Ethephon Accelerates Wound Healing in Ginger Rhizomes by Modulating Antioxidant Enzyme Activities and Secondary Metabolite Production
2022-04-01ZHUJieLICanyingSUNLeiGEYonghong
ZHU Jie, LI Canying, SUN Lei, GE Yonghong,*
(1. College of Food Science and Engineering, Bohai University, Jinzhou 121013, China; 2. National and Local Joint Engineering Research Center of Storage, Processing and Safety Control Technology for Fresh Agricultural and Aquatic Products, Jinzhou 121013, China)
Abstract: Ginger rhizomes are subject to mechanical damage during harvest, handling, and transportation, and ethephon has been reported to promote plant wound healing. In this study, the effect and mechanism of ethephon treatment after harvest on wound healing in ginger rhizomes were investigated. The results indicated that 50 mg/L ethephon treatment retarded mass loss, and enhanced the contents of hydrogen peroxide, total phenolic compounds and lignin in ginger rhizomes during the wound healing process. The activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) involved in reactive oxygen species (ROS) metabolism were increased in the ethephon-treated ginger rhizomes. The accumulation of phenolic compounds and lignin during wound healing were correlated with a significant increase in peroxidase (POD) and phenylalanine ammonia lyase (PAL) activities. These findings suggest that ethephon treatment can promote the wound healing process of ginger rhizomes by modulating key enzyme activities involved in ROS metabolism and the phenylpropanoid pathway to enhance antioxidant capacity and accumulate secondary metabolites.
Keywords: ethephon; ginger rhizomes; wound healing; reactive oxygen species; phenylalanine ammonia lyase
Ginger (Zingiber officinaleRosc.) is perennial herb of the ginger family, and widely cultivates in the central,southeastern and southwestern China[1]. It not only contains bioactive substances such as zingiberol, zingiberene,phellandrene, camphene and citral, but also is abundant in crude protein, amino acids, aspartic acid, carbohydrates,vitamins and minerals[2]. Old rhizome, fresh rhizome,stem and leaf were widely studied in order to improve the comprehensive utilization of ginger, among them fresh rhizome had the best antioxidant effect including preventing lipidemia, hyperglycemic, and relieving cough[3-4].However, pathogen infection happens seriously during storage especially absent of well wound-healing. The main postharvest diseases of ginger are rhizome rot caused byFusarium oxysporum, bacterial wilt caused byRalstonia solanacearumand anthracnose caused byColletotrichum capsiciandC. gloeosporioides[5]. Wound injury is inevitable during harvest, transportation and storage, and the wounds provide the approach to pathogen invasion and cause decay.Therefore, wound-healing after harvest is very important to prolong storage period and maintain quality during conventional storage including bury, cellar and refrigeration[6].Traditionally, ginger rhizomes finish wound-healing themselves under field conditions before storage. However, natural healing takes a longer period. Therefore, it is quite necessary to develop a convenient and effective healing method to reduce the quality loss and deterioration of ginger.
Many evidences proved that postharvest abscisic acid,nitric oxide, benzo-(1,2,3)-thiadiazole-7-carbothioic acidS-methyl ester (BTH), chitosan treatments promote the wound-healing of sweet potatoes[7], muskmelons[8], potato tubers[9]and citrus fruit[10]. The mechanisms involved in wound-healing including activation the generation of reactive oxygen species (ROS), phenylpropanoid and energy metabolisms, and suberization[11], in which ROS has the dual function in signal transduction and oxidative cross-linking of healing formation[12].
