It was observed during the experiments that the rhodium was completely dissolved in the reaction solution at 1500 μg/g. The results revealed that both the space-time yield and the selectivity increased with increasing rhodium concentration in the range of 500 - 1500 μg/g. However,the rate of increase slowed down considerably at rhodium concentrations exceeding 1000 μg/g. For achieving high activity while ensuring effective catalyst utilization, 1000 μg/g was determined to be the optimum rhodium concentration.

Figure 5 Influence of catalyst concentration
3.6 Influence of ethyl iodide concentration
The relationship between the ethyl iodide content and the space-time yield and selectivity was next explored.As shown in Figure 6, the space-time yield and selectivity rapidly increased with increasing ethyl iodide content over the studied range. It is generally believed that the rate-determining step of ethanol carbonylation is the oxidative addition of ethyl iodide, such that the ethyl iodide content would be expected to directly affect the rate of reaction[13]. However, because the content of ethyl iodide in the ethanol carbonylation reaction remains essentially unchanged until the ethanol and ethyl propionate are consumed, an excessive ethyl iodide content may lead to higher downstream separation costs. Therefore, the optimal ethyl iodide content was determined to be 10%.
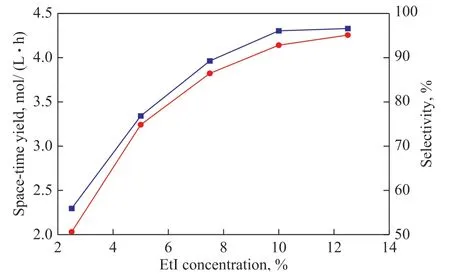
Figure 6 Influence of ethyl iodide content
3.7 Influence of lithium iodide concentration
Experiments were conducted to examine the effect of lithium iodide addition in the range of 2.5% - 12.5%, and the results are presented in Figure 7. Over the investigated range, both the space-time yield and the selectivity increased with increasing lithium iodide content.However, increasing the lithium iodide content from 10%to 12.5% had only a minimal influence. Therefore, the optimal lithium iodide content was determined to be 10%.In our earlier study, we reported that lithium iodide addition promoted ethanol carbonylation[22]. Lithium iodide helps to maintain the stability of the active rhodium species at low water concentrations during the carbonylation of both methanol and ethanol.

Figure 7 Influence of lithium iodide concentration
4 Kinetic Analysis
4.1 Mechanism of ethanol carbonylation
As part of this work, a reaction mechanism and kinetic model were proposed for the homogeneous rhodiumcatalyzed carbonylation of ethanol to produce propionic acid in the presence of dppmS2.
The experimental results demonstrated that the reaction rate was independent of the CO pressure above 3.0 MPa but increased with increasing temperature and increasing concentrations of ethyl iodide, lithium iodide, and rhodium. As discussed above, the highest space-time yield was obtained for a dppmS2:Rh ratio of 0.5:1. The complexes formed between dppmS2and rhodium may be similar to those previously reported for Ph3P=S[29]. They may have the same mechanism of promoting the reaction.We previously studied the reaction mechanism of ethanol carbonylation experimentally and proposed a possible mechanism in the presence of the dppmS2ligand[17,22]. The reaction mechanism is summarized in equations (5)-(10)and Figure 8.
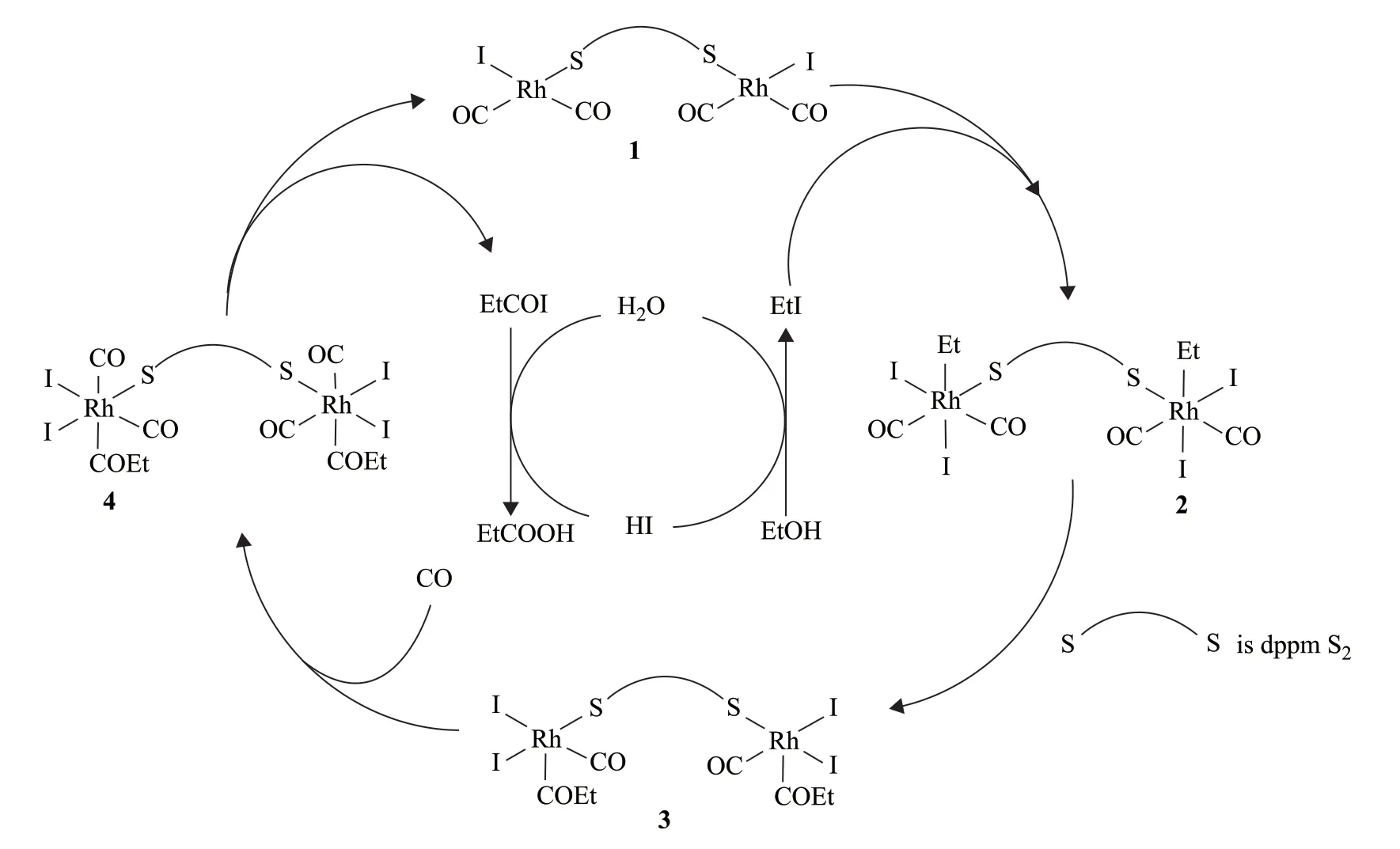
Figure 8 Reaction mechanism of ethanol carbonylation in the presence of dppmS2

