褪黑素处理对梨果实采后黑斑病及贮藏品质的影响
2022-04-21向妙莲吴帆李树成王印宝肖刘华彭文文陈金印陈明
向妙莲,吴帆,李树成,王印宝,肖刘华,彭文文,陈金印,2,陈明
褪黑素处理对梨果实采后黑斑病及贮藏品质的影响

1江西农业大学农学院/江西省果蔬采后处理关键技术与质量安全协同创新中心/江西省果蔬保鲜与无损检测重点实验室,南昌 330045;2萍乡学院,江西萍乡 337055
【】探究褪黑素(Melatonine,MT)处理对梨果实采后黑斑病及贮藏品质的影响,为外源物质调控果实抗采后病害及贮藏品质提供理论依据和参考。以‘翠冠’梨果实为试验材料,喷施0.1 mmol·L-1MT溶液置室温,48 h后沿梨果实赤道两侧刺直径1 mm、深度3 mm大小两个小孔,待伤口晾干后注入20 µL浓度为1.0×106spores/mL黑斑病菌()孢子悬浮液,以无菌水处理作为对照。接种后果实置于25℃,分析梨果实病斑直径、诱导效果及过氧化氢酶()、过氧化物酶()多酚氧化酶()、铜-锌超氧化物歧化酶()等防御酶相关基因和几丁质酶()、-1, 3葡聚糖酶()等病程相关基因的表达量,研究MT诱导梨果实抗黑斑病的效应和机理。此外,梨果实喷施0.1 mmol·L-1MT溶液晾干后于(5±1)℃、相对湿度85%—90%贮藏42 d,以无菌水处理为对照,定期测定果实腐烂率、失重率、呼吸强度、硬度、可溶性固形物、可滴定酸、维生素C、总酚和丙二醛含量,从而探讨MT处理对梨果实贮藏效果和品质的影响。梨果实接种后,病斑直径随接种时间延长而逐渐增大,MT处理组果实病斑直径显著小于对照组(<0.05),接种后第3、5和7天,MT对梨果实抗黑斑病的诱导效应分别为29.16%、45.03%和23.26%;梨果实、、和相对表达量在接种后第4—7天均显著高于对照,最大值分别为对照的1.35、2.08、2.28、2.02、2.89和3.45倍,其中和在接种后第1—6天表达量持续上升,且MT处理可显著提高表达量,表明MT处理诱导梨果实抗黑斑病可能与其提高防御酶基因和病程相关蛋白基因表达密切相关。在低温贮藏期间,MT处理组果实腐烂率与对照组差异不显著,或因病原菌在低温下生长受到抑制,果实腐烂降低;梨果实硬度在贮藏期内逐渐下降,但MT处理组果实硬度均高于对照组,在28 d时差异显著,MT处理组为对照组的1.06倍;梨果实呼吸强度在贮藏前期(7—14 d)上升后下降,但与对照组相比,MT处理抑制果实呼吸强度,延缓果实衰老的效果在贮藏前期较后期更明显;此外,MT处理也可显著降低果实失重率,维持较高水平的可溶性固形物,延缓可滴定酸和维生素C降解,同时促进果实总酚含量积累,增强果实抗氧化能力,抑制MDA含量积累,减轻细胞膜脂过氧化伤害。以上结果揭示MT可能通过调节梨果实糖、酸和细胞壁代谢,从而增强果实品质与耐贮性。0.1 mmol·L-1MT处理诱导了梨果实对采后黑斑病的抗性,激发了果实防御酶和病程相关蛋白基因的表达,且能显著提高梨果实贮藏品质。
褪黑素;梨;果实;黑斑病;诱导抗性;贮藏品质
0 引言
【研究意义】‘翠冠’梨(cv Cuiguan)属早熟品种,目前在中国南方地区多有栽培,具有皮薄肉脆汁多,早熟高产质优的特点[1]。成熟期在七月中下旬,正值高温高湿环境,果实采后极易快速衰老,感染病害而劣变,从而造成严重经济损失。黑斑病是由链格孢()引起的梨常见病害之一[2],该病害在梨生长发育、果实运输贮藏过程中均可发生,给梨产业造成很大经济损失。【前人研究进展】随着我国冷藏和气调贮藏设施及技术的不断完善,可通过调控贮藏条件有效维持梨果实采后品质[3-5],也有研究表明通过生防菌和外源诱导剂处理能显著减少果实采后病害的发生,提高贮藏品质[6-8]。