丝素蛋白纤维提取技术的研究
2022-04-21冯燕萍董志红张颖程丽佳唐璐归艳华
冯燕萍 董志红 张颖 程丽佳 唐璐 归艳华

摘要: 丝素蛋白(SF)以其优异的性能,如较好的生物相容性、生物降解性和机械性能等,被广泛应用于生物医学领域。天然的SF需要经过脱胶处理(去除蚕丝中的丝胶)方可获得,不同的脱胶方法和工艺参数会对SF结构和性能产生不同的影响,选择合理的高效脱胶工艺方法,为制备生物医用材料提供较高性能的SF。本研究从化学、物理和生物技术几方面论述了蚕丝脱胶的方法,包括碱性、酸性、高温高压(HTHP)、超声波、微波和酶处理等,阐述了SF纤维的提取技术,以期获得高纯度、表面光滑、可降解性好和机械性能优异的SF,制备出SF新型材料,为SF在生物医用材料领域的应用提供較好的前期研究基础。
关键词: 丝素蛋白纤维;丝胶蛋白;脱胶方法;生物医用材料;机械性能
中图分类号: TS102.33文献标志码: A文章编号: 10017003(2022)04000907
引用页码: 041102DOI: 10.3969/j.issn.1001-7003.2022.04.002(篇序)
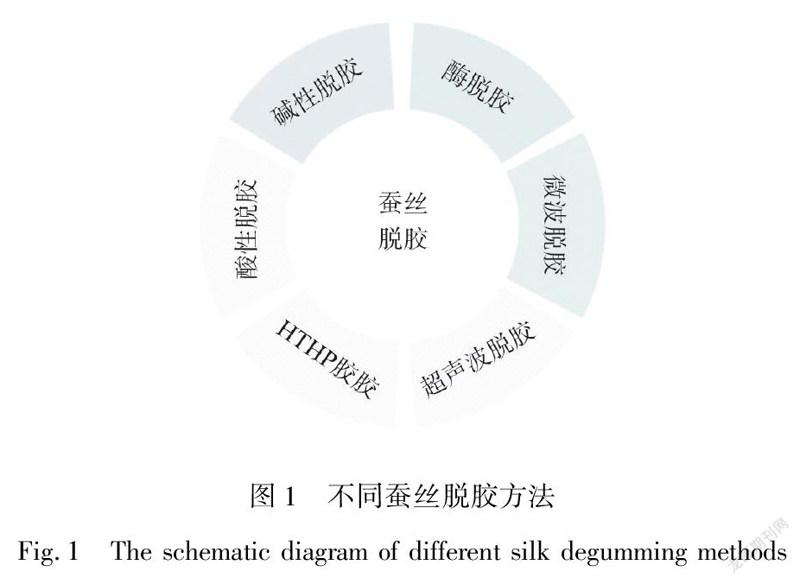
蚕丝是天然的纤维材料。无论何种类别的蚕丝,一般均由质量为70%左右的丝素蛋白(SF)和25%左右的丝胶所组成,剩余的5%左右则是一些杂质。早期蚕丝常被用在纺织领域上,随着新材料的开发和利用,蚕丝在军工、制药、生物医药材料领域上也逐渐展现出其优异的特性,尤其是蚕丝中的SF,其具有良好的力学性能、生物相容性、抗菌性等优点,同时它的物理和生物学特性也优于丝胶。因此,人们对SF的研究越来越深入,从制备方法到将其复合制备成新材料,无一不体现出它优异的特性,如将SF制成粉末[1]、凝胶[2]、薄膜状[3],通过静电纺丝制成SF纤维膜[4],也制备成SF纤维增强复合材料[5]等。在生物医疗领域,SF可作为药物释放的载体[6]、人工骨组织支架[7]、伤口敷料[8]、人工耳膜[9]及神经导管[10]等。脱胶是一种表面改性手段,可通过改变脱胶手段、调节脱胶参数等,利于保存SF完好的结构和性能,开发出具备独特的生物降解性、良好的机械性能的新型生物医用材料。而如何在无残留丝胶的同时,不损伤SF本身结构和性能,是蚕丝脱胶的首要问题。