Ethephon is an effective plant growth regulator,which can regulate the ripening and senescence of fruit and vegetables, enhance the activity of enzymes related to fruit ripening, induce the expression of defense genes. Study has showed that soaking ginger rhizomes with ethephon at different concentration could improve the number of plants, branches, leaves and rhizome yield[13]. Ethylene treatment could expedite the rapid lignification of wound sites and enhance the ability of wound cells to form callus[14].Studies have proved that the application of ethephon could induce the increase of phenylalanine ammonia lyase (PAL) activity,accelerate the deposition of phenolic compounds and lignin at the wound site, and promote the healing of broccoli florets[15],tomatoes[16], and bamboo shoots[17]. Ethephon treatment(10 μg/L) can also increase the ethylene release and accelerate the healing process of apples[18]. However, the effect of applying ethephon postharvest on wound-healing of ginger rhizomes and its possible mechanism involved were not well investigated yet.
The purposes of this study were to investigate the effect of postharvest ethephon treatment on wound-healing of ginger rhizomes and explore the primary mechanisms involved in the process of wound-healing.
1 Materials and Methods
1.1 Materials and chemicals
Ginger rhizomes were collected from Beizhen (Liaoning Province), China. Ginger rhizomes with good texture, free of disease and pest, and undamaged were selected and transported to the laboratory at the same day with cartons.
Ethephon, ascorbic acid,L-phenylalanine, oxidized glutathione, nicotinamide adenine dinucleotide phosphate Beijing Solarbio Life Sciences; Acetone, titanium tetrachloride, ethanol, guaiacol Tianjin Fuchen Chemical Reagent Factory; Hydrochloric acid, H2O2, methanol Jinzhou Gucheng Chemical Reagent Co., Ltd..
1.2 Instrumental and equipment
UV-2250 Ultraviolet and visible spectrophotometer Shandong Arcane Technology Co., Ltd.; H1650 high speed refrigerated centrifuge Shanghai Huyueming Scientific Instrument Co., Ltd.; ML204 Precision electronic balance Hebei Ruipu Instrument Co., Ltd.; ZBS-20 Mini ice maker Shanghai Anting Scientific Instrument Factory;Thermo Forma 900 Refrigerator Beijing Jinye Dexiang Biotechnology Co., Ltd..
1.3 Methods
1.3.1 Sample treatment
Selected ginger rhizomes were washed with tap water,immersed in 1.0 mL/L sodium hypochlorite for 1 min for disinfection and rinsed with distilled water. Then ginger rhizomes were dipped in 50 mg/L ethephon solution(containing 0.1 mL/L Tween 20) for 10 min and desiccated at room temperature. The ethephon concentration used in this study was screened by the preliminary experiments.Distilled water treatment was used as the control under the same conditions. After air-drying, the treated rhizomes were put into plastic trays and kept at (22 ± 1) ℃ with 80%-85%relative humidity.
1.3.2 Measurement of mass loss rate
The mass of ginger rhizomes were measured on day 0,7, 14, 21, 28, 35 and 42, and mass loss rate was calculated according to the formula as following.
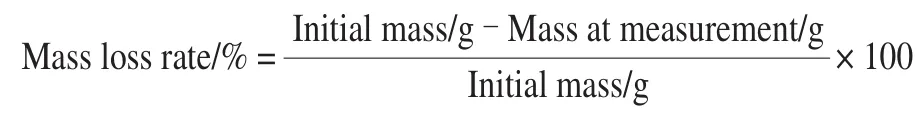
1.3.3 Sample collection
Sample was collected on day 0, 7, 14, 21, 28, 35 and 42 by peeling and mincing 15 of the ginger rhizomes treated with ethephon and control group. Tissues were frozen with liquid nitrogen and then kept at -80 ℃ for the following analysis.
1.3.4 Measurement of H2O2content
The content of H2O2was determined by the method of Ge Yonghong et al[19-20]. Briefly, 3.0 g of frozen tissues were ground into homogenates with 3.0 mL cold acetone, and then centrifuged for 20 min (12 000 ×g, 4 ℃). The reaction mixture included 2.0 mL of supernatant, 40 μL of 20 mL/L titanium tetrachloride solution and 50 μL of concentrated ammonia solution, and centrifuged for 10 min at the same conditions after 5 min reaction. Finally, the precipitation was dissolved in 3.0 mL of sulfuric acid solution (1.0 mol/L), and absorbance of the solution was determined at 410 nm. H2O2content was indicated as nmol/g (results in fresh mass).