褪黑素(Melatonin,MT)作为一种植物内源多功能生物信号分子,在植物生长发育,成熟衰老等生理代谢和植物生物与非生物胁迫应答过程中有着重要作用[9-12]。近年来,褪黑素在果蔬采后贮藏保鲜的作用已成为研究热点。研究者发现当植物遭遇逆境胁迫时,外源MT可通过调控生理代谢信号通路,激发抗逆基因表达,从而增强植物抗逆性。如外源MT通过调控活性氧代谢和抗氧化酶系统抑制荔枝[13]果实褐变,增强桃[14]、杏[15]果实抗冷性,保持梨[16]、石榴[17]、苹果[18]、芒果[19]果实的品质,从而延长贮藏时间。此外,生吉萍等[20]发现MT可通过激活抗病相关基因、等的表达提高番茄对灰霉病的抗性。MT还可诱导激活茉莉酸通路中的茉莉酸合成关键酶基因启动,促进其表达,最终影响茉莉酸含量,从而增强梨果实轮纹病抗性[21]。【本研究切入点】目前国内外有关褪黑素处理对梨采后黑斑病及贮藏品质影响的报道很少。笔者课题组前期试验初步解析了0.025—0.3 mmol·L-1MT处理对梨果实抗黑斑病的影响,结果表明0.1 mmol·L-1MT处理能显著诱导‘翠冠’梨果实抗采后黑斑病,可能与其增强梨果实抗病防御酶活性、调控活性氧代谢、促进病程相关蛋白有关,但MT对梨果实抗病相关基因及冷藏品质的影响还有待深入研究。【拟解决的关键问题】在MT处理梨果实后,通过测定病斑直径、关键防御酶及病程相关蛋白基因表达,分析抗病相关基因在整个病程中的表达趋势和梨果实冷藏品质的变化,为研发MT在生产实际中调控梨果实采后品质的方法提供理论依据和参考。
1 材料与方法
1.1 试验材料
试验用果:‘翠冠’梨果实于2019年7月15日(盛花期后114 d)采自江西省吉安市峡江县金坪乡精品富兴果业良种示范园,采摘后挑选无病虫害、大小均匀的果实,置于阴凉通风处36 h,充分散去田间热后备用。
供试菌株:由江西农业大学植物病理实验室提供。黑斑病菌()分离自典型黑斑病‘翠冠’梨果实,单孢分离后-80℃保存。
供试试剂:MT购自美国Sigma公司,先使用少许0.1% Tween80和乙醇混合均匀,后加无菌水配置浓度为0.1 mmol·L-1的溶液,保存于4℃冰箱备用。Hifair®Ⅲ试剂盒购自翌圣生物科技(上海)有限公司,SYBR®Premix购自宝生物工程(大连)有限公司。
1.2 试验方法
1.2.1 链格孢孢子悬浮液制备链格孢()于PDA培养基上培养5—7 d后,用无菌水洗脱孢子,经无菌脱脂棉过滤后用血球计数板计数,配置浓度为1.0×106spores/mL孢子悬浮液,现配现用。
1.2.2 MT诱导梨果实抗黑斑病的效应 取1.1所述梨果实,用0.1%次氯酸钠溶液浸泡果实1—2 min,经自来水冲洗干净,室温晾干,喷施0.1 mmol·L-1MT溶液,置于25℃恒温,48 h后用75%的酒精擦拭表面,无菌接种针沿梨果实赤道两侧各刺直径1.0 mm、深度3.0 mm的小孔,待伤口晾干后分别注入20 µL孢子悬浮液,每组处理60个果,3次重复,以无菌水处理作为对照。接种果实于25℃培养。
诱导效果:处理和对照随机选取12个果实,逐日观察梨果实发病情况,采用十字交叉法测量病斑直径,按以下公式计算诱导效果:诱导效果=(对照病斑直径-处理组病斑直径)/对照病斑直径×100%。
RNA提取与检测:接种后第0—7天逐日随机选取4个果实,取梨果实病健交界处果肉,液氮迅速冷冻后置-80℃备用。使用CTAB法提取梨果实总RNA。使用微量核酸分析仪和1%琼脂糖凝胶电泳并检测RNA的质量。使用Hifair®Ⅲ试剂盒(翌圣,上海)反转录RNA合成cDNA第一链。制备的cDNA储存在−80℃超低温冰箱用于后续RT-qPCR试验。