脱胶的实验方法及工艺参数直接影响SF的特性,如脱胶过程中,脱胶的完整性、均匀性,直接影响脱胶后SF的柔软性、光滑程度及光泽度等。本文从不同的角度总结阐述脱胶方案,主要从化学、物理和生物三方面阐述不同的提取方法,包括用碱、肥皂、尿素、有机酸、离子液体、高温高压、超声波、微波和酶等脱胶方法(图1)。无论是碱性、酸性还是物理/生物脱胶方法,都是将丝胶蛋白分解,从蚕丝上自行脱落下来,但是不同的脱胶方法对SF的生物医用会产生不同的影响。本文在SF制备方法上进行合理阐述,以便在生物医用材料领域上,为选取合适地提取方案提供清晰的思路及参考。
1.1碱性处理
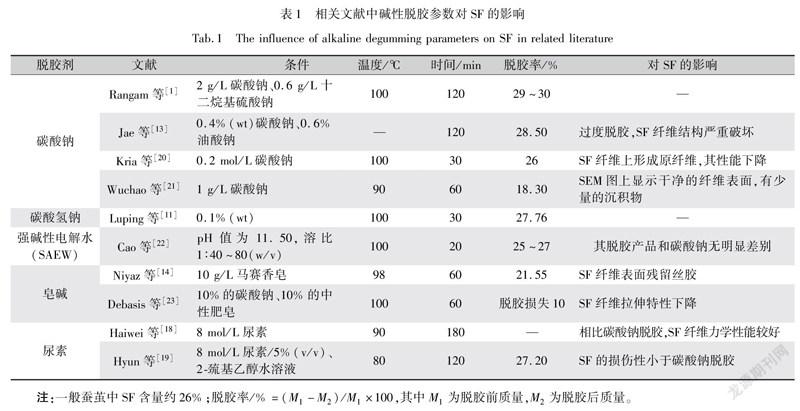
碱性脱胶主要包括碳酸钠(NaCO)、碳酸氢钠(NaHCO)、强碱性电解水(SAEW)、皂碱和尿素脱胶等,影响其脱胶的工艺参数主要有时间、温度和脱胶剂的浓度,它们对SF影响对比如表1所示。
丝胶不溶于冷水,易溶于热水。然而在水热碱性条件下,SF会发生热降解和水解,所以脱胶温度不易过高,一般不超过100 ℃(沸水点);且温度不低于60 ℃,否则脱胶过程无法完成。强碱(NaCO/NaOH)处理对SF腐蚀性很强,不利于获得较优异的SF[11],碳酸钠的强碱性会破坏SF的分子链,导致其分子量降低,SF的分子量和其制备生物材料(如SF纤维膜)的降解率有关,较高的相对分子质量显示出高效的降解行为。采用NaHCO脱胶会保留较高的相对分子质量,对SF损伤性更小,虽NaHCO脱胶率高,但仍有一部分SF被丝胶黏接,不利于制备高纯度的SF[12-13]。为了保持碱性的恒定性,强碱性电解水(SAEW)脱胶可以稳定脱胶过程的pH值。如表1所示,当pH值为11.5时,SF纤维上的丝胶完全脱去。
皂碱法脱胶后的SF纤维很柔软,具有良好的弹性,但是时间长了容易变色发黄,因此不利于长时间储存。肥皂的蚕丝脱胶原理,即肥皂经过水解形成碱,再与丝胶蛋白相互作用,生成溶于水的物质。当单独采用带有一定碱性的肥皂脱胶时,可以较短时间完成脱胶,且对SF的损伤性比碳酸钠更小[14]。
从NaCO到SAEW再到肥皂脱胶,碱性逐渐减弱,虽然脱胶率随之下降(NaCO>SAEW>肥皂),但随之逐渐保持较高SF的相对分子质量,且拉伸率上升(NaCO<SAEW<肥皂)[15]。虽然这种肥皂苏打法可以有效地脱胶,碱性也较弱,但其脱胶周期有限[16]。