1.3.5 Measurement of superoxide dismutase activity
Superoxide dismutase (SOD) activity was assayed as described by Ge Yonghong et al.[20]with some modifications.Ginger tissues (3.0 g) was pulverized with a triturator and homogenized with 3.0 mL of 100 mmol/L phosphate buffer(pH 7.5), and then centrifuged at 12 000 ×gat 4 ℃ for 30 min. The supernatant was collected and used for assaying SOD activity. The activity of SOD was defined as an enzyme activity unit (U) of 50% of the photoreduction of nitroblue tetrazolium (NBT) per milligram protein.
1.3.6 Measurement of catalase activity
Crude enzyme extraction and catalase (CAT) activity measurement were conducted as mentioned by Wang Yousheng et al[21]. The reaction mixture was composed of 800 μL of 3.0 mmol/L ascorbic acid, 200 μL of crude enzyme extract, and 2.0 mL of 100 mmol/L phosphate buffer (pH 7.5).Absorbance of the reaction solution at 240 nm was recorded.CAT activity was represented as U, where one unit was defined as the changes of absorbance at 240 nm for 0.01 per minute per milligram protein.
1.3.7 Measurement of ascorbate peroxidase and glutathione reductase activities
The method described by Ren Yaling et al.[22]was used to extract crude enzyme and assay the activities of ascorbate peroxidase (APX) and glutathione reductase (GR).The reaction solutions for APX included 800 μL of 3.0 mmol/L ascorbic acid, 200 μL of crude enzyme, 2.0 mL of 100 mmol/L phosphate buffer (pH 7.5) and 500 μL of 0.5 mmol/L H2O2.GR reaction system was consisted of 3.0 mL of 100 mmol/L phosphate buffer (pH 7.5), 100 μL of 5.0 mmol/L oxidized glutathione (GSSG), 200 μL of crude enzyme and 30 μL of 3.0 mmol/L nicotinamide adenine dinucleotide phosphate (NADPH).APX and GR activities were represented as U, where one unit was defined as the changes of absorbance at 290 nm and 340 nm for 0.01 per minute per milligram protein.
1.3.8 Measurement of peroxidase activity
Crude enzyme extraction and peroxidase (POD) activity determination were conducted following the method of Liu Hongxia et al[23]. The reaction solutions were consisted of 200 μL of 250 mmol/L H2O2, 2.5 mL of 25 mmol/L guaiacol and 200 μL of crude enzyme. POD activity was represented as U, where one unit was defined as the changes of absorbance at 470 nm for 0.01 per minute per milligram protein.
1.3.9 Measurement of phenylalanine ammonia-lyase activity
The modified method of Assis et al.[24]was used to extract crude enzyme and determine phenylalanine ammonialyase (PAL) activity. The reaction mixtures included 500 μL of 20 mmol/LL-phenylalanine, 500 μL of crude enzyme extract, and 3.0 mL of sterile deionized water. One unit of PAL activity was defined as the change of 0.01 in the absorbance at 290 nm per minute per milligram protein, and represented as U.
1.3.10 Measurement of the protein content
The method of Bradford[25]was used to assay the protein content in crude enzymes.
1.3.11 Measurement of total phenolic compounds and lignin contents
Total phenolic compounds content was analyzed by Liu Yaoyao et al[26]. Briefly, 3.0 g of frozen tissues were ground into homogenate in 5.0 mL of pre-cooled 1% hydrochloric acid-methanol, and then centrifuged at 12 000 ×gfor 15 min at 4 ℃. Absorbance of the supernatant was measured at 280 nm, and total phenolic compounds content was represented as OD280nmper gram of fresh tissue.