基因表达分析:以为内参[22],使用TB Green®™(Takara,大连)通过RT-qPCR方法检测6种抗病相关基因的表达,引物序列如表1所示。PCR反应程序设定为:95℃预变性进行30 s,95℃持续5 s,60℃退火持续30 s,72℃延伸30 s,进行40个循环。反应体系为10 μL,包括5 μL TB Green,上、下游引物各0.3 μL,3.4 μL ddH2O,1 μL cDNA。每个样品进行3次重复。基因表达结果采用2-ΔΔCt计算[23]。
1.2.3 MT对梨果实贮藏效果和品质的影响 取1.1所述梨果实,喷施0.1 mmol·L-1MT溶液晾干后于5℃、相对湿度85%—90%贮藏,以无菌水处理为对照,每处理120个果,3次重复。分别随机取20个果实用于腐烂率和失重率测定,另外,每隔7 d随机选取6个果实,去皮后取果肉切碎混匀,液氮迅速冷冻后置-80℃备用。
腐烂率(%)=腐烂个数/总数×100。失重率:随机选取20个梨果实编号并称重。失重率(%)=(贮藏前重量-贮藏后重量)/贮藏前重量×100。每7 d测量一次指标并统计数据。

表1 引物序列
采用手持数显糖度计(RA250-WE)测定果实可溶性固形物(TSS,%),可滴定酸含量采用酸碱滴定法(%),总糖含量采用蒽酮比色法(%),维生素C含量(Vc)采用2, 6-二氯靛酚滴定法测定(mg/100 g)。
使用TA.XT Plus型质构仪(英国SMS公司)测定果实硬度,每个果实随机均匀取赤道部附近,去果皮测定6个点,每组处理测定6个果实,3次重复;使用果蔬呼吸测定仪(GHX-3051H)测定梨果实呼吸速率,脱CO2的空气为载气,以标准CO2(1 040 μL·L-1)校准;MDA含量测定采用硫代巴比妥酸法;总酚含量根据Folin-Ciocalteu法测定,以没食子酸作标准曲线计算总酚含量,样品总酚含量换算为每100克鲜重样品没食子酸含量。
1.3 数据处理与统计分析
采用Excel 2013和SPSS20.0软件对数据进行处理和分析,使用-test和Duncan新复极差法进行差异显著性分析。
2 结果
2.1 MT诱导梨果实抗采后黑斑病的效应
如图1-A所示,对照组与0.1 mmol·L-1MT处理组果实病斑直径随接种时间延长而逐渐增大,在第3天时开始出现显著性差异,且MT处理组果实病斑直径在第3—7天均显著小于对照(<0.05)。MT处理组果实诱导效应呈先上升后下降趋势(图1-B),在第5天时达最大值45.03%,与其余时间点相比差异显著,分别是第3和7天的1.54和1.94倍(<0.05)。对照和MT处理果实病斑大小见图1-C。
2.2 MT对梨果实防御酶基因表达的影响
如图2-A所示,损伤接种后梨果实相对表达量于0—2 d上升,2—4 d下降,5—7 d上升。且与对照相比,MT处理组相对表达量均显著提高,第2天时差异极显著,相对表达量是对照的2.49倍(<0.01),而在第5天其表达量达到最高,是对照的1.35倍(<0.01)。
损伤接种后,梨果实相对表达量在0—6 d呈上升趋势,第7天时下降(图2-B)。MT处理组的表达量在1—3 d与对照无显著性差异,但在4—7 d显著高于对照,分别是对照的1.47、1.76、2.08和1.32倍(<0.01)。
从图2-C可知,梨果实相对表达量随接种时间延长整体呈上升趋势。与对照相比,MT处理组相对表达量第1天不显著,第3天显著降低,其他均显著高于对照,在第6天相对表达量达到最高,为对照的2.28倍(<0.01)。