尿素作为一种温和的脱胶剂,有一定的碱性作用,研究人员[17]发现在相同质量浓度下,相比NaCO脱胶,尿素脱胶后的SF溶液黏度更高,即SF相对分子质量更高。尿素也分碱性和非碱性,当采用非碱性尿素,得到相对分子质量较高的SF,有较好的生物降解性,但对SF有一定的损伤[18]。由表1可见,其脱胶率相对偏高,SF部分被脱掉,可能是由于其水解等因素引起。但当非碱尿素和肥皂脱胶相比,脱胶后SF相对分子质量和黏度为非碱尿素>肥皂[19]。
1.2酸性处理
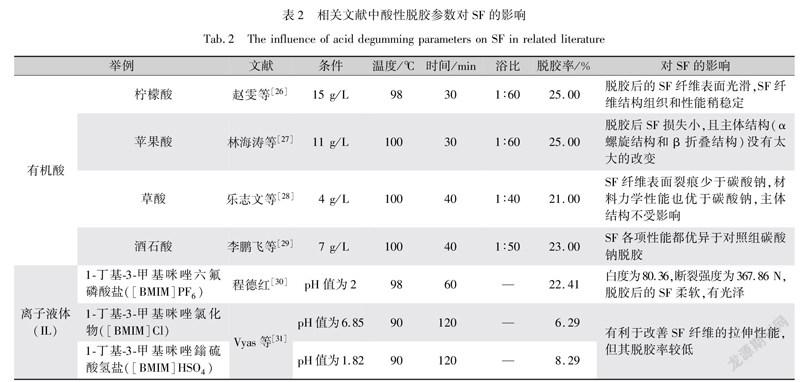
酸性脱胶不亚于碱性脱胶,特别是有机酸(如琥珀酸、柠檬酸、苹果酸、草酸、酒石酸等),其作用较碱性更温和。与肥皂碱脱胶法相比,使用柠檬酸在高温下脱胶得到的SF和肥皂脱胶一样柔软,但有机酸得到的SF纤维表面更光滑,稳定性更好[24]。然而与大多数碱处理一样,随着酸性强弱变化,SF仍然易受到攻击,例如琥珀强酸与柠檬弱酸相比,前者对SF的损伤性更大[16]。表2展现不同的有机酸脱胶参数及对SF产生的影响。相比于碱性脱胶,有机弱酸脱胶对SF纤维造成更小的损伤,在结构和性能上最大程度地保持了SF纤维的完整性,但其脱胶率不够,在SF纤维上仍残留不少丝胶蛋白,不利于制备高纯度的SF。
在酸性条件下,以离子液体(IL)对蚕丝进行脱胶,可充分发挥其特殊性能,如热稳定性、高溶解性等。由表2可见,以pH值、温度和时间为脱胶实验工艺参数,呈酸性的离子液体进行脱胶处理,使SF纤维表面比碱性处理更加丝滑[25]。虽然酸性离子液体相比碱性脱胶和有机酸更有利于改善SF纤维的拉伸性能,但其脱胶率太低,无法满足高纯度SF提取的要求[14]。
1.3物理/生物脱胶
除上述化学脱胶,现今主要的脱胶处理还有高温高压、超声波、微波辅助等物理方法和酶等生物方法,其保证了脱胶的高效环保。
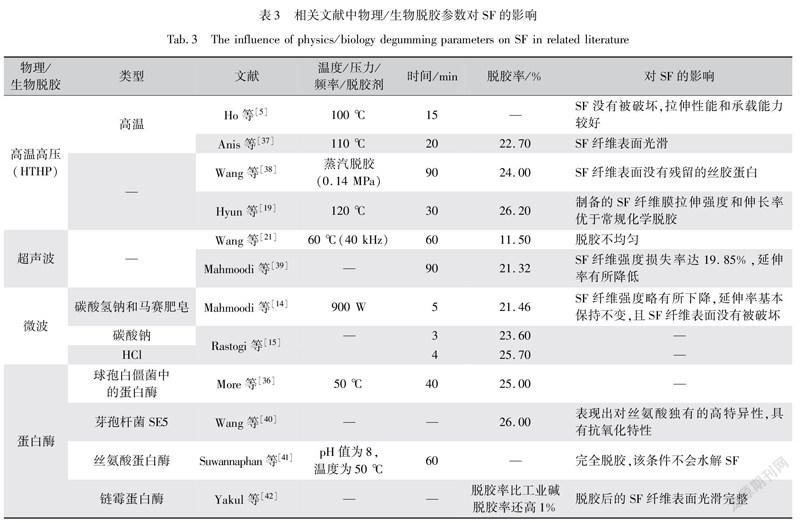