Figure 9 Comparison of fitted and experimental reaction rates

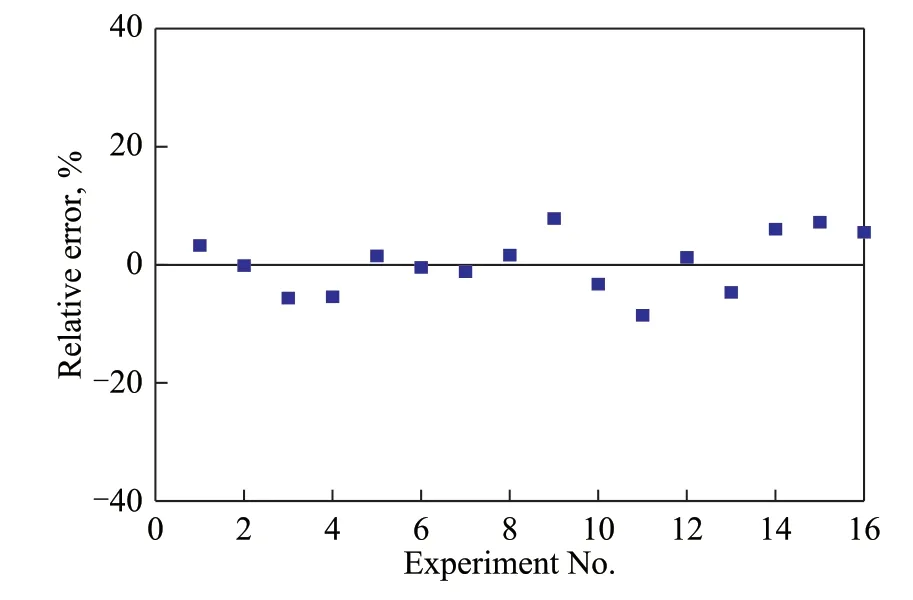
Figure 10 Relative error between the fitted and experimental reaction rates
The reaction mechanism of homogeneous ethanol carbonylation in the presence of dppmS2is similar to other methanol and ethanol carbonylation mechanisms. The main catalytically active species is Rh2(CO)4(μ-dppmS2)I2(1), which generates the intermediate Rh2(CO)4(Et)2(μdppmS2)I2(2). This is the rate-determining step of the reaction. Intermediate (2) is then converted to Rh2(CO)2(COEt)2(μ-dppmS2)I4(3) through a migration/insertion process, after which a CO ligand inserts into each rhodium center to afford Rh2(CO)4(COEt)2(μdppmS2)I4(4). Intermediate (4) is then reduced back to Rh2(CO)4(μ-dppmS2)I2(1) by elimination to generate propionyl iodide, which is hydrolyzed to propionic acid.
4.2 Kinetic model
The kinetics of the reaction were established based on the mechanism of homogeneous rhodium-catalyzed ethanol carbonylation in the presence of dppmS2. If all of the above reactions were to be considered, the rate equations for the reaction kinetics would be highly complex.Therefore, the apparent reaction rate was expressed by a power-function-type rate equation. The effects of the lithium iodide and hydroiodic acid concentrations on the reaction were represented by the iodide ion concentration.Finally, the following rate equation was obtained[13,17,22]:
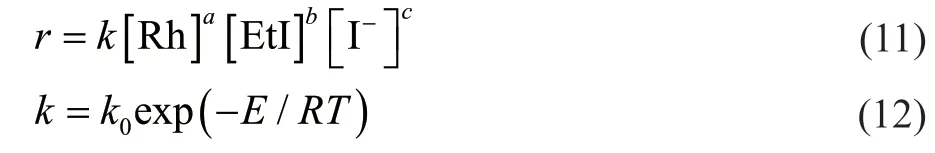
In the kinetic equation,ris the reaction rate expressed as the space-time yield (mol/(h·L));Erefers to activation energy;Rstands for Avogadro’s constant. Under the condition of a dppmS2:Rh molar ratio of 0.5:1, a temperature of 442 - 463 K, an ethyl iodide content of 2.5% - 12.5%, a lithium iodide content of 2.5% -12.5%, and a catalyst concentration of 500 - 1250 μg/g, the proposed kinetic equation was valid. The kinetic study data are shown in Table 1. Regression analysis was performed on the data in Table 1 using the MATLAB software, and the following reaction kinetic equation was obtained:
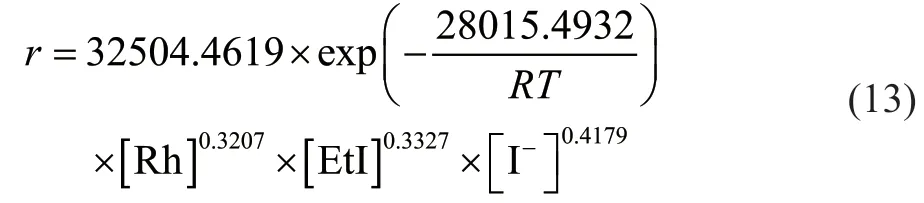
where [Rh], [EtI] and [I-] refers to the concentration of rhodium, iodine ethane and iodine ion respectively.The data at all temperatures were fitted according to the Arrhenius formula, and the activation energy of the reaction was determined to be 28.01 kJ/mol. This value is lower than that for the ligand-free reaction system[13],demonstrating that the addition of dppmS2enhances the reactivity.