链格孢损伤接种后梨果实相对表达量与相对表达量趋势相同,随接种时间延长,整体呈上升趋势(图2-D)。与对照相比,MT处理组相对表达量除第3天为对照的71.79%外,其余时间均显著高于对照,且在第7天达到最高,为对照的2.02倍(<0.01)。
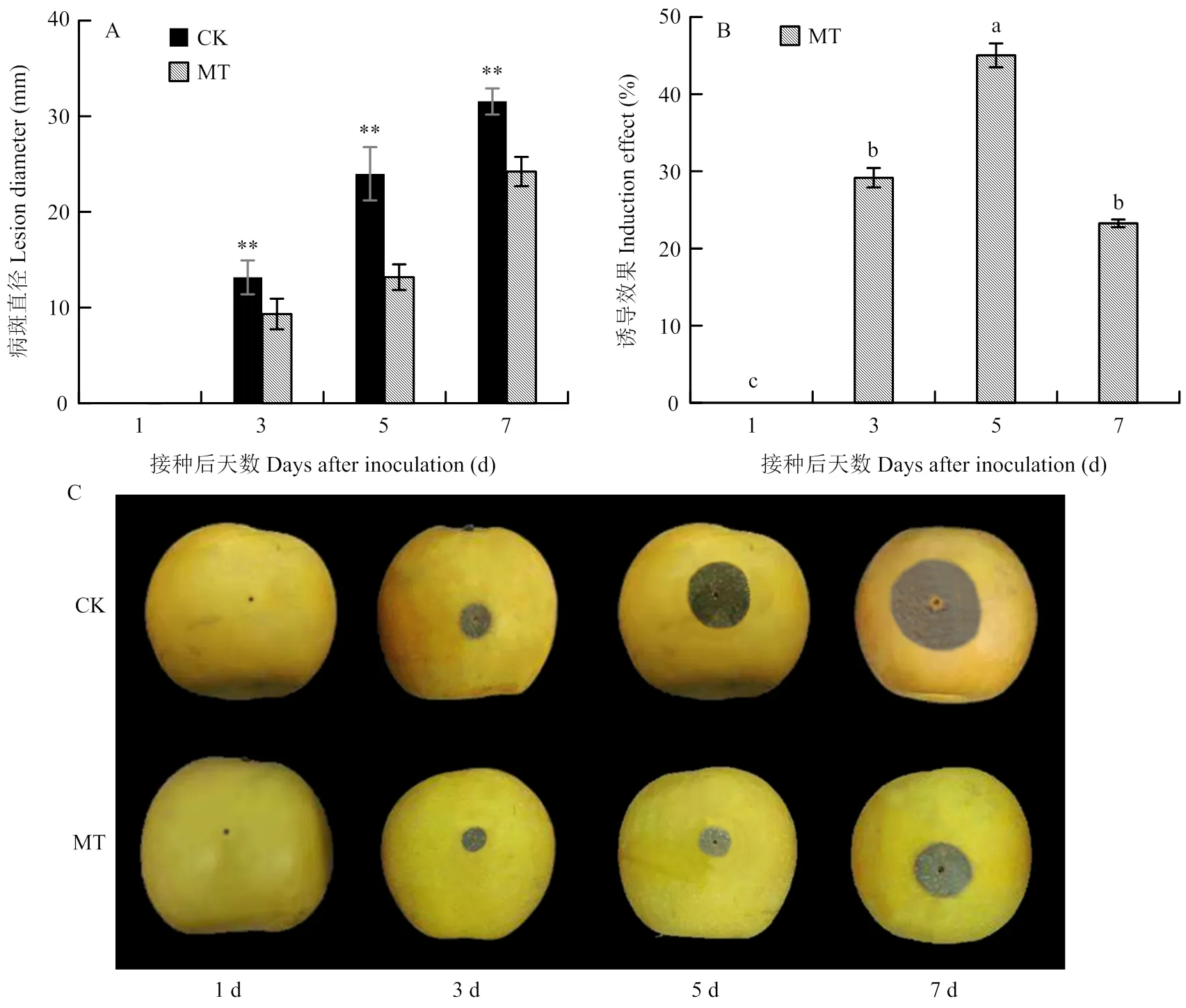
**表示同一时间MT处理和对照之间差异极显著(P<0.01),不同小写字母表示经Duncan新复极差检验在P<0.05水平上有显著性差异。下同
2.3 MT对梨果实病程相关蛋白基因表达的影响
由图3-A可知,梨果实损伤接种后,相对表达量随时间延长逐渐上升。与对照相比,MT处理组梨果实相对表达量除第1和3天外均显著高于对照,在第4天急剧上升,于第6天达到峰值,分别是对照的2.97和2.89倍(<0.01)。梨果实相对表达量随时间增加呈先上升后下降再上升的趋势(图3-B)。在第6天达到峰值,为对照的3.45倍,与对照相比,2—7 d表达量均显著高于对照(<0.01)。
2.4 MT处理对果实冷藏期间腐烂率和失重率的影响
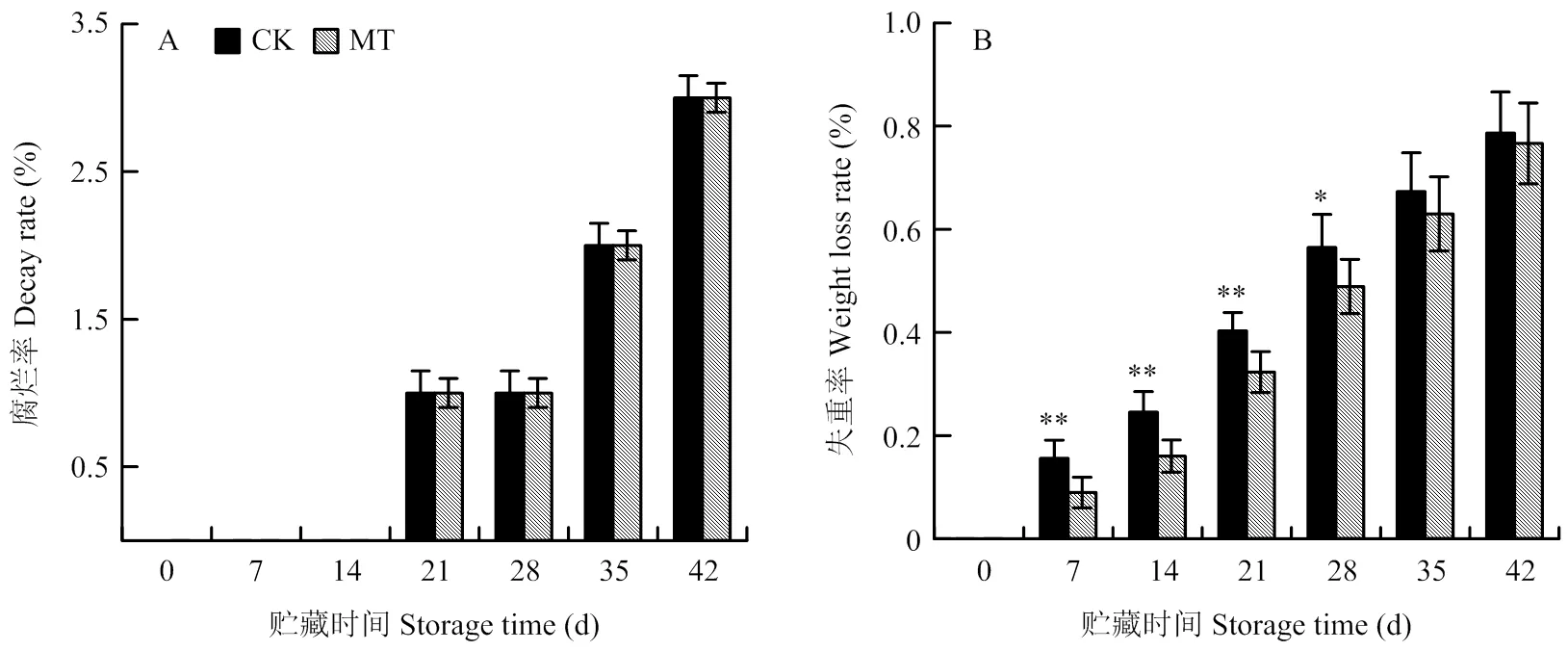
由图4-A可知,冷藏期间果实腐烂率随贮藏时间延长而逐渐上升,MT处理组与对照之间无显著差异,在整个贮藏期都处于较低水平,42 d时约为3.00%。失重率随贮藏时间延长而不断升高(图4-B)。在贮藏前期(7—28 d),MT处理组果实失重率均显著低于对照,分别为0.09%、0.16%、0.32%和0.49%;后期(35—42 d)与对照无显著差异。
2.5 MT处理对果实冷藏期间TSS、TA、VC和总糖含量的影响
由图5-A所示,果实可溶性固形物含量随贮藏时间延长呈先上升后下降再上升趋势,除35 d外,MT处理组可溶性固形物均显著高于对照(<0.05),前期(7—28 d)极显著高于对照,分别为对照的1.07、1.09、1.05和1.06倍(<0.01)。
可滴定酸含量在贮藏期间先上升后下降(图5-B)。在14、28和35 d,MT处理组可滴定酸含量极显著高于对照,分别为对照的1.17、1.22和1.33倍(<0.01)。在7和21 d,其含量低于对照,但无显著性差异。
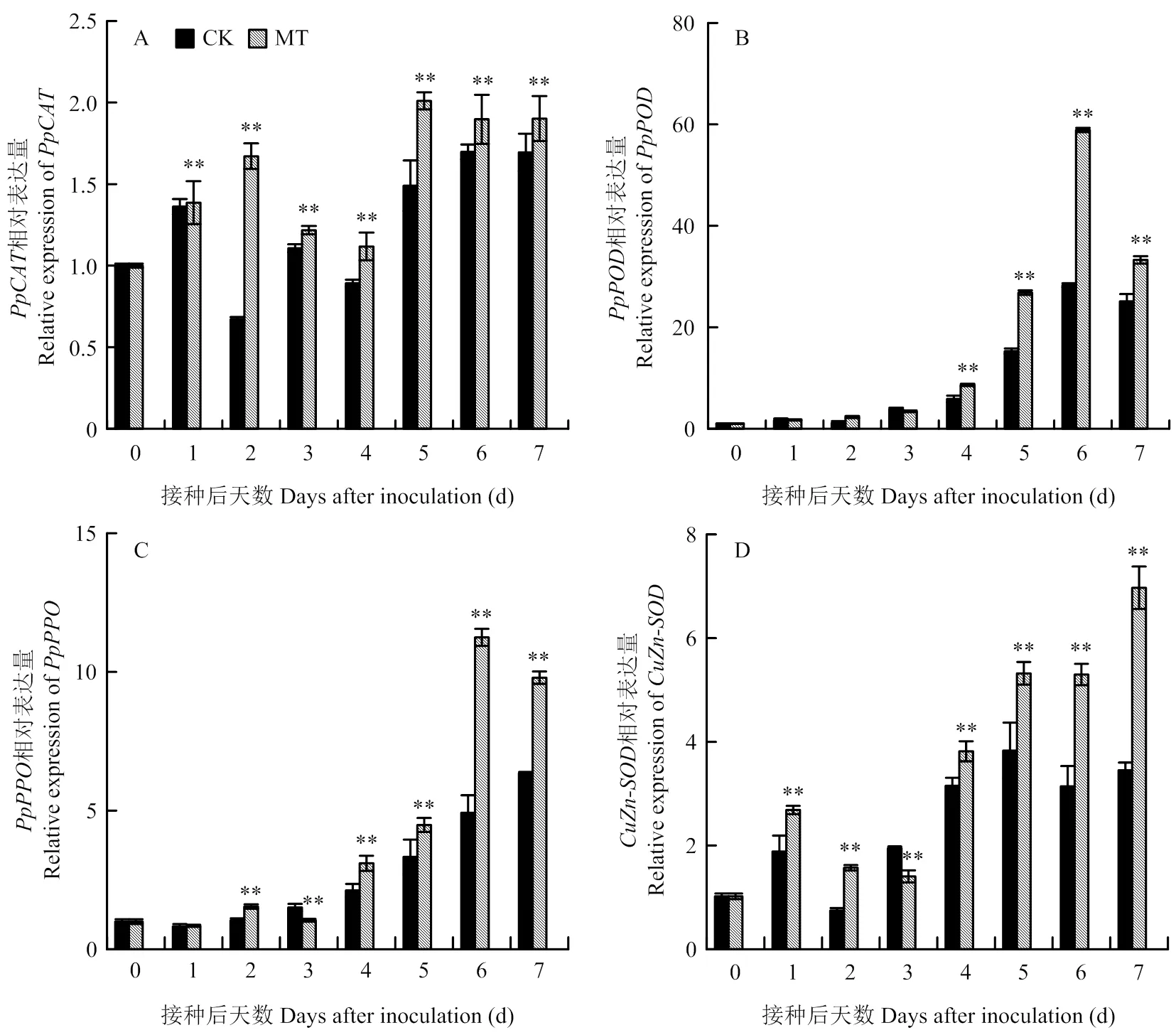
图2 褪黑素处理对梨果实防御酶基因相对表达量的影响

图3 褪黑素处理对梨果实病程相关蛋白CHI和GLU基因相对表达量的影响
由图5-C可知,整个贮藏期内VC含量逐渐降低。除35 d外,MT处理组果实VC含量均显著高于对照(<0.05),在7和21 d极显著高于对照,分别为对照的1.20和1.18倍(<0.01)。
贮藏期间果实总糖含量整体呈上升趋势(图5-D)。与对照相比,MT处理组果实总糖含量在35 d显著低于对照(<0.05),其他时间也低于对照,但无显著性差异。
*表示处理间差异显著(P<0.05)。下同 *indicate significant difference (P<0.05). The same as below
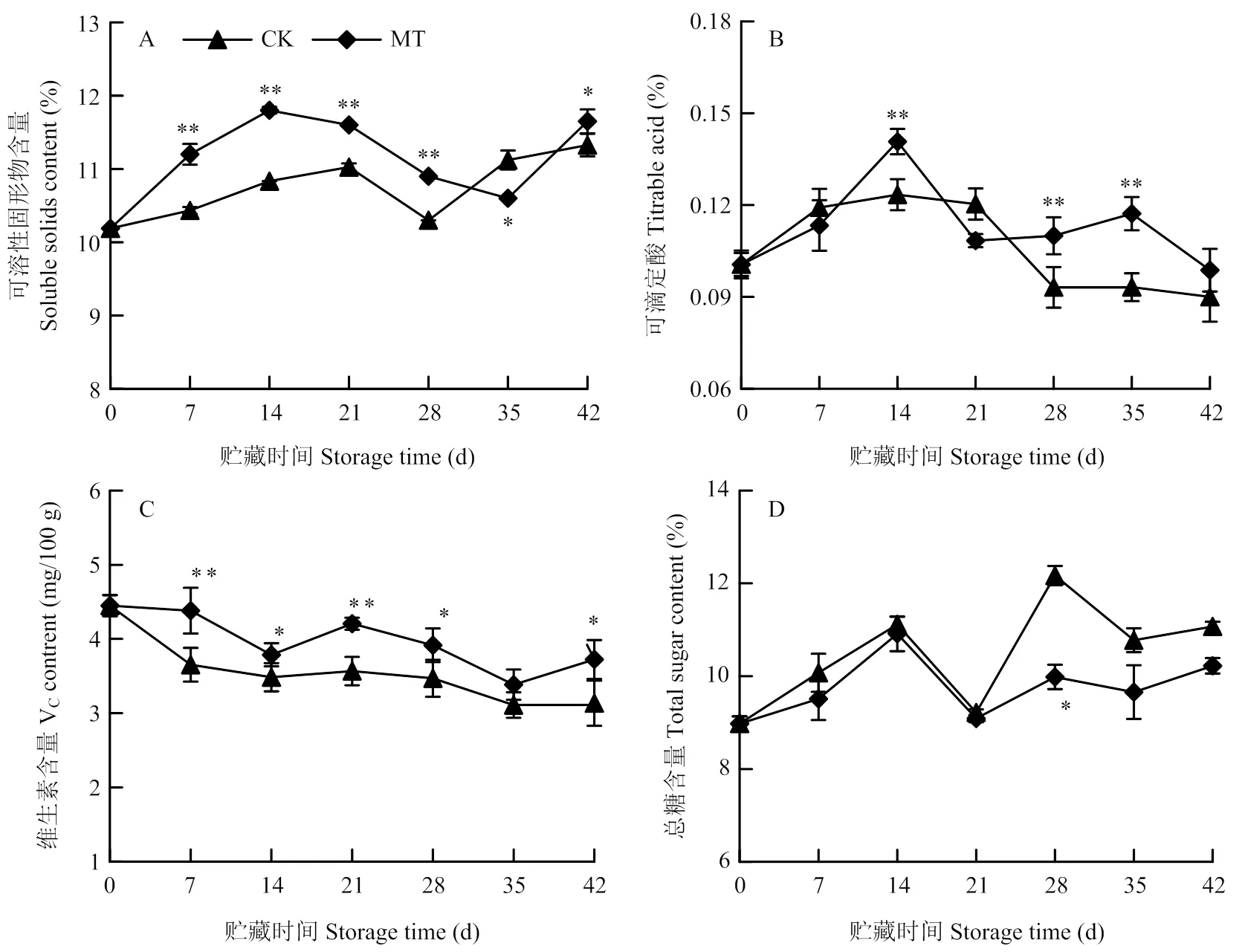
图5 MT处理对梨果实冷藏期间TSS、TA、VC和总糖含量的影响
2.6 MT处理对梨果实冷藏期间硬度、呼吸强度、总酚和MDA含量的影响
由图6-A可知,果实硬度在贮藏期内逐渐下降,MT处理组果实可维持较高硬度,在28 d时显著高于对照,为对照的1.06倍(<0.05)。果实呼吸强度在贮藏前期(7—14 d)上升,后期下降(图6-B)。与对照相比,MT处理组果实呼吸强度在7—14 d和42 d时显著低于对照(<0.05),中期略有上升,但与对照无显著性差异。总酚含量在贮藏期内逐渐上升,MT处理组果实总酚含量稍高于对照,21 d有显著性差异,其余时间均无显著性差异(图6-C)。MDA含量在第7天急剧上升后又迅速下降,14—42 d稳步上升(图6-D)。整个贮藏期间MT处理组的MDA含量均明显低于对照,7—28 d与对照相比有显著性差异(<0.05)。