The modified method of Ge Yonghong et al.[27]was used to assay lignin content. Tissues (1.0 g) were homogenized in 3.0 mL of 95% cold ethanol and then centrifuged at 12 000 ×gfor 15 min at 4 ℃. The sediment was washed 3 times with 95% ethanol and ethanol:n-hexane (1:2,V/V), respectively.After centrifugation, the precipitate was dissolved in 1.0 mL of 25% acetic acid, and then heated for 30 min at 70 ℃. The termination reaction occurred after adding 1.0 mL of 2.0 mol/L sodium hydroxide. The absorbance of the reaction mixtures was determined by colorimetry at 280 nm, and the lignin content was expressed as OD280nmper gram of fresh tissue.
1.4 Statistical analysis
All triplicates data were performed by one-way analysis of variation using SPSS 19.0 software.P< 0.05 were considered significant by least significant difference (LSD).
2 Results and Analysis
2.1 Effect of ethephon treatment on mass loss rate of ginger rhizomes
Mass loss rate of the ethephon-treated and control ginger rhizomes increased with the extension of wound-healing period (Fig. 1). Ethephon treatment restrained the increase of mass loss rate during wound-healing period. However, there is no distinct difference in the mass loss rate between the control group and ethephon-treated ginger rhizomes.

Fig. 1 Effect of ethephon treatment on mass loss of ginger rhizomes during wound healing
2.2 Effects of ethephon treatment on H2O2 content, SOD and CAT activities in ginger rhizomes
SOD activity increased in the ginger rhizomes treatment with ethephon on day 0—21 and descended in the following period, but ascended on day 0—7 in the control group and peaked on day 7 (Fig. 2A). Postharvest applying ethephon notably increased SOD activity in ginger rhizomes on day 14—42. The maximum level of SOD activity was determined on day 21 as 1.65-fold higher in the ethephon-treated group than that of the control group.

Fig. 2 Effect of ethephon treatment on SOD activity (A), H2O2 content (B),and CAT activity (C) in ginger rhizomes during wound healing
H2O2content in the ginger rhizomes treated with ethephon increased on day 0—21, and decreased on day 21—42. Similar trend was found in the control group, but peaked on day 14 (Fig. 2B). On day 21, H2O2content in the ethephontreated group was 1.49-fold higher than the control group.
CAT activity in the ginger rhizomes treatment with ethephon and the control group indicated similar trends which increased on day 0—7 and decreased on day 7—42 (Fig. 2C).The significant differences between the control and ethephontreated ginger rhizomes were observed on day 7—35.
2.3 Effects of ethephon treatment on the activities of APX,GR and POD in ginger rhizomes
As shown in Fig. 3A, the activity of APX increased on day 0—14 and day 21—35 but decreased on day 14—21 and day 35—42 in the control group. In the ethephon-treated ginger rhizomes, APX activity increased on day 0—14 and declined on day 14—42. Ethephon treatment resulted in a considerable augment in APX activity in the ginger rhizomes which was notably higher than the control group on day 7—28. The highest level of APX activity in the ethephon-treated ginger rhizomes was observed on day 14 as 1.20-times higher than that of the control group.
GR activity in the ginger rhizomes treatment with ethephon increased on day 0—14 and decreased in the subsequent period, while in the control group GR activity increased on day 0—21 and decreased on day 21—42 (Fig. 3B).Ethephon treatment significantly promoted GR activity on day 7—28 in comparison with the control group.
POD activity was peaked on day 14 and 35 in both the ethephon-treated ginger rhizomes and control group(Fig. 3C). Ethephon treatment distinctly enhanced POD activity on day 7, 14, 28 and 35 compared to the control group. After 35 day of storage, the POD activity in the ethephon-treated ginger rhizomes reached the highest level,which was 1.19-times higher than the control group.