若不添加任何脱胶剂处理,即用常规物理方法对蚕丝进行脱胶,如高温高压(HTHP)脱胶,既可以使丝胶蛋白在高温下自行解体,又无废水处理,更加环保。利用高温高压的蒸汽法就是一种有效的脱胶方法,在热加压蒸汽中含有大量强渗透性的高能水分子,其可能导致蛋白质-蛋白质氢键的进一步被破坏。因此,丝胶蛋白可以逐渐水解并变得高度溶于水,从而使其与蚕丝蛋白分离。通常,高温高压(HTHP)制备的SF纤维膜的拉伸强度和伸长率优于常规化学脱胶,如肥皂苏打脱胶法,且在相同的浓度下,SF溶液黏度的顺序如下:HTHP脱胶>酸脱胶(柠檬酸)>肥皂/苏打脱胶[19]。但是温度和压力过高会使丝素蛋白中的氢键、肽键分解,不利于控制[26]。而且天然的蚕丝组织复杂,若长期暴露在空气和高温状态下,其微观组织和结构难免会产生一定的损伤,同时高温处理时间过长、过短都不利于SF表面的光滑性[20]。
随着超声波和微波技术的成熟,超声波和微波处理对蚕丝进行脱胶逐渐引起了人们的兴趣。超声波脱胶处理,即在SF和丝胶蛋白之间的交点处发生声空化效应,通过高频率声能,将丝胶蛋白团聚体破碎成小颗粒,而无需高温,避免对SF产生破坏。超声波频率、温度和时间等参数会影响脱胶效果,但是超声波脱胶处理脱胶效率不高,无法获得高纯度的SF。
无论是何种脱胶方式,都需要一定的热量,而微波处理可通过微波能量间接为材料加热,利用其进行脱胶处理,避免了传热问题。其最大的优点就是快速升温,快速为蚕丝脱去丝胶蛋白。常用的微波处理是需要和其他化学脱胶剂混合使用,无论是碱性还是酸性微波辅助脱胶,都可以在3~5 min完成脱胶,非常高效。其最大的优点就是脱胶时间最短,耗能最低。若单独使用微波处理,虽然能减少丝素纤维强度的损失,但是脱胶率比其辅助脱胶低[32],且微波处理脱胶不能应用于工业规模。从脱胶率可见,仍会有些许残留的丝胶蛋白,不利于获得高纯度的SF。
由微生物分泌的蛋白酶也会水解丝胶蛋白,蛋白酶将丝胶蛋白分解成肽,再经肽酶水解成氨基酸,使得丝胶蛋白脱落。如丝氨酸蛋白酶常被用作蚕丝脱胶,它将丝胶大分子蛋白质中的肽键断裂,使之成为小分子蛋白质,从蚕丝中脱掉。酶在较温和的环境下对蚕丝进行脱胶,在SF水解之前将丝胶蛋白瓦解,具有针对性,其脱胶后的废水可以回收利用,也在很大程度上避免了丝胶蛋白的浪费,环保节约。用蛋白酶脱胶后,SF质软,有光泽,操作温度低,生产效率高[33-34]。
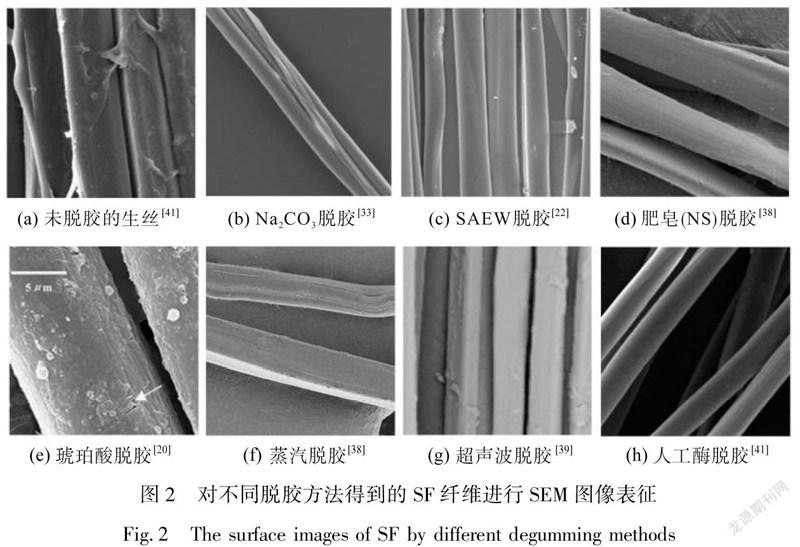