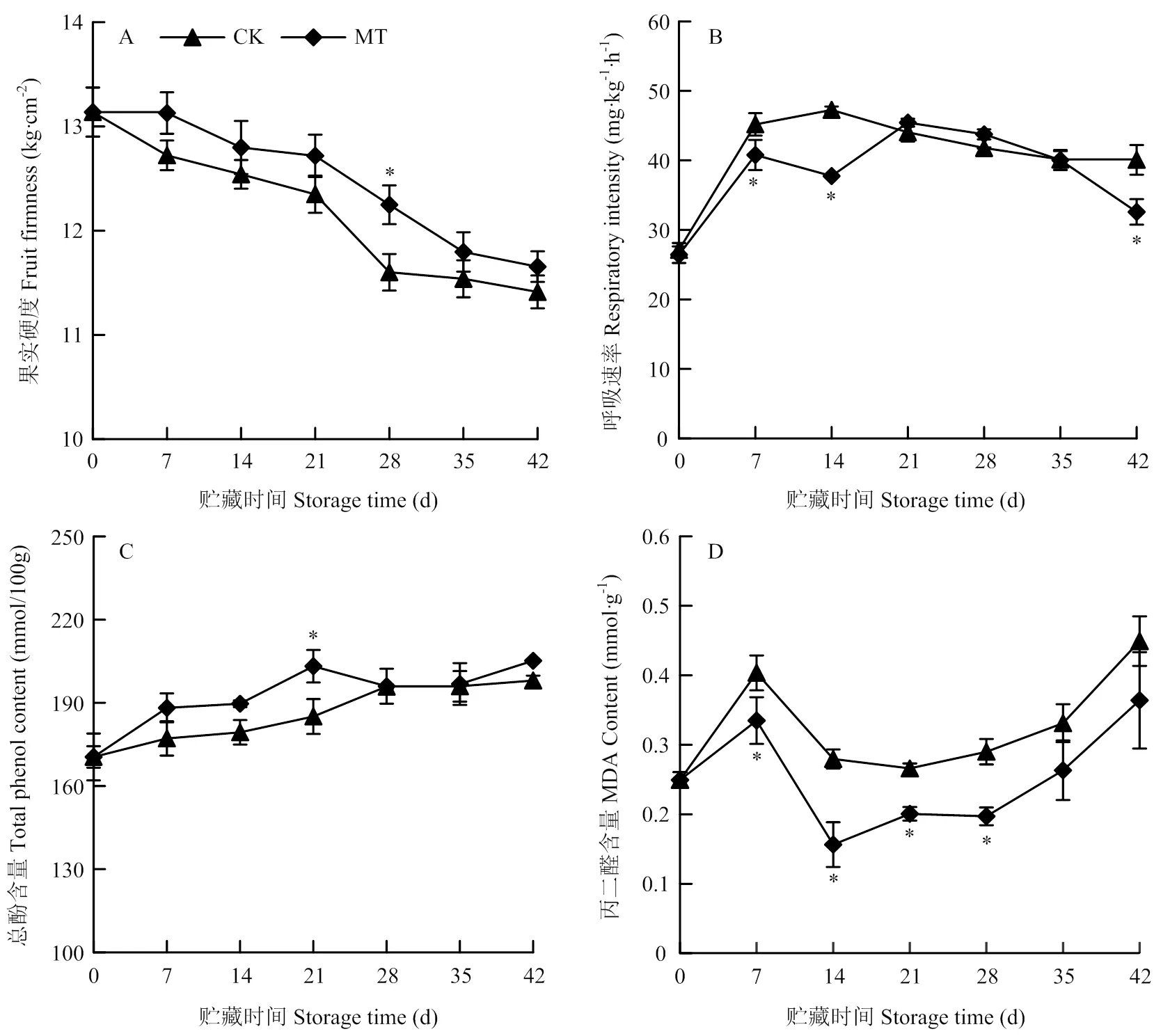
图6 MT处理对梨果实冷藏期间硬度、呼吸强度、总酚和MDA含量的影响
3 讨论
3.1 外源物质诱导植物抗病基因表达
CAT、PPO、SOD、POD、GLU和CHI等是寄主植物关键防御酶,植物受到病原微生物侵染时,外源诱导物质可通过调控上述酶编码基因表达来诱导植物产生抗病性,如猕猴桃果实经茉莉酸甲酯(MeJA)熏蒸处理后,和表达量显著高于对照,抗病性显著提高[24]。李磊等[25]发现马铃薯经水杨酸诱导后抗晚疫病效果增强,过氧化物酶基因和多酚氧化酶基因表达量在施用早期显著高于对照。2, 4-表油菜素内酯处理葡萄果实,和抗病基因表达显著增加,果实采后灰霉病发病率降低[26]。
3.2 MT处理对植物抗病基因表达的影响
MT作为近年来诱导植物抗病研究领域的新热点,可通过调控活性氧代谢,激发防御酶活性和病程相关蛋白基因表达等提高植物抗逆性。孙子荀等[27]发现外源MT处理通过提高草莓抗病相关基因和等的表达量,从而提高草莓黑斑病抗性。MT处理感锈病小豆品种激发诱导了水杨酸通路关键基因表达,进而激活下游病程相关蛋白(PR1、PR5、CHI、GLU)的高水平应答,使其获得对锈病的抗性[28]。本研究结果表明,MT预处理激活了梨果实中防御酶基因、、、和的表达。这些防御相关基因均在后期维持较高水平表达,且与对照相比差异显著,其中和的表达量明显高于其余基因。MT处理诱导梨果实对黑斑病的抗性可能与其防御酶基因和病程相关蛋白基因表达密切相关。
3.3 MT处理对梨果实贮藏品质的影响
梨属呼吸跃变型果实,采后贮藏期间果实呼吸作用增强,糖、酸、VC等营养物质逐渐降解,硬度下降,衰老速度加快,品质降低[29]。本研究中,MT处理能维持梨果实硬度和TSS在较高水平,延缓可滴定酸和VC的降解,抑制呼吸速率,促进总酚积累。MT处理甜樱桃[30]、石榴[31]和猕猴桃[32]等果实也得出类似结论,从而有效延缓果实采后衰老。TSS的变化可能与贮藏期间糖的转化有关[33],有研究表明MT处理可通过抑制枇杷[34]和桃果实[35]蔗糖下降,减缓葡萄糖和果糖含量变化,提高酚类物质和有机酸含量,增强木质素生物合成相关酶和抗氧化酶活性,延缓果实衰老,提高果实品质。MT处理枣还可抑制与果实软化相关酶(PME、PG、Cx和-glu)的活性,减缓可溶性果胶产生,保持果实硬度[36]。推测MT可能通过增强抗氧化酶活性,调节果实糖、酸和细胞壁代谢,从而增强果实品质与耐贮性。本研究中,果实腐烂率在整个贮藏期都处于较低水平,但MT处理组与对照之间无显著差异,这可能是由于病原菌在低温下生长受到抑制,从而延缓病害发生时间[37-38]。
4 结论
翠冠梨果实经0.1 mmol·L-1MT处理,可能通过激活防御相关酶基因和病程相关蛋白基因的表达,从而诱导梨果实抗采后黑斑病。MT处理显著抑制低温贮藏期间果实呼吸强度,延缓果实衰老,维持TSS在较高水平,延缓可滴定酸和VC的降解;同时促进果实总酚含量的积累,增强果实抗氧化能力,抑制MDA含量积累,减轻细胞膜脂过氧化伤害,从而提高了果实品质。
[1] 邓秀新, 王力荣, 李绍华, 张绍铃, 张志宏, 丛佩华, 易干军, 陈学森, 陈厚彬, 钟彩虹. 果树育种40年回顾与展望. 果树学报, 2019, 36(4): 514-520.