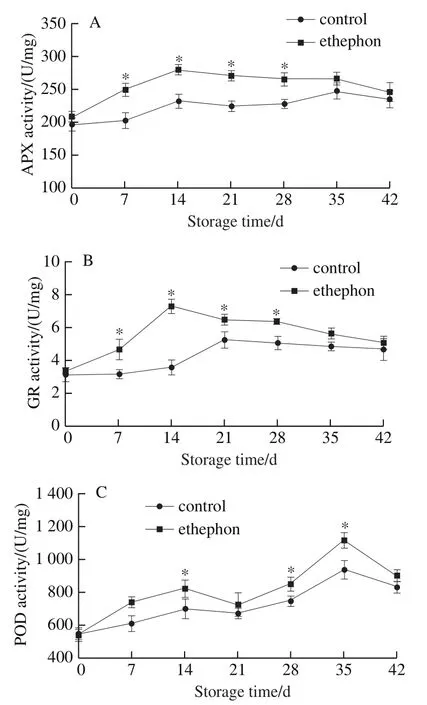
Fig. 3 Changes in the activities of APX (A), GR (B) and POD (C) in ginger rhizomes treated with ethephon during wound healing
2.4 Effects of ethephon treatment on PAL activity in ginger rhizomes
Fig.4 shows that PAL activity in the ethephon-treated group increased on day 0—35 and decreased in the following period. However, PAL in the control group did not change significantly on day 0—42. Postharvest ethephon treatment notably increased PAL activity in ginger rhizomes on day 21—42 in comparison with the control group. The highest value of PAL activity was assayed on day 35, which was 1.31-times higher in the ethephon-treated ginger rhizomes than that of the control group.
Fig. 4 Effect of ethephon treatment on PAL activity in ginger rhizomes during wound healing
2.5 Effect of ethephon treatment on the contents of total phenolic compounds and lignin
Fig. 5A shows that the content of total phenolic compounds inclined in the ethephon-treated groups on day 0—42. Ethephon treatment dramatically improved total phenolic compounds content in ginger rhizomes on day 21,28, 35, 42 in comparison with the control group, which was 1.13-, 1.28-, 1.18-, and 1.13-times higher than that of the control group, respectively. Lignin content increased in the ethephon-treated ginger rhizomes during the entire woundhealing period which was distinctly higher than that of the control group on day 14—42 (Fig. 5B). The highest level of lignin content in the ethephon-treated ginger rhizomes was determined on day 42 which was 1.55-times higher than the control group.

Fig. 5 Effect of ethephon treatment on the contents of total phenolic compounds (A) and lignin (B) in ginger rhizomes during wound healing
3 Discussion
Mass loss is one of the important factors that affect the appearance and quality of fruit and vegetables, and mass loss rate is also an important indicator for evaluating the effect of wound-healing[28]. Mass loss can be caused by water evaporation, respiration and other metabolism.The results of this study showed that 50 mg/L ethephon treatment reduced the mass loss of ginger rhizomes (Fig. 1),and also inhibited respiratory rate (data is not shown).These results suggest that ethephon treatment could reduce the consumption of nutrients and evaporation during wound-healing.Similar reports have been reported in soft pears[29]andMomordica charantia[30].
When the plant is subjected to stress conditions, the ROS generation capacity was activated, thereby releasing different types of ROS includingOH and H2O2[31]. The production of ROS could not only kill the pathogen directly, participate in the lignification of the wound to strengthen the cell wall,but also serve as a signal to trigger other defense responses[32].SOD is a natural superoxide radical scavenging enzyme in the cells, which can convert the unstableinto H2O2[33].This study demonstrated that ethephon treatment enhanced SOD activity in ginger rhizomes during wound-healing,therefore accelerated the accumulation of H2O2. Li Canying et al.[34]reported that the production of H2O2in ginger rhizomes could activate phenylpropanoid pathway to accumulate antifungal compounds. In addition, H2O2is also involved in the cross-linking of cell wall proteins and the oxidative polymerization of phenolic compounds during lignin formation, thereby indirectly reducing disease and promoting wound-healing[35]. Our results also showed that ethephon treatment distinctly enhanced PAL and POD activities in ginger rhizomes during wound-healing and accelerated lignin accumulation and phenolic compounds accumulation, which was in agreement with the study in methyl jasmonate-treated ginger rhizomes[34].