不同的蛋白酶有不同的脱胶效果。当采用天然的茧酶(蚕蛹在羽化时成虫分泌的蛋白酶)和枯草杆菌蛋白酶(Alcalase)比较脱胶效果,Alcalase会导致一定SF水解,对其提取不利。茧酶对SF几乎无影响,但是其脱胶率不高,即需要更多的茧酶(2ISU)和更多时间(48 h),才可完全除去丝胶[35]。而经过人工处理的蛋白酶比天然蛋白酶具有更好的脱胶功能,人工处理后的蛋白酶脱胶时间短,脱胶率高,脱胶效果好,如表3所示。图2是将几种脱胶方法进行对比,经表征可见酶脱胶效果较好。采用芽孢杆菌新分离的C4菌株产生的丝氨酸蛋白酶,脱胶后SF表面无残留的丝胶蛋白,SF纤维表面光滑细腻。可见人工处理的蛋白酶脱胶可解决酶脱胶耗时长的局限性,SF纤维强度可以保持不变,且处理温度低,降低了SF纤维的软化程度[36];且相比其他脱胶方法,人工酶脱胶毒理性更小,对于创伤愈合等生物医用材料的制备提供了潜在参考价值。
2结语
本文通过碱性、酸性、物理/生物处理等技术来对蚕丝进行脱胶,以获得SF纤维。生物医用材料领域对SF要求高,而脱胶方法会影响其生物性质和理化性能。碱性过强,对SF损伤性过大,虽然可通过减弱碱性来进行调整,但其脱胶率又无法接近理想值;尿素脱胶后的SF性能较好,但其已损失了部分SF;有机酸和离子液体在酸性脱胶中相对温和,但是脫胶不完全;高温高压及微波脱胶虽然环保节约,但超过了正常脱胶温度(100 ℃以下),加上压力的作用,影响SF的结构和性能;相比其他脱胶方法,人工酶处理技术在几乎完全脱去丝胶的同时,保证了SF的结构完整性,SF纤维表面光滑平整、质软,获得的SF新型材料可降解、抗氧化性能好。人工酶脱胶为SF在生物医用材料领域的使用提供了较好的技术支撑。
参考文献:
[1]RANGAMR, LIJING W, JAGAT R K, et al. Molecular weight and secondary structure change in eri silk during alkali degumming and powdering[J]. Journal of Applied Polymer Science, 2011, 119(3): 1339-1347.
[2]李慧君, 马彦龙, 贾兰, 等. 丝素蛋白水凝胶作为药物载体材料的研究进展[J]. 化工新型材料, 2017, 45(3): 230-232.