DENG X X, WANG L R, LI S H, ZHANG S L, ZHANG Z H, CONG P H, YI G J, CHEN X S, CHEN H B, ZHONG C H. Retrospection and prospect of fruit breeding for last four decades in China. Journal of Fruit Science, 2019, 36(4): 514-520. (in Chinese)
[2] 李丙新, 何锋, 刘娟娟, 赵延存, 孙伟波, 操海群, 刘凤权. 梨黑斑病菌遗传操作体系的建立与RFP标记转化子的致病性分析. 植物病理学报, 2018, 48(5): 648-655. doi:10.13926/j.cnki.apps.000182.
LI B X, HE F, LIU J J, ZHAO Y C, SUN W B, CAO H Q, LIU F Q. Established genetic transformation ofand pathogenicity analysis of the RFP labeled transformants. Acta Phytopathologica Sinica, 2018, 48(5): 648-655. doi:10.13926/j.cnki. apps.000182. (in Chinese)
[3] 王志华, 王文辉, 贾朝爽, 姜云斌. CO2体积分数对气调贮藏‘红香酥’梨果实货架期相关生理指标的影响. 果树学报, 2020, 37(10): 1562-1572. doi:10.13925/j.cnki.gsxb.20200272.
WANG Z H, WANG W H, JIA C S, JIANG Y B. Effects of carbon dioxide concentrations on the physiological indexes of 'Hongxiangsu'pears during shelf-life after controlled atmosphere storage. Journal of Fruit Science, 2020, 37(10): 1562-1572. doi:10.13925/j.cnki.gsxb.20200272. (in Chinese)
[4] 杜艳民, 王文辉, 贾晓辉, 佟伟, 王阳, 张鑫楠. 不同O2浓度对鸭梨采后生理代谢及贮藏品质的影响. 中国农业科学, 2020, 53(23): 4918-4928.
DU Y M, WANG W H, JIA X H, TONG W, WANG Y, ZHANG X N. The effects of different oxygen concentration on postharvest physiology and storage quality of yali pear. Scientia Agricultura Sinica, 2020, 53(23): 4918-4928. (in Chinese)
[5] LI M, ZHI H H, DONG Y. The influence of pre- and postharvest 1-MCP application and oxygen regimes on textural properties, cell wall metabolism, and physiological disorders of late-harvest ‘Bartlett’ pears. Postharvest Biology and Technology, 2021, 173: 111429.
[6] 马强, 石雅君, 李正男, 王文辉, 孙平平.PGLY-1的分离、鉴定及对梨青霉病的抑制作用评价. 中国果树, 2020(5): 50-54. doi:10.16626/j.cnki.issn1000-8047.2020.05.009.
MA Q, SHI Y J, LI Z N, WANG W H, SUN P P. Isolation and identification ofPGLY-1 for the inhibition of pear green mold. China Fruits, 2020(5): 50-54. doi:10.16626/j.cnki. issn1000-8047.2020.05.009. (in Chinese)
[7] 张靖国, 陈启亮, 杨晓平, 范净, 胡红菊. 1-MCP处理对翠冠梨货架期品质的影响. 湖北农业科学, 2020, 59(21): 121-123. doi:10.14088/j.cnki.issn0439-8114.2020.21.025.
ZHANG J G, CHEN Q L, YANG X P, FAN J, HU H J. Effects of 1-MCP treatment on shelf life quality of Cuiguan pear. Hubei Agricultural Sciences, 2020, 59(21): 121-123. doi:10.14088/j.cnki. issn0439-8114.2020.21.025. (in Chinese)
[8] KAN C N, GAO Y, WAN C P, CHEN M, ZHAO X Y, LIU S J, CHEN J Y. Influence of different cold storage times on quality of ‘Cuiguan’pear fruits during shelf life. Journal of Food Processing and Preservation, 2019, 43(12): 14245.
[9] 王蕊, 杨小龙, 须晖, 李天来. 高等植物褪黑素的合成和代谢研究进展. 植物生理学报, 2016, 52(5): 615-627.
WANG R, YANG X L, XU H, LI Y L. Research progress of melatonin biosynthesis and metabolism in higher plants. Plant Physiology Journal, 2016, 52(5): 615-627. (in Chinese)
[10] 巩彪, 史庆华. 园艺作物褪黑素的研究进展. 中国农业科学, 2017, 50(12): 2326-2337. doi:10.3864/j.issn.0578-1752.2017.12.013.
GONG B, SHI Q H. Review of melatonin in horticultural crops. Scientia Agricultura Sinica, 2017, 50(12): 2326-2337. doi:10.3864/ j.issn.0578-1752.2017.12.013. (in Chinese)
[11] WANG L, LUO Z S, BAN Z J, JIANG N, YANG M Y, LI L. Role of exogenous melatonin involved in phenolic metabolism ofjujuba fruit. Food Chemistry, 2021, 341(pt 2): 128268. doi:10.1016/ j.foodchem.2020.128268.
[12] 卞凤娥, 肖秋红, 郝桂梅, 孙永江, 陆文利, 杜远鹏, 翟衡. 根施褪黑素对NaCl胁迫下葡萄内源褪黑素及叶绿素荧光特性的影响. 中国农业科学, 2018, 51(5): 952-963.
BIAN F E, XIAO Q H, HAO G M, SUN Y J, LU W L, DU Y P, ZHAI H. Effect of root-applied melatonin on endogenous melatonin and chlorophyll fluorescence characteristics in grapevine under NaCl stress. Scientia Agricultura Sinica, 2018, 51(5): 952-963. (in Chinese)
[13] 乔沛, 殷菲胧, 王雨萱, 李静, 董新红. 外源褪黑素处理对采后荔枝褐变及活性氧代谢的影响. 食品工业科技, 2021, 42(6): 282-287. doi:10.13386/j.issn1002-0306.2020060279.