Although H2O2helps to enhance disease resistance of the fruit to some extent, but excessive H2O2is toxic to the cells[36]. CAT, APX, and GR are crucial components of the cell protective enzyme system to eliminate H2O2. CAT is an significant ROS-detoxifying enzyme and its activity is closely correlated with redox status of ginger rhizomes[36].It can decompose H2O2in ginger rhizomes into O2and H2O, and slow down oxidative damage[37]. CAT activity enhancement was observed during the early wound-healing period in this study (Fig. 2C), which was consistent with the result described in the acibenzolar-S-methyl-treated potato tuber during wound-healing. APX and GR are two important enzymes in the ascorbate-glutathione (AsA-GSH) cycle and contribute to protecting cells from oxidative damage[34].GR plays an important role in maintaining glutathione content and redox state of cells in plants[38]. With AsA as the substrate, APX catalyzes the reduction of H2O2into H2O and oxygen. We found that ethephon treatment significantly induced the activity of APX and GR within 14 days during wound-healing of ginger rhizomes, suggesting that APX and GR might participate in the elimination of H2O2in the early stage of wound-healing[39]. Therefore, ethephon treatment can elevated the ability of the healing tissue to scavenge excess ROS during healing, indicating that the production and scavenging of ROS is possibly involved in the wound healing in ginger rhizomes elicited by ethephon. However, more study is still needed to fully elucidate the role of AsA-GSH cycle in wound-healing of ginger rhizomes.
Biotic or abiotic stresses can activate the phenylpropanoid pathway and produce a series of secondary metabolites, such as phenols, lignins, flavonoids and alkaloids[40]. These substances are deposited at the wound site and participate in the formation of callus to enhance disease resistance. The phenolic compounds, lignin and other metabolites could enhance cell wall structure and antioxidants. Similar results have been reported in the woundhealing process of muskmelons, tomatoes and kiwifruits[8,16,41].PAL is the initiator of phenylpropanoid pathway and its activity is proportional to the wound-healing ability of the damaged site. Recent studies have shown that H2O2can induce an increase in the activity of PAL, while removal of H2O2can decrease PAL activity[42]. POD, as a key enzyme in the formation of healing sealant layer, takes H2O2as a substrate and participates in the oxidative polymerization of phenolic substances and the synthesis of lignin to strengthen the cell wall[43]. POD also works with CAT and other enzymes to prevent ROS from damaging the cell membrane. Study in apples and pineapples reported that the accumulation of H2O2at the early stage of wound-healing was closely related to the induction of PAL and POD activities. The current study found that ethephon treatment enhanced the content of H2O2, and the activities of POD and PAL in ginger rhizomes. Phenolic compounds are precursors of lignin synthesis and can form a physical barrier against pathogen infection[44]. Chemically induced lignin and phenolic compounds accumulation increase have already been observed in many wounded fruit and vegetables, such as sweet potatoes, cherimoya, and carrots[7,28,45], and this is consistent with our observation that ethephon treatment promoted the accumulation of phenolic compounds and lignin in ginger rhizomes. Therefore, we speculated that ethephon treatment can weaken membrane lipid peroxidation and improve antioxidant capacity, promote the accumulation of phenolic substances, and accelerate the callus process. Similar results have also been reported that ROS are involved in the accumulation of phenolic substances in carrot after mechanical injury, and may play a role as signal molecules[45]. All these results suggest that ethephon treatment activate ROS generation at the early stage of wound-healing, and then trigger phenylpropanoid pathway to accumulate metabolites and enhance disease resistance in ginger rhizomes.
4 Conclusions
Postharvest ethephon treatment could accelerate the wound-healing of ginger rhizomes by modulating the production of H2O2and enzyme activity in H2O2metabolism to maintain redox status in the cells. Ethephon treatment also enhances the key enzyme in phenylpropanoid pathway to accumulate phenolic compounds and lignin, therefore formation healing tissues to enhance disease resistance and prolong storage period.