LI Huijun, MA Yanlong, JIA Lan, et al. Research progress of silk fibroin hydrogel used as drug carrier material[J]. New Chemical Materials, 2017, 45(3): 230-232.
[3]HAO D, BAOQI Z. Effect of sodium carbonate concentrations on the degumming and regeneration process of silk fibroin[J]. The Journal of the Textile Institute, 2015, 106(3): 311-319.
[4]KYUNGHWAN Y, HA NI L, CHANG SEOK K, et al. Effects of degumming conditions on electro-spinning rate of regenerated silk[J]. International Journal of Biological Macromolecules, 2013, 61: 50-57.
[5]MEI-PO H, HAO W, KIN-TAK L. Effect of degumming time on silkworm silk fibre for biodegradable polymer composites[J]. Applied Surface Science, 2012, 258(8): 3948-3955.
[6]KIRA N, OLIVER G. Silk fibroin degumming affects scaffold structure and release of macromolecular drugs[J]. European Journal of Pharmaceutical Sciences, 2017, 106: 254-261.
[7]J M, M R, V NITHYA P, et al. Substantial effect of silk fibroin reinforcement on properties of hydroxyapatite/silk fibroin nanocomposite for bone tissue engineering application[J]. Journal of Molecular Structure, 2020, 1206: 127739.
[8]K MURUGESH B, N S, DV A, et al. Silk fibroin coated antimicrobial textile medical products[J]. Journal of the Textile Institute, 2020(2): 1-9.
[9]REZA G, SHARON R, ZAINUDDIN, et al. Advancing towards a tissue-engineered tympanic membrane: Silk fibroin as a substratum for growing human eardrum keratinocytes[J]. Journal of Biomaterials Applications, 2010, 24(7): 591-606.
[10]XIUFANG L, LUPING W, LIANG L, et al. Water-stable silk fibroin nerve conduits with tunable degradation prepared by a mild freezing-induced assembly[J]. Polymer Degradation and Stability, 2019, 164: 61-68.
[11]LUPING W, ZUWEI L, QIANG Z, et al. Effect of degumming methods on the degradation behavior of silk fibroin biomaterials[J]. Fibers and Polymers, 2019, 20(1): 45-50.
[12]BENJAMIN J A, RANGAM R, RODNEY J D, et al. The impact of degumming conditions on the properties of silk films for biomedical applications[J]. Textile Research Journal, 2016, 86(3): 275-287.
[13]JAE S J, KYUNGHWAN Y, CHANG S K, et al. Effect of degumming condition on the solution properties and electrospinnablity of regenerated silk solution[J]. International Journal of Biological Macromolecules, 2013, 55(2): 161-168.
[14]NIYAZ MOHAMMAD M, FERESHTEH M, MOKHTAR M, et al. Silk degumming using microwave irradiation as an environmentally friendly surface modification method[J]. Fibers and Polymers, 2010, 11(2): 234-240.
[15]SHIVANI R, BALASUBRAMANIAN K. Processing trends of silk fibers: Silk degumming, regeneration and physical functionalization[J]. Journal of the Textile Institute, 2020, 111(1): 1-17.
[16]ZONGQIA W, HAIWEI Y, WEI L, et al. Effect of silk degumming on the structure and properties of silk fibroin[J]. Journal of the Textile Institute, 2019, 110(1): 134-140.
[17]VIKAS P, TANWEER H, ASHOK R, et al. Surface modified silk fibroin nanoparticles for improved delivery of doxorubicin: Development, characterization, in-vitro studies[J]. International Journal of Biological Macromolecules, 2020, 164: 2018-2027.
[18]HAIWEI Y, ZONGQIA W, MINGRONG W, et al. Structure and properties of silk fibroin aerogels prepared by non-alkali degumming process[J]. Polymer, 2020, 192: 122298.
[19]HYUN JU K, MOO KON K, KI HOON L, et al. Effect of degumming methods on structural characteristics and properties of regenerated silk[J]. International Journal of Biological Macromolecules, 2017, 104: 294-302.