QIAO P, YIN F L, WANG Y X, LI J, DONG X H. Effects of exogenous melatonin on browning and active oxygen metabolism of postharvest. Science and Technology of Food Industry, 2021, 42(6): 282-287. doi:10.13386/j.issn1002-0306.2020060279. (in Chinese)
[14] GAO H, LU Z M, YANG Y, WANG D N, YANG T, CAO M M, CAO W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chemistry, 2018, 245: 659-666. doi:10.1016/j. foodchem.2017.10.008.
[15] 何欢, 刘昭雪, 张亚琳, 张欢欢, 芦玉佳, 朱璇. 外源褪黑素调控活性氧代谢对减轻采后杏果实冷害的分析. 食品科学, 2021. https:// kns.cnki.net/kcms/detail/11.2206.TS.20210322.1129. 018.html.
HE H, LIU Z X, ZHANG Y L, ZHANG H H, LU Y J, ZHU X. Effects of exogenous melatonin on reactive oxygen species metabolism and chilling injury of postharvest apricot fruit. Food Science, 2021. https:// kns.cnki.net/kcms/detail/11.2206.TS.20210322.1129.018.html. (in Chinese)
[16] 王纪忠, 童瑶, 史云勇, 魏树伟. 外源褪黑素处理对常温货架期梨果实贮藏品质的影响. 果树学报, 2021, 38(4): 569-579.
WANG J Z, TONG Y, SHI Y Y, WEI S W. Effects of exogenous melatonin treatment on storage quality of pear fruits during shelf life at room temperature. Journal of Fruit Science, 2021, 38(4): 569-579. (in Chinese)
[17] AGHDAM M S, LUO Z, LI L, JANNATIZADEH A, FARD J R, PIRZAD F. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chemistry, 2020, 303: 125385. doi:10.1016/j.foodchem.2019.125385.
[18] ONIK J C, WAI S C, LI A, LIN Q, SUN Q Q, WANG Z D, DUAN Y Q. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chemistry, 2021, 337: 127753. doi:10.1016/j.foodchem.2020.127753.
[19] 刘帅民, 胡康琦, 刘港帅, 张善英, 潘永贵, 史学群, 张正科. 外源褪黑素处理对鲜切芒果贮藏品质的影响. 食品科学, 2020, 41(21): 160-166. doi:10.7506/spkx1002-6630-20191031-358.
LIU S M, HU K Q, LIU G S, ZHANG S Y, PAN Y G, SHI X Q, ZHANG Z K. Effect of exogenous melatonin treatment on storage quality of fresh-cut mango. Food Science, 2020, 41(21): 160-166. doi:10.7506/spkx1002-6630-20191031-358. (in Chinese)
[20] 生吉萍, 赵瑞瑞, 陈玲玲, 申琳. 褪黑素采前喷施对采后番茄果实抗病性和贮藏品质的影响. 食品科学, 2020, 41(9): 188-193. doi:10.7506/spkx1002-6630-20190416-204.
SHENG J P, ZHAO R R, CHEN L L, SHEN L. Effect of pre-harvest melatonin spraying on the post-harvest disease resistance and storage quality of tomato fruit. Food Science, 2020, 41(9): 188-193. doi:10.7506/spkx1002-6630-20190416-204. (in Chinese)
[21] 刘建龙. 外源褪黑素对梨果实发育、采后品质和抗轮纹病的影响及其调控机制研究[D]. 杨凌: 西北农林科技大学, 2019.
LIU J L. Regulatory function of exogenous melatonin on fruit development, postharvest fruit quality and ring rot disease resistance in pears [D]. Yangling: Northwest A & F University, 2019. (in Chinese)
[22] 余辰.-氨基丁酸对梨果实青霉病抗性的诱导作用及相关机理研究[D]. 杭州: 浙江大学, 2014.
YU C. Induced effect of-aminobutvric acid on host resistance against blue mold and defense-related mechanism in pear fruit [D]. Hangzhou: Zhejiang University, 2014. (in Chinese)
[23] LIVAK K J, SCHMITTGEN T D S. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCtmethod. Methods, 2001, 25(4): 402-408.
[24] PAN L Y, ZHAO X Y, CHEN M, FU Y Q, XIANG M L, CHEN J Y. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chemistry, 2020, 305: 125483. doi:10.1016/j.foodchem.2019.125483.
[25] 李磊, 陆杰, 包亚洲, 李勇, 吕文河, 王晓丹. 四种化合物诱导马铃薯抗晚疫病的效果及其相关防御基因表达分析. 植物保护学报, 2020, 47(6): 1277-1286. doi:10.13802/j.cnki.zwbhxb.2020.2020019.
LI L, LU J, BAO Y Z, LI Y, LÜ W H, WANG X D. Resistant effects of potato against late blight and expression analysis of potato-related defense genes induced by four kinds of compounds. Journal of Plant Protection, 2020, 47(6): 1277-1286. doi:10.13802/j.cnki.zwbhxb.2020. 2020019. (in Chinese)
[26] 杨艺琳, 张正敏, 李美琳, 赵立艳, 金鹏, 郑永华. 2, 4-表油菜素内酯对葡萄果实采后灰霉病的抑制作用机理. 食品科学, 2019, 40(15): 231-238. doi:10.7506/spkx1002-6630-20180821-222.
YANG Y L, ZHANG Z M, LI M L, ZHAO L Y, JIN P, ZHENG Y H. Modes of action of 2, 4-epibrassionolide against postharvest gray mold decay of grapes. Food Science, 2019, 40(15): 231-238. doi:10. 7506/spkx1002-6630-20180821-222. (in Chinese)
[27] 孙子荀, 倪照君, 高志红, 乔玉山, 万春雁, 古咸彬. 外源褪黑素提高草莓黑斑病抗性的效果和作用机制初探. 西北植物学报, 2020, 40(10): 1679-1687. doi:10.7606/j.issn.1000-4025.2020.10.1679.
SUN Z X, NI Z J, GAO Z H, QIAO Y S, WAN C Y, GU X B. Effect and mechanism of exogenous melatonin on improvement of black rot disease resistance in strawberry. Acta Botanica Boreali-Occidentalia Sinica, 2020, 40(10): 1679-1687. doi:10.7606/j.issn.1000-4025.2020. 10.1679. (in Chinese)
[28] 郭博铖, 柯希望, 高尚雨, 于昕卉, 孙启明, 左豫虎. 褪黑素诱导小豆抗锈病机理的初步研究. 植物保护, 2020, 46(1): 145-150, 156. doi:10.16688/j.zwbh.2018521.
GUO B C, KE X W, GAO S Y, YU X H, SUN Q M, ZUO Y H. A preliminary study on the mechanisms of melatonin-induced rust resistance of adzuki bean. Plant Protection, 2020, 46(1): 145-150, 156. doi:10.16688/j.zwbh.2018521. (in Chinese)
[29] ADHIKARY T, GILL P P S, JAWANDHA S K, BHARDWAJ R D, ANURAG R K. Efficacy of postharvest sodium nitroprusside application to extend storability by regulating physico-chemical quality of pear fruit. Food Chemistry, 2021, 346: 128934. doi:10.1016/ j.foodchem.2020.128934.