To ensure the accuracy of the kinetic model, statistical analysis was performed on the data and the residuals and systematic errors were calculated. It is generally considered that a kinetic model is suitable whenρ2> 0.9 andF> 10F0.05(95% confidence). As shown in Table 2,the statistical analysis confirmed the high reliability of the developed kinetic model.

Table 1 Kinetic data in the presence of dppmS2

Table 2 Statistical parameters for the kinetic reaction rate formula
Experimental rate refers to the space-time yield measured experimentally. Fitted rate refers to the space-time yield calculated by the kinetic equation. The relative error is the value of fitted rate minus experimental rate.
5 Conclusion
In this work, five bis(diphenylphosphine) ligands were prepared and evaluated in the rhodium-catalyzed carbonylation of ethanol to produce propionic acid.The rhodium complex formed with the dppmS2ligand exhibited the best catalytic performance by reducing the activation energy and increasing the reaction rate. For this catalyst, the optimal conditions for the synthesis of propionic acid included a rhodium content of 1000 μg/g,a dppmS2:Rh molar ratio of 0.5:1, an ethyl iodide content of 10%, a lithium iodide content of 10%, a reaction temperature of 463 K, and a CO pressure of 3.0 MPa,which afforded a maximum space-time yield of 4.30 mol(h·L). A mechanism for the homogeneous ethanol carbonylation mediated by the dppmS2complex was also proposed. Finally, a kinetic model was established, and the results revealed an activation energy of 28.01 kJ/mol and reaction orders for the iodide ion, ethyl iodide, and rhodium of 0.3207, 0.3327, and 0.4179, respectively.
杂志排行
中国炼油与石油化工的其它文章
- Amorphous Catalysts for Electrochemical Water Splitting
- Tetralin Hydrocracking Reaction Network to Single-Ring Aromatics on Bifunctional Catalysts
- Upgradation of Heavy Crude Oil Via Hydrodynamic Cavitation Through Variations in Asphaltenes
- Wet Desulfurization of High-Sulfur Petroleum Coke Improved via Pre-calcination, H2O2 Treatment, and Ultrasound
- Removal of Heteroaromatic Sulfur Compounds by a Non-noble Metal Fe Single-atom Adsorbent
- A High-Performance Composite Epoxy Coating Based on the Cooperative Enhancement of 2D Co2(OH)2BDC and Electrospun PAN Nanofiber Membrane