[20]KRIA N, LIVIA K B, MURIEL N, et al. Effects of silk degumming process on physicochemical, tensile, and optical properties of regenerated silk fibroin[J]. Macromolecular Materials and Engineering, 2018, 303(12): 1800408.
[21]WUCHAO W, YI P, KANG G, et al. A comparative study of ultrasonic degumming of silk sericin using citric acid, sodium carbonate and papain[J]. Coloration Technology, 2019, 135(3): 195-201.
[22]CAO T T, WANG Y J, ZHANG Y Q. Effect of strongly alkaline electrolyzed water on silk degumming and the physical properties of the fibroin fiber[J]. Plos One, 2013, 8(6): e65654.
[23]DEBASIS C, ARIJIT C, S M C. Studies on degumming of eri silk cocoons[J]. Journal of the Textile Institute, 2017, 108(8): 1327-1339.
[24]VYAS S K, SHUKLA S R. Comparative study of degumming of silk varieties by different techniques[J]. Journal of the Textile Institute, 2016, 107(2): 191-199.
[25]VYAS S K, SHUKLA S R. Degumming of tasar silk using imidazolium-based ionic liquids[J]. Journal of the Textile Institute, 2020, 111(9): 1364-1370.
[26]赵雯, 陈国强. 蚕丝的柠檬酸脱胶[J], 印染助剂, 2012, 29(7): 36-38.ZHAO Wen, CHEN Guoqiang. Degumming raw silk with citric acid[J]. Textile Auxiliaries, 2012, 29(7): 36-38.
[27]林海涛, 封宝山, 陶立全, 等. 蚕丝的苹果酸脱胶[J]. 丝绸, 2013, 50(10): 1-5.LIN Haitao, FENG Baoshan, TAO Liquan, et al. Degumming of silk with malic acid[J]. Journal of Silk, 2013, 50(10): 1-5.
[28]乐志文, 李鹏飞, 李金环, 等. 生絲的草酸脱胶工艺研究[J]. 纺织高校基础科学学报, 2017, 30(4): 445-450.
YUE Zhiwen, LI Pengfei, LI Jinhuan, et al. Research on degumming of raw silk with oxalic acid[J]. Basic Sciences Journal of Textile Universities, 2017, 30(4): 445-450.
[29]李鵬飞, 蒋娟娟, 凌新龙. 生丝的酒石酸脱胶研究[J]. 广西科技大学学报, 2018, 29(1): 94-99.
LI Pengfei, JIANG Juanjuan, LING Xinlong. Degumming of raw silk with tartaric acid[J]. Journal of Guangxi University of Science and Technology, 2018, 29(1): 94-99.
[30]程德红. 离子液体在桑蚕丝脱胶及染色中的应用[J]. 化工学报, 2011, 62 (S2): 169-172.CHENG Dehong. Application of ionic liquid on degummed and dyeing of silk[J]. CIESC Journal, 2011, 62 (S2): 169-172.
[31]VYAS S K, SHUKLA S R. Degumming of eri silk using ionic liquids and optimization through response surface methodology[J]. Journal of the Textile Institute, 2016, 107(9): 1096-1111.
[32]MD MAJIBUR R, MASUHIRO T, YASUO G, et al. Physical properties and dyeability of silk fibers degummed with citric acid[J]. Bioresource Technology, 2010, 101(21): 8439-8445.
[33]MEI-PO H, HAO W, KIN-TAK L, et al. Interfacial bonding and degumming effects on silk fibre/polymer biocomposites[J]. Composites Part B, 2012, 43(7): 2801-2812.
[34]S V M, H B K, M A J, et al. Enzymatic degumming of silk with microbial proteases[J]. Journal of Natural Fibers, 2013, 10(2): 98-111.
[35]PRANGPRAPAI R, DUMRONGKIET A, UTAI U, et al. Functional expression of a bombyx mori cocoonase: Potential application for silk degumming[J]. Acta Biochim Biophys Sin, 2012, 44(12): 974-983.