[30] MIRANDA S, VILCHES P, SUAZO M, PAVEZ L, GARCÍA K, MÉNDEZ M A, GONZÁLEZ M, MEISEL L A, DEFILIPPI B G, DEL POZO T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chemistry, 2020, 319: 126360. doi:10.1016/j. foodchem.2020.126360.
[31] LORENTE-MENTO J M, GUILLÉN F, CASTILLO S, MARTÍNEZROMERO D, VALVERDE J M, VALERO D, SERRANO M. Melatonin treatment to pomegranate trees enhances fruit bioactive compounds and quality traits at harvest and during postharvest storage. Antioxidants, 2021, 10(6): 820.
[32] 胡苗, 李佳颖, 饶景萍. 褪黑素处理对采后猕猴桃果实后熟衰老的影响. 食品科学, 2018, 39(19): 226-232. doi:10.7506/spkx1002- 6630-201819035.
HU M, LI J Y, RAO J P. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Food Science, 2018, 39(19): 226-232. doi:10.7506/spkx1002-6630-201819035. (in Chinese)
[33] ZHAO H D, WANG B G, CUI K B, CAO J K, JIANG W B. Improving postharvest quality and antioxidant capacity of sweet cherry fruit by storage at near-freezing temperature. Scientia Horticulturae, 2019, 246: 68-78.
[34] WANG D, CHEN Q Y, CHEN W W, GUO Q G, XIA Y, WU D, JING D L, LIANG G L. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Scientia Horticulturae, 2021, 284: 110126
[35] 徐利伟, 岑啸, 李林香, 沈子明, 陈景丹, 陈馨, 陈伟, 杨震峰. 外源褪黑素对低温胁迫下桃果实蔗糖代谢的影响. 核农学报, 2017, 31(10): 1963-1971. doi:10.11869/j.issn.100-8551.2017.10.1963.
XU L W, CEN X, LI L X, SHEN Z M, CHEN J D, CHEN X, CHEN W, YANG Z F. Effect of exogenous melatonin on sucrose metabolism in peach fruit exposed to low temperature stress. Journal of Nuclear Agricultural Sciences, 2017, 31(10): 1963-1971. doi:10.11869/j.issn. 100-8551.2017.10.1963. (in Chinese)
[36] TANG Q, LI C Y, GE Y H, LI X, CHENG Y, HOU J B, LI J R. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT-Food Science and Technology, 2020, 127: 109431.
[37] POSE G, PATRIARCA A, KYANKO V, PARDO A, FERNÁNDEZ PINTO V. Water activity and temperature effects on mycotoxin production byon a synthetic tomato medium. International Journal of Food Microbiology, 2010, 142(3): 348-353. doi:10.1016/j.ijfoodmicro.2010.07.017.
[38] 黄伟, 冯作山, 白羽嘉, 张培岭, 郑峰. 采后果实链格孢属真菌病害防治方法研究进展. 食品与机械, 2016, 32(3): 247-252.
HUANG W, FENG Z S, BAI Y J, ZHANG P L, ZHENG F. Advances on methods to control fungal diseases ofin postharvest. Food & Machinery, 2016, 32(3): 247-252. (in Chinese)
Effects of Melatonin Treatment on Resistance to Black Spot and Postharvest Storage Quality of Pear Fruit

1College of Agronomy, Jiangxi Agricultural University/Collaborative Innovation Center of Postharvest Key Technology and Quality Safety of Fruits and Vegetables in Jiangxi Province/Jiangxi Key Laboratory for Postharvest Technology and Non-destructive Testing of Fruits & Vegetables, Nanchang 330045;2Pingxiang University, Pingxiang 337055, Jiangxi
【】The aim of this study was to explore the effects of melatonine (MT) treatment on postharvest black spot disease and storage quality of pear fruit, so as to provide the theoretical basis and reference for exogenous substances regulating postharvest disease and storage quality of pear fruit.【】‘Cuiguan’ pears were used as the experimental material, which were sprayed with 0.1 mmol·L-1MT solution and then kept at room temperature for 48 h. The treated fruits were inoculated with two 1 mm diameter × 3 mm depth small holes along both sides of the fruit equator. Twenty µLspore suspension with 1.0×106spores/mL were injected into the two holes, and the sterile water treatment was used as control. The fruit was placed at 25℃ after inoculation, and then the lesion diameters and induced effects as well as the genes expression of defense enzymes, such as catalase (), peroxidase (), polyphenol oxidase (), and copper-zinc superoxide dismutase (), and pathogenesis-related protein including chitinase () and-1,3 glucanase (), were analysed to demonstrate the effect and mechanism of MT-induced pear fruit against black spot disease. In addition, the pear fruits were sprayed with 0.1 mmol·L-1MT solution, and then stored at (5±1)℃, 85%-90% relative humidity for 42 d. The sterile water treatment was used as the control. The decay rate, weight loss rate, respiration rate, firmness, total soluble solids, titratable acid, vitamin C, total phenols and malondialdehyde contents were measured at fixed period, and the effect of MT treatment on the storage effect and quality of pear fruit were discussed. 【】The lesion diameters of pear fruits inoculated withgradually increased with the time, while the lesion diameters under MT treatment was significantly smaller than those under the control (<0.05). The MT-induced resistance effects on pear fruits black spot were 29.16%, 45.03% and 23.26% on the 3rd, 5th and 7th day, respectively. The relative expression levels of,,,,andin MT-treated groupwere significantly higher than those of the control group during 4-7 days post inoculation (dpi), and the maximum values of them were 1.35, 2.08, 2.28, 2.02, 2.89 and 3.45 times of control fruits, respectively. The induced expression of,andindicated that MT treatment inducing resistance of pear fruit to black spot disease possible depended on these defense enzyme and pathogenesis-related proteins. For the low temperature storage, the fruit decay rate was not significantly different between MT treatment group and control group, which was probably caused by the inhibition growth of pathogen under low temperature. All of fruit firmness gradually decreased during storage, while the fruit firmness under MT treatment was higher than that under the control with 1.06 times of significant difference at 28 d. Furthermore, the fruit respiration rate increased in the early storage stage (7-14 d) and decreased in the later stage. In comparison with the control group, the effects of MT treatment inhibiting fruit respiration and delaying fruit senescence were more significantly in the early storage stage than in the later stage. In addition, MT treatment also significantly reduced the fruit weight loss rate, maintained the high levels of total soluble solids, and delayed the degradation of titratable acid and vitamin C. Also, it promoted the accumulation of total phenolic content, enhanced the fruit antioxidant capacity, inhibited the accumulation of MDA content, and reduced the damage of cell membrane lipid peroxidation. The above results indicated that MT might enhance fruit quality and storage resistance by regulating fruit sugar, acid, and cell wall metabolism.【】0.1 mmol·L-1MT treatment induced the resistance of pear fruit to black spot, stimulated the relative expression of defense enzymes and pathogenesis-related protein coded-genes, and finally improved the storage quality of pear fruit.
melatonin; pear; fruit; black spot; induced resistance; storage quality

10.3864/j.issn.0578-1752.2022.04.013
2021-06-08;
2021-08-20
国家自然科学基金(31360466)、江西省自然科学基金(20192BAB204018)、江西省果蔬采后处理关键技术及质量安全协同创新中心项目(JXGS-02)
向妙莲,E-mail:mlxiang@jxau.edu.cn。通信作者陈明,E-mail:mingchen@jxau.edu.cn
(责任编辑 赵伶俐)