[36]SNEHALV M, SAKALYA C, ASMITA A P. Silk degumming and utilization of silk sericin by hydrolysis using alkaline protease from beauveria sp (MTCC 5184): A green approach[J]. Journal of Natural Fibers, 2018, 15(3): 373-383.
[37]PERVIN A, TUBA T, EYUPHAN Y, et al. Investigation of the effects of environmentally friendly degumming methods on silk dyeing performance[J]. Textile Research Journal, 2019, 89(7): 1286-1296.
[38]RUI W, YAOFENG Z, ZHUO S, et al. Degumming of raw silk via steam treatment[J]. Journal of Cleaner Production, 2018, 1216: 492-497.
[39]NIYAZ MOHAMMAD M, MOKHTAR A, FIROOZMEHR M, et al. Degradation of sericin (degumming) of Persian silk by ultrasound and enzymes as a cleaner and environmentally friendly process[J]. Journal of Cleaner Production, 2010, 18(2): 146-151.
[40]XIAO W, YIWEI Q, ANDREW J C, et al. Improved human tenocyte proliferation and differentiation in vitro by optimized silk degumming[J]. Biomedical Materials, 2011, 6(3): 035010.
[41]SUNISA S, EKKASIT F, AMORNRAT P, et al. A serine protease from newly isolated Bacillus sp for efficient silk degumming, sericin degrading and colour bleaching activities[J]. International Biodeterioration and Biodegradation, 2017, 117: 141-149.
[42]KAMON Y, SHINJI T, KENSUKE N, et al. Characterization of thermostable alkaline protease from Bacillus halodurans SE5 and its application in degumming coupled with sericin hydrolysate production from yellow cocoon[J]. Process Biochemistry, 2019, 78: 63-70.
Study on extraction of silk fibroin fiberFENG Yanping DONG Zhihong ZHANG Ying CHENG Lijia TANG Lu GUI Yanhua(1a.School of Mechanical Engineering; 1b.School of Preclinical Medicine, Chengdu University, Chengdu 610106, China;
2.Sichuan Industrial Institute of Antibiotics, Chengdu 610051, China; 3.Affiliated Hospital of Chengdu University,
Chengdu 610081, China; 4.Sichuan Innovation Biotechnology Co., Ltd., Chengdu 610213, China)
Abstract: Silk fibroin (SF) is used in various fields, such as air sensing, filtration and biomedical engineering fields, especially in biomedicine. SF has good biocompatibility and low immunogenicity, which prevents rejection and inflammation in human body. At the same time, it exhibits good biodegradability, certain hydrophobicity and permeability, which may improve cell adhesion and proliferation. Besides, it is widely used in wound, nerve, bone and cartilage repair for its good mechanical properties. To obtain the ideal SF, the natural SF containing sericin and a small amount of impurities needs to be degummed. The purpose of this paper is to summarize different extraction methods for degumming treatment. SF with different structures can be obtained by reasonably selecting process parameters, which provides some guidance for the application of SF in biomedical materials.
In this paper, different degumming methods and technological parameters are studied. The degumming methods of silk are discussed from three aspects of chemistry, physics and biotechnology, including alkaline, acidic, HTHP, ultrasonic, microwave and enzyme treatment. Take sodium carbonate in alkali degumming as an example, in the process of degumming, sodium carbonate can react with sericin to dissolve it, so as to obtain SF. However, different degumming methods and degumming parameters, including concentration, temperature, pH, degumming time and pressure value, can destroy SF structure and affect its performance. The selection of relatively good degumming methods and parameters is the key to obtaining high performance. By comparing with other degumming methods, it is found that the artificial enzyme degumming scheme can obtain SF with better structure, smooth surface and better mechanical properties. It provides a good preliminary research foundation for the application of SF in the field of preparation of new biomedical materials.
This article is expected to provide a reasonable comprehensive reference for the selection and optimization of silk degumming processes, as well as the control and improvement of SF structure and performance, so as to better play its SF function in the biomedical field.
Key words: silk fibroin fiber; sericin; degumming method; biomedical materials; mechanical property
